Introduction
The horse chestnut leafminer, Cameraria ohridella Deschka & Dimic (Lepidoptera: Gracillariidae), was first found in Macedonia in 1984 (Deschka & Dimić, Reference Deschka and Dimić1986) and has now established itself as a part of almost all of Central European fauna (Butin & Führer, Reference Butin and Führer1994; Heitland et al., Reference Heitland, Kopelke, Freise and Metzger1999; Kindl et al., Reference Kindl, Kalinová, Freise, Heitland, Augustin, Guichard, Avtzis and Svatos2002). Although the trees are not severely damaged (Salleo et al., Reference Salleo, Nardini, Raimondo, Assunta Lo Gullo, Pace and Giacomich2003), the infestation of horse chestnut trees creates an undesirable image for the public. This is due to leaf wilting and the early loss of leaves in summer as a result of high infestation rates. The infestation situation has developed heterogeneously in recent years and varied strongly between years and locations. The problem will continue in the future (Heitland et al., Reference Heitland, Kopelke and Freise2003).
Although the horse chestnut leafminer can be found everywhere in Europe since the end of the nineties, native predators such as ants (Radeghieri, Reference Radeghieri2004) and birds (Kehrli & Bacher, Reference Kehrli and Bacher2002; Grabenweger et al., Reference Grabenweger, Kehrli, Schlick-Steiner, Steiner, Stolz and Bacher2005b) already exploit C. ohridella but adapt slowly to this neozoic species. However, the most important natural enemies of leafminers are parasitic Hymenoptera (Askew & Shaw, Reference Askew and Shaw1974). The composition of the parasitoid complex of C. ohridella has been examined in several studies (Grabenweger & Lethmayer, Reference Grabenweger and Lethmayer1999; Hellrigl, Reference Hellrigl2001; Freise et al., Reference Freise, Heitland and Tosevski2002). At the moment, 37 species of ecto- and endoparasitoids are known to exploit C. ohridella larvae as hosts (Grabenweger, Reference Grabenweger2003a). The dominant parasitoids are often the same in different regions and the species composition is quite typical for a leafminer in Europe (Grabenweger, Reference Grabenweger2003a). However, until now parasitation rates are generally low (Grabenweger & Lethmayer, Reference Grabenweger and Lethmayer1999), ranging mostly from 0.5 to 5.0% (Freise & Heitland, Reference Freise and Heitland2003). The low parasitation rates can be partly explained by the asynchrony in the life cycles of native parasitoid species and the horse chestnut leafminer (Grabenweger, Reference Grabenweger2004). Most parasitoids that have overwintered in horse chestnut leaf litter emerge before the host, i.e. moth of the horse chestnut leafminers (Grabenweger, Reference Grabenweger2004). Consequently, it is likely that most adult parasitoids are dead before suitable host larval stages are available on the horse chestnut trees.
In contrast, the parasitoid complex of other native leafminer species is adapted to the life cycle of its host species and parasitation rates of 50% or more have been frequently recorded. These high parasitation rates limit the population development of the leafminers and prevent them from reaching pest status (Askew & Shaw, Reference Askew and Shaw1979; Maier, Reference Maier1984; Mey, Reference Mey1993). Since it is likely that the adaptation process of native parasitoid species to the horse chestnut leafminer will take decades, augmentative biological control methods are of major interest. Two major problems have to be solved to facilitate an inundative release of parasitoids. First, simple biotechnological methods have to be developed for the parasitoid extraction and the retention of leafminers from horse chestnut leaf litter. Second, the parasitoids have to be released at a time when hosts suitable for parasitation are present. The first investigations to solve these problems have been done by a Swiss working group (Kehrli, Reference Kehrli2004; Kehrli et al., Reference Kehrli, Lehmann and Bacher2005). They collected leaf litter and stored it in a mass rearing device. This device consists of a container filled with leaf litter and openings covered with gauze with a defined mesh size to allow smaller parasitoid species to pass and retain the larger moth individuals. Moreover, to optimise the release schedule, devices were stored in cold storage houses. Overall, the results were promising, but due to several problems only a minor impact on the horse chestnut leafminer could be detected in the field. Although this technique is a good starting point, further development is necessary to meet concerns about applicability in urban greens, where huge amounts of infested horse chestnut leaves are collected each year.
