Introduction
The mustard aphid Lipaphis erysimi (L.) Kaltenbach (Hemiptera: Aphididae) is one of the most important pests of brassica crops, mainly in tropical and subtropical climate areas (Lamb et al., Reference Lamb, Smith and Bodnaryk1993; Liu & Meng, Reference Liu and Meng2000; Rana, Reference Rana2005). These aphids quantitatively and qualitatively affect plant production, through sap sucking, toxin injection and transmission of viruses from the Luteoviridae family, leading to leaf curling, shrivelling and yellowing (Sylvester, Reference Sylvester, Minks and Harrewijn1987). In addition, these aphids produce honeydew, a medium for the growth of sooty mold that negatively effects photosynthesis, leaf durability, and crop market value (Ram et al., Reference Ram, Gupta and Maurya1989).
From a pest management standpoint, it is very important to know the main natural factors (i.e., key mortality factors) that regulate insect pest populations, since the magnitude of these factors (climatic elements, natural enemies or top-down forces, and host-plant attributes or bottom-up effects) varies considerably with the pest species (Pereira et al., Reference Pereira, Picanço, Bacci, Crespo and Guedes2007, Reference Pereira, Neves, Campos, Santana, Hunt and Picanço2018; Semeão et al., Reference Semeão, Martins, Picanço, Chediak, Silva and Silva2012; Silva et al., Reference Silva, Silva, Silva, Milagres, Bacci and Picanço2017). In addition, the application of control measures at the stages that most influence the size of a pest population (i.e., critical stages) increases control efficiency and allows the reduction of insecticide use and environmental impacts (Wilby & Thomas, Reference Wilby and Thomas2002; D'Auria et al., Reference D'Auria, Wohleb, Waters and Crowder2016).
In this context, ecological life tables are very useful tools because, through the qualification and quantification of the mortality factors at each stage of a pest life cycle, they identify the key factors and the critical mortality stages (Harcourt, Reference Harcourt1969; Podoler & Rogers, Reference Podoler and Rogers1975; Southwood & Henderson, Reference Southwood and Henderson2000). The information obtained by life table studies also allows the identification of new natural biological control agents and provides data to determine potential sources of plant resistance to pests.
In this study, we report the critical stage and key mortality factors for L. erysimi using an ecological life table aiming to better understand the population ecology and role of natural factors in regulating populations of this important pest.
Materials and methods
Study site
The study was carried out on cabbage crops (Brassica oleracea var. capitata, hybrid Sekai F1) in the experimental area of the university (20°46′10″S; 42°52′10″W; altitude 750 m), Minas Gerais state, Brazil. The climate of the study region is tropical and corresponds to the Köppen class Cwb (Peel et al., Reference Peel, Finlayson and McMahon2007), with rainy summers and dry winters. The average annual temperature is 19.4°C, ranging between 13 and 30°C. The mean annual rainfall is 1170 mm with rains concentrated between October and March (INMET, 2017). The study site was located near fragments of native vegetation (seasonal semi-deciduous forest).
The cabbage crops were constituted by five rows of 30 plants spaced 1 × 0.5 m2. Cabbage seedlings were transplanted in the field 30 days after sowing. The fields were grown as recommended by Filgueira (Reference Filgueira2000). No pesticide was applied during the study.
Insects
The aphids used in the experiments were obtained from an established laboratory population. To carry out this rearing, cabbage leaves infested with L. erysimi were collected in commercial cabbage fields from Viçosa County, Minas Gerais, Brazil. Adults from these colonies were transferred to cabbage leaves and placed in wooden cages (45 × 45 × 45 cm3) covered with organza fabric. Twenty-four hours after the transfer, the females were removed and only the nymphs generated were left on the leaves, in order to avoid infestation of parasitoids and fungi during rearing. Every 3 days, new cabbage leaves were added to the cages and the yellow leaves were removed.
Cohort establishment
Life table data of L. erysimi were obtained in seven periods, as presented in table 1. These periods were selected to allow the evaluation of the factors regulating L. erysimi populations in all seasons of the year. The whole cycle of the aphids was monitored in the field to determine the critical stage and the mortality factors of these insects at each stage. The experimental design was completely randomized with 10 plots. Each plot consisted of two cabbage plants in stage 3 (6–8 leaves): one designated to evaluate the mortality caused by physiological disturbances and the other to determinate total mortality.