The aims of our greenhouse and field experiments were to quantify the potential efficiency of the parasitoid complex emerging from leaf litter of the previous year under controlled conditions. Therefore, parasitoid species were released at different densities to investigate (i) their contribution to biological control, (ii) the impact of parasitoids on leafminer population development, and (iii) the establishment of species by a single release at a time when suitable host developmental stages are present on trees.
Material and methods
Insect breeding
Since C. ohridella and all parasitoid species overwinter inside the mine, i.e. in the fallen horse chestnut leaf litter, all insects used in the experiments were reared from leaf litter collected in the city of Braunschweig in autumn 2004. Leaf litter was stored in a cold room until experimental use. Prior to the experiments, the number of insects emerging from 100 g of dry leaf litter was determined with photoeclectors in a climatic chamber (22°C, 80% RH, 16:8 L:D). The results show that approximately 250 C. ohridella adults and 50 parasitoid individuals emerged from 100 g of dry leaf litter. The parasitoid complex was dominated by the species Minotetrastichus frontalis, Closterocerus trifasciatus, and Pnigalio agraules (fig. 1). Since leaf litter quality changes during cold storage (unpublished data), the exact parasitoid density and species composition was monitored again under experimental conditions.

Fig. 1. Parasitoid complex emerging from 100 g of horse chestnut leaf litter infested with the horse chestnut leafminer C. ohridella. Leaf litter was collected in the city of Braunschweig in 2004; n=10 (■, female; ![]() , male).
, male).
Greenhouse experiments
The effect of host density on parasitation rates was tested in the greenhouse in 32 gauze tents (1.8×2.0 m; 2 m high) covering single 1–2-m-high potted six-year-old white-flowering horse chestnut trees (Aesculus hippocastanum) infested with C. ohridella larvae. Four different treatments, each replicated eight times, were tested: (i) few parasitoids – low leafminer density; (ii) few parasitoids – high leafminer density; (iii) many parasitoids – low leafminer density; and (iv) many parasitoids – high leafminer density.
To obtain the different leafminer infestation levels, 10 g (i.e. 25 adult moth (low leafminer density)) and 50 g (i.e. 125 adult moth (high leafminer density)) of leaf litter was used to infest 4–5 horse chestnut trees. At the presence of the first visible mines on the leaves, single trees were placed in the gauze tents for different treatments. Horse chestnut leaf litter was also used to introduce the parasitoid species complex. Therefore, 35 g of leaf litter (approx. 17.5 parasitoid individuals) were introduced in the few, and 350 g of leaf litter (approx. 175 parasitoid individuals) were introduced in the many parasitoids treatments. The leaf litter was stored in a climate chamber (22°C, 80% RH, 16:8 L:D) for six days prior to its introduction in the experimental units in order to accelerate emergence of parasitoids.
The emergence pattern of parasitoids from the leaf litter and species composition was monitored with three photoeclectors in the same greenhouse, each containing 25 g of leaf litter. The emerging parasitoids were collected and determined every second day. The main emergence period of the parasitoids was ten days after the introduction of leaf litter in the experimental units. At that time, the age distribution and natural mortality of horse chestnut larvae was quantified in two additional gauze tents, which contained either three horse chestnut trees of the low or high leafminer density treatments.
All experimental units were randomly distributed in two greenhouses of 10×24 m in size. During the experiment, temperature in both greenhouses was monitored with data loggers. The experiment was terminated 22 days after the introduction of leaf litter containing parasitoids and all leaves were collected. At this time, we expected that parasitized leafminers would be easy to detect and that the chances for successful breeding would be high. To estimate parasitisation rates, samples of six infested leaves, i.e. two with low, intermediate and high mine density, were taken from three replicates per treatment and dissected. Upon dissection, the number of living and dead C. ohridella larvae, as well as pupal or larval stages of parasitoids were counted and the parasitation rate (parasitized hosts×100) per (hosts alive+hosts parasitized+hosts dead) was calculated (Freise, Reference Freise2001). Dead larvae were taken into account, because the dissection period lasted three weeks. Superparasitism was counted as a single parasitation event. Parasitoid larvae, pupae and C. ohridella larvae containing endoparasitoids were transferred into small plastic vials equipped with a piece of moist filter paper. All vials were checked on a daily basis for emerging parasitoids. Adult parasitoids were identified and counted.
Field experiments
Experiments took place at three different study sites on the terrain of the Federal Biological Research Centre for Agriculture and Forestry in Braunschweig, Hötzum and Essehof, Germany. At each site several planted groups of five eight-year-old white-flowering horse chestnut trees (Aesculus hippocastanum, 1.9±0.2 m high) were enclosed by a 3-m-high gauze tent covering a base area of 2.0 m×1.8 m. To protect the trees from heavy rainfall, the roof of all tents was build of transparent plastic tarpaulin. All trees were attached to a watering system. On all study sites, abiotic conditions, such as wind speed and air temperature, were monitored.
To create the same initial number of mines in all experimental units, 8 g of leaf litter, i.e. 20 adult moths, were introduced into each of the 22 gauze tents. Approximately four weeks after the introduction of leaf litter with C. ohridella pupae, mines were counted on all leaves. The mine density in each experimental unit, i.e. on five trees, was 693.0 (±49.7 SE). Parasitation rates and impact of parasitoid species on horse chestnut leafminer population development was estimated in three treatments, i.e. control without parasitoid release, low and high parasitoid release density. All treatments were equally distributed among the different study sites.
Parasitoids were extracted from dry leaf litter in a climate chamber (22°C, 80% RH, 16:8, L:D). Therefore, photoeclectors were prepared with either 28 g (low parasitoid density, approx. 14 individuals) or 280 g (high parasitoid density, approx. 140 individuals) of leaf litter. Emerging parasitoids were collected every second day and immediately released in the corresponding treatments. The first parasitoids were released on July the 14th, at a time when late larval developmental stages of C. ohridella were already present.
To monitor the temporal pattern of parasitoid emergence under field conditions, additional photoeclectors were prepared with 28 g (n=3) and 280 g (n=3) of leaf litter. Emerging parasitoids were collected 1–2 days and stored in a freezer for later identification and counting of individuals.
In all treatments and replicates, mine densities and parasitation rates were estimated two times. At the presence of the first larval generation of the horse chestnut leafminer, the number of mines was counted in all experimental units. To determine the parasitation rate of the first moth generation and the species composition of the parasitoid complex, 10% of all mines equally distributed over five trees in each experimental unit were cut out of the horse chestnut leaves. Single mines were then transferred into small plastic vials, provided with moist filter paper and stored at 22°C and 80% RH in climatic chambers until parasitoid or moth emergence.
At the end of August, i.e. four weeks after the emergence of the first moths generation, the mine density was assessed a second time. Therefore, all mines on each of the five trees per experimental unit were counted. Finally, parasitation rates of the second moth generation were determined at mid-September by dissecting approximately 100 mines equally distributed over the leaves of the five trees in each experimental unit.
Statistical data analysis
Percentages were arcsine-transformed prior to calculations. Normality of data distribution was verified by a Kolmogorov-Smirnov test. Heterogenity of variance was tested by a Levene test. Parasitation rates of the different treatments were compared by a univariate analysis of variance. For data sets with non normal distributions, the Kruskal-Wallis H-test was chosen. For pairwise comparisons, we used Mann-Whitney U tests or t-tests.