Table 1. Periods of data collection of Lipaphis erysimi life table in cabbage crops in Viçosa, MG, Brazil.

Physiological disturbances are abnormalities observed in insects, such as incomplete molt and malformation of nymphs (Semeão et al., Reference Semeão, Martins, Picanço, Chediak, Silva and Silva2012). These disorders are related to bottom-up forces, including plant phenology, nutritional quality and defense compounds produced by the plant (Godoy & Cividanes, Reference Godoy and Cividanes2002; Chattopadhyay et al., Reference Chattopadhyay, Agrawal, Kumar, Singh, Roy, Khan, Bhar, Chakravarthy, Srivastava, Patel, Srivastava, Singh and Mehta2005). The plants used to assess mortality caused by physiological disturbances were covered with organza-enclosed wooden cages (45 × 45 × 45 cm3) to prevent the action of natural enemies. The cages were protected from rain by canvas sheeting attached to wooden supports. These plants were previously inspected for removal of aphids and other arthropods present.
For the initial establishment of the cohort, 35 2-day-old females were equally distributed on two medium leaves of the plants using a fine brush. To prevent predation of females during the infestation period, all plants were covered with cages. After 24 h, the females were removed and the 1st instar nymphs generated (130, on average) were left on the plant. The aphid infested leaves were numbered to facilitate evaluation and the cages covering the plants used for the assessment of total mortality were removed.
Assessment of mortality factors
Causes of mortality at each stage of L. erysimi development were monitored daily in the field from the establishment of the cohort until the adults entered the reproductive phase. Aphids were counted three times a day (8 am, 12 am, and 5 pm) and only at 5 pm for plants used to evaluate total mortality and mortality due to physiological disturbances, respectively. Lipaphis erysimi fecundity was determined by counting the nymphs produced by females, daily, in the plants designated to evaluate the mortality caused by physiological disturbances.
Aphids were also counted immediately after the occurrence of rains and those that disappeared during this period or died covered by mud were considered dead due to this factor. Mortality due to parasitism was evaluated by counting parasitized mummies (smooth, shiny and swollen mummies). Mortality due to fungal infection was evaluated by counting mummies covered by mycelium or aphids with infection symptoms (pinkish mummies). The mortality of aphids during the molting process in the cage-covered plants was attributed to physiological disturbances. Nymphs that died attached to their exuviae were considered dead by this factor. The same mortality rates caused by physiological disturbances in caged plants was adopted for unprotected plants since, in the latter ones, these rates are obscured by other factors (predation and rainfall, for instance). Mortality due to predation was directly evaluated in the field through the observation of arthropods feeding on aphids. The plants were observed for 15 min, at each evaluation time (8 am, 12 am, and 5 pm), to identify the predators.
Exemplars of parasitized L. erysimi were collected in the evaluated plants and in other plants of the crop and placed in 100-ml plastic pots for the emergence of the parasitoids in the laboratory. Specimens of predators and parasitoids were maintained in 70% alcohol and identified according to the literature (Auad & Trevizani, Reference Auad and Trevizani2005; Rakhshani et al., Reference Rakhshani, Tomanović, Starý, Talebi, Kavallieratos, Zamani and Stamenković2008). Fungi infected aphids were mounted on microscope slides to identify the entomopathogens.
Construction and analyses of life tables
Standard methods were used to generate the life tables (Varley et al., Reference Varley, Gradwell and Hassell1974; Southwood & Henderson, Reference Southwood and Henderson2000; Silva et al., Reference Silva, Silva, Silva, Milagres, Bacci and Picanço2017). Net reproductive rate (R 0) was estimated by dividing the number of first instar nymphs expected in the next generation (number of surviving adults from the original cohort × sex ratio × fecundity) by the initial number of 1st instar nymphs (l 0). Sex ratio (sr) was taken to be 1.0 since all individuals in the L. erysimi population are females, and fecundity (f) was obtained in the plants used to assess mortality from physiological disturbances.
Life tables were composed of the columns x, lx, dx, 100qx, and 100rx, where x is the developmental stage, lx is the number of individuals alive at the beginning of each stage, dx is the number of individuals that died during each stage, 100qx is the apparent mortality percentage (100qx = 100 × dx/lx), and 100rx is the real mortality percentage (100rx = 100 × dx/l 0).