Results
Greenhouse experiment: effects of host density on parasitation rates
Mean daily temperature in both greenhouses during the experiments was approximately 17°C (1. greenhouse: 17.1°C±0.2 SE; 2. greenhouse: 17.3°C±0.2 SE). As expected, nine different parasitoid species emerged from the leaf litter introduced into the experimental units. Nevertheless, monitoring of emerging parasitioid species revealed that slightly more parasitoid individuals (86.66±5.99 per 100 g leaf litter) as expected emerged. Minotetrastichus frontalis appeared to be the most abundant species with a mean number of 62.8 (±6.4 SE) individuals per 100 g leaf litter. As the second and third most abundant species, Closterocerus trifasciatus (10.8±4.8 SE individuals) and Pnigalio agraules (5.2±2.4 SE individuals) were identified. All other species occurred irregularly at very low densities (0.8–2 individuals per 100 g). The emerging period for P. agraules lasted from day 3–6, while M. frontalis and C. trifasciatus emerged from day 6–13 after introduction into the experimental units.
At the time of the main parasitoid emergence from the leaf litter, 96.8% (±1.3 SE) of C. ohridella larvae had reached late larval developmental stages or had pupated (29.7%±0.1 SE). Only 3.0% (±1.3 SE) of the individuals were L3 and 0.1% (±0.1 SE) were L2 larvae. Natural mortality of C. ohridella larvae was 1.6% (±1.6 SE) on trees with low and 4.9% (±2.4 SE) on trees with high leafminer density.
At an average density of 379 (±43.5 SE) mines per tree, the introduction of 30.33 (±2.10 SE) parasitoids resulted in a parasitation rate of 7.1% (±7.1 SE). Leaving the parasitoid density constant, a five-fold increase in the host density (1951±186.1 SE) did not influence the parasitation rate (9.9%±9.9 SE). In contrast, four-fold higher parasitation rates (34.3%±10.9 SE) were recorded if ten times more parasitoids (i.e. 300.66±20.97 SE) were released at a host density of 379 mines per tree. With an increasing host density, the parasitation rate remained nearly constant at 31.9% (±9.8 SE). Analysis of variance showed a significant influence of parasitoid densities (few and many) on the parasitation rate (table 1), whereas parasitation rates were not affected by numbers of C. ohridella larvae, i.e. 379 and 1951 mines, on the experimental trees (table 1, fig. 2).

Fig. 2. Mean parasitation rate in the different treatments in the greenhouse experiment. Parasitation rates were calculated on the basis of all C. ohridella larvae (dead and alive) recorded during dissection of six leaves per tree. See table 1 for significant difference (n=3).
Table 1. Summarised univariate analysis of variance of parasitation rates at two different parasitoid densities (few, many) and two different leafminer host densities (low, high).
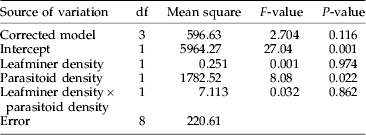
Dependent variable was parasitation rate per experimental unit, i.e. a single horse chestnut tree (n=3).
Emerging parasitoids were counted and identified to estimate their contribution to the total future parasitation rate. Not all species could be identified because of fungal infections or diapausing pupae. Nevertheless, 98.94% of the emerging species from parasitoid larvae of dissected leaves, and 98.59% of the emerging species from mined horse chestnut leaves collected at the end of the experiment were identified as the species P. agraules. The remaining species were M. frontalis (1.06 and 1.06%), Closterocerus trifasciatus (0.001 and 0.21%) and Colastes braconius (0.001 and 0.14%).
Semi-field experiment: impact of parasitoids on leafminer population development
Similar to the greenhouse experiment, the mean daily temperature was approximately 17°C at all experimental sites (Braunschweig: 17.9°C±0.3 SE; Hötzum: 17.3°C±0.4 SE; Essehof: 17.14°C±0.4 SE).
Seven days after exposure of the leaf litter in the photoeclector, the first parasitoids emerged. From then onwards, emerging individuals were released every second day in the experimental units. At that time, 61% (±2.4 SE) of the leafminer population were in the fourth larval developmental stage. The rest of the population was in the L3- (34.1%±1.3 SE) and L2-stage (4.2%±1.9 SE).