Marginal mortality (the expected mortality of a factor as if this was the only acting factor) was calculated. This concept is important since mortality factors like rainfall and predation kill quickly and are easily observed while physiological disturbances, parasitism, and entomopathogens usually take longer to kill (Elkinton et al., Reference Elkinton, Buonaccorsi, Bellows and Van Driesche1992). Mortality due to rainfall and physiological disturbances was not obscured by any other factor and therefore their marginal mortality was considered equal to the apparent mortality. The same probability of predation of parasitized or fungi-infected aphids and healthy aphids was assumed.
For the subsequent analyses, mortality was expressed as a k-value (k = −log (1−MMx/100)) where MMx is the marginal mortality (%) for a given factor at a given developmental stage. The use of k-value is convenient because it is additive through stages and mortality factors. The total mortality (K) of the developmental stage in question can be obtained by the sum of the k-values (K = Σk). For the identification of critical stages and key mortality factors, correlation analyses were performed between partial mortality (k) and total mortality (K) (Varley et al., Reference Varley, Gradwell and Hassell1974). When a positive, significant correlation (P < 0.05) existed between mortality in a particular stage and total mortality, that stage was considered the critical mortality stage. When more than one stage showed significant correlation, partial mortality (k) were regressed on total K, and the critical stage was the one presenting the largest significant regression angular coefficient (slope) at P < 0.05 (Podoler & Rogers, Reference Podoler and Rogers1975; Naranjo & Ellsworth, Reference Naranjo and Ellsworth2009; Pereira et al., Reference Pereira, Neves, Campos, Santana, Hunt and Picanço2018). Difference between slopes in the regression analyses was verified by the confidence interval at 95% probability. Key mortality factors were determined at the critical stage through the same procedures described above (Podoler & Rogers, Reference Podoler and Rogers1975). Correlation and regression analyses were performed using PROC CORR and PROC REG (SAS 9.0, SAS Institute, Cary, USA). Assumptions of normality and homoscedasticity were checked using PROC UNIVARIATE and PROC GLM (SAS 9.0).
Results
Mortality factors of Lipaphis erysimi
The mean mortality of the entire L. erysimi cycle was 90.21%. Mortality was 45.38% in the 1st instar; 36.02% in the 2nd instar; 27.59% in the 3rd instar; 20.27% in the 4th instar; 36.77% in the 5th instar and 23.77% in the adult phase. On average, of 130 initial individuals, 17 reached adulthood and 13 reached the reproductive phase. Based on the fecundity obtained (46.42 nymphs/female), the net reproductive rate (R 0) of L. erysimi was 4.95 (table 2).
Table 2. Ecological life table of Lipaphis erysimi in cabbage crops in Viçosa, MG, Brazil.

lx = number of insects alive at the beginning of each stage, dx = number of insects killed by each factor at each stage, 100qx = apparent or non-cumulative mortality (%), 100rx = actual mortality or cumulative mortality (%), MM = marginal mortality (%), k = −log(1−MM/100), and R 0 = net reproductive rate. The presented values represent an average of 55 life tables.
Mortality of 1st instar nymphs was caused by physiological disturbances, rainfall, Syrphidae larvae, Coccinellidae adults, and ants. In the 2nd instar, causes of mortality were physiological disturbances, rainfall, spiders, Syrphidae larvae, Coccinellidae larvae and adults, and ants. Mortality in the 3rd instar was caused by physiological disturbances, rainfall, spiders, Chrysoperla externa Hagen larvae (Neuroptera: Chrysopidae), Aphidoletes sp. larvae (Diptera: Cecidomyiidae), Syrphidae larvae and Entomophthorales fungi. In the 4th nymphal instar, mortality was caused by physiological disturbances, rainfall, spiders, Syrphidae larvae, Aphidoletes sp. larvae, Coccinellidae larvae and adults, Entomophthorales and parasitism by Diaeretiella rapae (M'Intosh) (Hymenoptera: Braconidae). In the 5th instar, causes of mortality were physiological disturbances, rainfall, spiders, Syrphidae larvae, Aphidoletes sp. larvae, Coccinellidae larvae and adults, ants, Entomophthorales and parasitism by D. rapae and Aphidius colemani Viereck (Hymenoptera: Braconidae). In the adult phase, mortality factors were rainfall, spiders, Syrphidae larvae, Aphidoletes sp. larvae, Coccinellidae larvae, Entomophthorales, D. rapae, and A. colemani (table 2).