The monitoring of parasitoid emergence in the field revealed that parasitoid density in the leaf litter was lower than expected. On average 110 (±1.51 SE) parasitoids emerged from 280 g and 12 (±1.53 SE) from 28 g of leaf litter and were introduced in the experimental units in the field. Nevertheless, the released parasitoid complex was composed of eight different species. Similar to the greenhouse experiment, M. frontalis (67.8%), P. agraules (12.3%), and C. trifasciatus (7.38%) were the most abundant species.
Parasitation rate of the first moth generation
On average 693 (±49.7 SE) mines were distributed on the leaves of the five horse chestnut trees in each of the experimental units (fig. 3). The introduction of 12 parasitoids, i.e. at a parasitoid to mine ratio of 1:57.75, resulted in a mean parasitation rate of 1% (±0.4 SE). With a ten-fold increase in released parasitoids, i.e. a parasitoid to mine ratio of 1:6.3, the mean parasitation rate increased significantly to 11% (±3.8 SE). In experimental units without parasitoid release, none of the horse chestnut leafminers were parasitized (fig. 4).

Fig. 3. Mean number of mines per experimental unit, i.e. five horse chestnut trees, during the first and second moth generation for the different treatments in the semi-field experiment. Different letters indicate significant difference among treatments for the second moth generation (U test, p<0.05, n=5) (■, first leafminer generation; ![]() , second leafminer generation).
, second leafminer generation).

Fig. 4. Mean parasitation rates for the first and second moth generation in the different treatments of the semi-field experiment. Parasitation rates were calculated on the basis of all C. ohridella larvae (dead and alive) found during dissection of 10% of all mines (first generation) or 100 mines (second generation) per experimental unit, i.e. five horse chestnut trees. Different letters indicate significant difference within each leafminer generation (U test, p<0.05, n=9) (■, first leafminer generation; ![]() , second leafminer generation).
, second leafminer generation).
The breeding success of the total number of parasitoid individuals isolated by dissections was 68%. Although it was not possible to rear the remaining 32% of the isolated parasitoid to adulthood, we assume that most of them belong to the species Pnigalio agraules because of similar larval morphology. P. agraules was identified as the dominant species parasitizing the first leafminer generation in the low parasitoid (66.7%±33.3 SE), as well as in the high parasitoid density treatment (92.4%±5.1 SE). Only three other parasitoid species, Cirrospilus viticola, Closterocerus trifasciatus and Pteromalus sp., were identified. In total, 77% of the P. agraules individuals found in all treatments emerged from leafminer larvae and the remaining 23% from pupae.
Development of the leafminer population and parasitation rates
The leafminer density increased in the control treatment, i.e. without introduction of parasitoids, from 693 (±49.7 SE) to an average of 3789 (±593.2 SE) mines per experimental unit in the second leafminer generation (fig. 3). The leafminer population development was similar to the control if 12 parasitoids were released in the experimental units. In contrast, the introduction of 110 parasitoids resulted in a leafminer density in the second generation that was significantly lower compared to the control and the introduction of 12 parasitoids (H-test, Chi-square=9.3, df=2, p=0.010). Compared to the control, the average mine density was reduced by 39% (±17.1 SE), i.e. 2309 (±652.4 SE) mines per experimental unit (fig. 3).
Most of the leafminers were at the L3- (39%±2.6 SE) and L4- (30%±3.7 SE) developmental stages at the time of the parasitation rate estimation. The parasitation rates of the second leafminer generation were, in general, low and without significant differences among the treatments (fig. 4) (ANOVA, F=2.459, df=2, p=0.107). In treatments with an initial release of 12 parasitoids, the parasitation rate recorded for the second leafminer generation was similar to the first leafminer generation (fig. 4) (t-test, t=1,213, df=16, p=0.243). In contrast, in the treatment with an initial release of 110 parasitoids, the parasitation rate decreased ten-fold compared to parasitation rates achieved for the first leafminer generation (fig. 4) (t-test, t=2.753, df=16, p=0.014). We successfully reared 6% of the total amount of parasitoid individuals isolated by dissections in this generation. P. agraules was the only species. It was not possible to rear the remaining 94% of the isolated parasitoids to adulthood. Because of similar larval morphology, it is likely that most of them also belonged to the species P. agraules.
Discussion
In their natural habitats, phytophagous insects, especially leafminers, are effectively controlled by parasitoids. In many cases, the parasitation rates exceed a level of 50% (Askew & Shaw, Reference Askew and Shaw1979; Mey, Reference Mey1993). If leafminers invade new host plants in habitats far away from their place of origin, they are most likely able to build up huge population densities because a specialized parasitoid complex is lacking (Cornell & Hawkins, Reference Cornell and Hawkins1993). Different examples show that, after a certain time, parasitoids in the new invaded habitat also exploit the new host species, but mostly play only a limited role in the regulation of the population dynamics of the invader (Stojanović & Marković, Reference Stojanović and Marković2005; Vercher et al., Reference Vercher, Costa-Comelles, Marzal and García-Marí2005). Only if specialised parasitoid species follow the invader, as was observed, for example, for Phyllonorycter platani in England (Godfray et al., Reference Godfray, Agassiz, Nash and Lawton1995), herbivore population densities are under natural control within a short period of time. If natural enemies do not follow the invader, the introduction of new species of parasitoids from the place of origin of the leafminer is often suggested in classical biological control programs. For example, Garcia-Marí et al. (Reference García-Marí, Vercher, Costa-Comelles, Marzal and Villalba2004) were able to show that the establishment of the eulophid parasitoid Citrostichus phyllocnistoides between 1996 and 1999 as a natural enemy of the citrus leafminer Phyllocnistis citrella (Lepidoptera: Gracillariidae) in Spain was successful. In 2000 and 2001, the parasitoid expanded and became the most abundant species in all the citrus orchards and the mean percentage of parasitism increased from 20–25% to nearly 60%.
The invasion of Europe by the horse chestnut leafminer started in 1984 from Macedonia (Deschka & Dimić, Reference Deschka and Dimić1986), and today the leafminer is an inherent part of the European fauna (Heitland et al., Reference Heitland, Kopelke, Freise and Metzger1999). Classical biological control, i.e. the introduction of natural enemies of the horse chestnut leafminer, was discussed years ago (Kenis, Reference Kenis1997), but until now the area of origin of the leafminer remains unclear. None of the possible options could be completely discarded; but, most likely, C. ohridella originated from another host genus in a non-European region (Kenis et al., Reference Kenis, Avtzis, Freise, Girardoz, Grabenweger, Heitland, Lakatos, Lopez Vaamonde, Svatos and Tomov2004; Kenis et al., Reference Kenis, Tomov, Svatos, Schlinsog, Lopez Vaamonde, Heitland, Grabenweger, Girardroz, Freise and Avtzis2005). For this reason, the biocontrol potential and adaptation process of the native parasitoid complex is of major interest from an economic (i.e. to tree nurseries, local communities responsible for leaf litter removal), as well as from an ecological (population dynamics, adaptation of natural enemies) point of view.
Currently, only the removal and decomposition of horse chestnut leaf litter, which contains the overwintering leafminer, can be recommended to reduce horse chestnut leafminer densities in public and private greens. Although this method is quite successful, it affects not only the leafminer density but also the adaptation process of native parasitoids in a dramatic way, since not only horse chestnut leafminer but also the natural enemies, i.e. hymenopteran parasitoids, overwinter in the leaf litter. At the moment, the impact of native parasitoid species on horse chestnut leafminer population development is small, but it is only a matter of time until parasitoids are better adapted and start to limit the population growth of C. ohridella. In this sense, the removal of leaf litter is counterproductive, because each year the parasitoid selection process restarts.