Critical mortality stage of Lipaphis erysimi
Lipaphis erysimi mortality curve during the nymphal stage was the one that best represented the total mortality curve, as indicated by the positive and significant correlation coefficient (r = 0.93, P < 0.0001, n = 55). Mortalities of all nymph stages showed positive and significant correlations (P < 0.05, n = 55) with the nymphal total mortality. The mortality curve with the largest significant slope (b) was that for 1st instar (table 3). Therefore, the critical mortality stage of L. erysimi was the 1st instar nymph.
Table 3. Pearson correlation and simple linear regression analyses for determination of the critical mortality stage of Lipaphis erysimi in cabbage crops in Viçosa, MG, Brazil.

r, correlation coefficient; b, angular coefficient of the mortality curve; CI95% = Confidence interval at 95% probability.
1 Higher angular coefficient based on the confidence interval at 95% probability.
Key mortality factors of Lipaphis erysimi
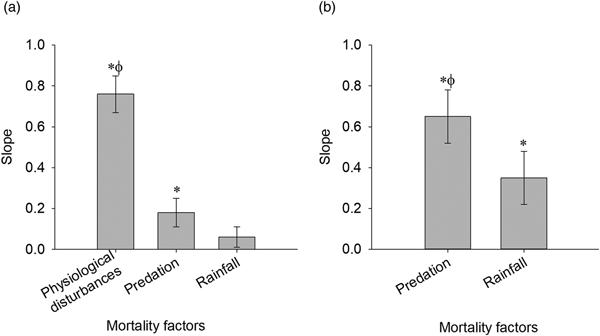
The mortality factors of 1st instar L. erysimi nymphs were physiological disturbances, predation, and rainfall. The L. erysimi partial mortality curve for physiological disturbances presented the largest significant slope (b), followed by the partial mortality curve for predation (fig. 1a, b).

Fig. 1. Slopes (b) of the simple linear regression curves for determination of key mortality factors for 1st instar nymphs of Lipaphis erysimi in cabbage crops (Viçosa, MG, Brazil). (a) The factor with significant and greater slope based on confidence interval at 95% probability was selected. Physiological disturbances: b = 0.76 (0.67–0.85), r 2 = 0.85, F = 296.09, P < 0.0001; Predation: b = 0.18 (0.11–0.26), r 2 = 0.32, F = 24.95, P < 0.0001; Rainfall: b = 0.06 (0.00–0.12), r 2 = 0.06, F = 3.29, P = 0.08. (b) The key mortality factors were submitted again to this analysis, excluding the previously selected factor. Predation: b = 0.65 (0.52–0.78), r 2 = 0.65, F = 99.04, P < 0.0001; Rainfall: b = 0.35 (0.22–0.48), r 2 = 0.35, F = 29.44, P < 0.0001. Numbers in parentheses represent 95% confidence interval for the slope of the curves. *Significant angular coefficient (P < 0.05); ϕGreater slope based on confidence interval at 95% probability. n = 55.
Syrphidae larvae, ants, and Coccinellidae adults were the predators that caused mortality to 1st instar L. erysimi nymphs. The partial mortality curve for Syrphidae presented the largest significant slope (b), followed by the partial mortality curve for adults of Coccinellidae (fig. 2a, b). Therefore, the key mortality factor for 1st instar L. erysimi nymphs was physiological disturbances, followed by predation and rainfall. The predator that caused the highest mortality to L. erysimi was Syrphidae larvae.

Fig. 2. Slopes (b) of the simple linear regression curves for determination of the main predators of 1st instar nymphs of Lipaphis erysimi in cabbage crops (Viçosa, MG, Brazil). (a) The predator with significant and greater slope based on confidence interval at 95% probability was selected. Syrphidae larvae: b = 0.61 (0.48–0.75), r 2 = 0.61, F = 86.12, P < 0.0001; Coccinellidae larvae: b = 0.31 (0.18–0.43), r 2 = 0.31, F = 24.24, P < 0.0001; Ants: b = 0.08 (0.00–0.14), r 2 = 0.08, F = 4.42, P = 0.04. (b) The predators were submitted again to this analysis, excluding the previously selected predator. Coccinellidae larvae: b = 0.65 (0.52–0.78), r 2 = 0.65, F = 99.04, P < 0.0001; Ants: b = 0.35 (0.22–0.48), r 2 = 0.35, F = 29.44, P < 0.0001. Numbers in parentheses represent 95% confidence interval for the slope of the curves. *Significant angular coefficient (P < 0.05); ϕGreater slope based on confidence interval at 95% probability. n = 55.
Discussion
Knowledge about the natural mortality factors and their magnitude in the population dynamics of insect pests is fundamental for the development of efficient management systems of these organisms. Populations of L. erysimi are determined by biotic and abiotic factors that caused a 92% reduction in L. erysimi cohorts. However, in spite of this significant mortality, L. erysimi had a population increase during the year (R 0 > 1), principally due to the reproductive advantage of these organisms, that reproduce by parthenogenesis (Powell et al., Reference Powell, Tosh and Hardie2006). This shows that the causes of natural mortality are not sufficient to reduce densities of these insects. Therefore, other methods and management strategies should be adopted in order to maximize or complement the action of natural mortality factors in cabbage crops.
Lipaphis erysimi nymphs are more vulnerable than the adult stage. This is due to the longer development period of this stage compared with the adult phase. In addition, earlier stages are more vulnerable to desiccation, low plant quality, and climatic variability, especially temperature (Aschehoug et al., Reference Aschehoug, Sivakoff, Cayton, Morris, Haddad and Reeve2015; Sultana et al., Reference Sultana, Baumgartner, Dominiak, Royer and Beaumont2017). Thus, control measures should be taken early during L. erysimi development, since the critical mortality stage for this pest is the 1st instar nymph.
The most important mortality factor for 1st instar nymphs of L. erysimi is physiological disturbances. Plants defense compounds, including total phenols, O-OH phenols, glucosinolates, and lectins, have been shown to cause physiological disorders to L. erysimi (Rana, Reference Rana2005; Newton et al., Reference Newton, Bullock and Hodgson2009; Kumar et al., Reference Kumar, Atri, Sangha and Banga2011). Therefore, the use of resistant plants is a promising strategy to manage this pest. Since cultivated cabbage genotypes have a low content of defense compounds, wild brassica expressing high levels of lectins, such as Brassica fruticulosa, B. montana, and Rorippa indica, have been shown to be promising sources of resistance to L. erysimi (Kumar et al., Reference Kumar, Atri, Sangha and Banga2011; Bandopadhyay et al., Reference Bandopadhyay, Basu and Sikdar2013). Our results may be a starting point for future research to determine which chemical(s) are responsible for the physiological disturbances.
The application of insect growth regulators (IGRs) is another tactic that can be adopted in L. erysimi management. The effect of juvenile hormone analogues, such as pyriproxyfen and methoprene, on this pest, has been studied (Rup & Gill, Reference Rup and Gill1993; Liu & Chen, Reference Liu and Chen2001). These products cause excessive molting and premature death of immature phases. When exposed to these molecules, nymphs of L. erysimi up to 3rd instar suffer the greatest effects, while nymphs of 4th instar normally molt to the adult phase (Liu & Chen, Reference Liu and Chen2001). There are reports of side effects of these insecticides on some natural enemies (Mendel et al., Reference Mendel, Blumberg and Ishaaya1994; Hattingh & Tate, Reference Hattingh and Tate1995), but not on others (Liu & Stansly, Reference Liu and Stansly2004; Cloyd & Dickinson, Reference Cloyd and Dickinson2006). In general, growth regulators are safer to beneficial organisms than the molecules commonly applied in the management of L. erysimi (pyrethroids, carbamates, and organophosphates) (Naranjo et al., Reference Naranjo, Ellsworth and Hagler2004; Cloyd et al., Reference Cloyd, Timmons, Goebel and Kemp2009; Naranjo & Ellsworth, Reference Naranjo and Ellsworth2009; Echegaray & Cloyd, Reference Echegaray and Cloyd2012). Since IGRs act primarily at the critical mortality stage of L. erysimi and are safer to natural enemies when compared to conventional alternatives, they fit well in this pest management.
Among the predators, Syrphidae larvae, Coccinellidae adults and ants were the most important organisms regulating L. erysimi. Ocyptamus gastrostactus Wiedemann, Allograpta exotica, and Pseudodorus clavatus Fabricius were the species of Syrphidae found. In Brazil, there are several reports of Syrphidae causing mortality of aphids including Allograpta neotropica Curran, O. gastrostactus, Syrphus phaetostigma Wiedemann, Ocyptamus dimidiatus Fabricius, and P. clavatus predating aphids in citrus, kale, cucumber, wheat and potato (Auad & Trevizani, Reference Auad and Trevizani2005). In general, Syrphidae larvae feed on 660 to 1140 third instar nymphs during their larval development (Tenhumberg & Poehling, Reference Tenhumberg and Poehling1995; Soleyman-Nezhadiyan & Laughlin, Reference Soleyman-Nezhadiyan and Laughlin1998) and play an important role in aphid regulation (Michaud & Belliure, Reference Michaud and Belliure2001). The species of Coccinellidae found predating L. erysimi were Cycloneda sanguinea (L.), Eriopis connexa (Germar) and Harmonia axyridis (Pallas). Adults and larvae of ladybugs are highly mobile and voracious predators. Although they are generalists, ladybugs are often associated with aphids (Snyder & Ives, Reference Snyder and Ives2003). Predation by ladybugs may also cause aphids to drop from the plants, an anti-predation behavior observed in several aphid species (Kunert et al., Reference Kunert, Otto, Röse, Gershenzon and Weisser2005; Francke et al., Reference Francke, Harmon, Harvey and Ives2008). As aphids have a thin cuticle layer and few defense strategies, this dropping can be advantageous as a defense strategy against ladybugs. However, once on the ground, they can be preyed on by soil-dwelling arthropods or die due to desiccation (Gish & Inbar, Reference Gish and Inbar2006). The ant species found preying on L. erysimi are from the genus Solenopsis. Mutualism of L. erysimi with these ants not being verified. In fact, the predation on aphids on the soil by these organisms was often observed during the evaluations.
In order to maximize the natural control of L. erysimi, habitat management strategies can be adopted to provide resources for its main natural enemies. More complex agroecosystems (e.g., bands of flowering plants near brassica plantations and intercropping) favor Coccinellidae and Syrphidae adults, and consequently L. erysimi suppression, since these organisms feed on pollen and nectar (White et al., Reference White, Wratten, Berry and Weigmann1995; Hickman & Wratten, Reference Hickman and Wratten1996; Obrycki et al., Reference Obrycki, Harwood, Kring and O'Neil2009; Ramsden et al., Reference Ramsden, Menéndez, Leather and Wäckers2014). Maintenance of weed coverage and soil moisture, in turn, are measures that favor S. saevissima in brassica crops (Harvey & Eubanks, Reference Harvey and Eubanks2004; Wang et al., Reference Wang, Wang, Zeng and Lu2016). Additionally, the use of selective insecticides, aiming to reduce the ecological impacts of these chemicals and insecticide applications (e.g., adoption of sampling and action thresholds) can contribute to the biological control of L. erysimi.
In conclusion, the nymphal stage, especially first instar nymphs, is critical for L. erysimi mortality. The key mortality factors during this stage in order of decreasing importance are physiological disturbances and predation by Syrphidae, Coccinellidae, and Solenopsis ants. Therefore, control measures should target early stages of L. erysimi and research aimed at developing cabbage varieties resistant to L erysimi should be prioritized. Finally, strategies aiming to maintain the action of the biological control agents might contribute to L. erysimi suppression in brassica crops.
Acknowledgements
Financial support was provided by the Coordination of Improvement of Higher Education Personnel (CAPES), National Council of Scientific and Technological Development (CNPq) and Research Support Foundation of the State of Minas Gerais (FAPEMIG). The authors are grateful to Dr Simon Elliot for his valuable assistance with the fungi identification. Mr Phillip John Villani (B.A. from The University of Melbourne, Australia) revised and corrected the English language used in this manuscript. The authors are also grateful to the anonymous reviewers for their helpful comments on an earlier draft of this paper.