Our results show that Minotetrastichus frontalis and Pnigalio agraules were the dominant species in the leaf litter collected at the end of the season in Braunschweig, Germany. For example, from 100 g dry horse chestnut leaf litter (volume of approx. 50 l) 60 M. frontalis and six P. agraulis individuals were extracted in addition to several other species. From the literature, we know that M. frontalis is a gregarious ectoparasitoid preferring larval developmental stages (Noyes, Reference Noyes2002; Grabenweger, Reference Grabenweger2003a; Lupi, Reference Lupi2005) and is able to develop as a facultative hyperparasitoid (Freise, Reference Freise2001; Noyes, Reference Noyes2002). In contrast, P. agraules is a solitary ectoparasitoid attacking larvae and pupae of the horse chestnut leafminer. Both species are polyphagous, attacking, as a minimum, more than 60 different leafminer species (Noyes, Reference Noyes2002). Parasitation rates in our experiments range between 1 and 35%. Although both parasitoids belong to the most frequently found species in urban and natural stands of infested white horse chestnuts throughout the year (Grabenweger & Lethmayer, Reference Grabenweger and Lethmayer1999; Hellrigl, Reference Hellrigl2001; Freise & Heitland, Reference Freise and Heitland2003; Grabenweger, Reference Grabenweger2003a), the species P. agraules almost exclusively was parasitizing the horse chestnut leafminer in our greenhouse and semi-field experiments.
The response of P. agraules to increasing host densities, i.e. the functional response, cannot be evaluated conclusively, especially since parasitism rates at low host densities were not covered by our experiments. However, at least at the quite high host densities tested in our experiments, the parasitation rate is independent of the leafminer density. This was observed for both tested parasitoid densities. Nevertheless, the parasitation rate increased four-fold if ten times more parasitoid individuals were released. This result was supported by results of our semi-field experiments. In total each parasitoid individual was able to parasitize approximately 30 horse chestnut leafminers, which seems to be the maximum realised fecundity of P. agraules. Nevertheless, it is likely that this parasitoid species frequently contributes to host mortality by host feeding. At the frequently observed high horse chestnut leafminer field densities, the missing functional response disqualifies P. agraules as an efficient antagonist; but, in the future, the parasitoid might be better adapted to either the host and/or high leafminer densities.
Parasitation rates for the horse chestnut leafminer of 10–20% have been reported at different European locations (Grabenweger & Lethmayer, Reference Grabenweger and Lethmayer1999; Freise et al., Reference Freise, Heitland and Tosevski2002; Grabenweger et al., Reference Grabenweger, Avtzis, Girardoz, Hrasovec, Tomov and Kenis2005a; Lupi, Reference Lupi2005; Volter & Kenis, Reference Volter and Kenis2006). However, Freise & Heitland (Reference Freise and Heitland2003) point out that it is more likely that the parasitation rates range between 1 and 5% and attribute the discrepancy to different methodologies in the estimation of parasitation rates. In a first attempt to investigate the effects of artificially synchronised parasitoids on the biocontrol of the horse chestnut leafminer, Kehrli et al. (Reference Kehrli, Lehmann and Bacher2005) used mass-emergence devices and recorded parasitation rates of up to 17%. However, due to technical problems, the high parasitation rates could not be attributed to the impact of parasitoid augmentation. Additionally, the authors stated that 77% of P. agraules were retained by the mesh size of 600 μm used in their experiments. In our study, P. agraules turned out to be the dominant parasitoid species parasitizing horse chestnut leafminer larvae. This underlines the importance of this species for the control of C. ohridella.
The maximum parasitation rates recorded in our study are in the range estimated for other leafminer species. For example, parasitation rates of 30–50% were recorded for native birch leafminers Coleophora serratella (Hymenoptera: Tenthredinidae) in northern Germany (Pschorn-Walcher, Reference Pschorn-Walcher1980) and 30–67% for the locust leafminer Phyllonorycter robiniella (Lepidoptera: Gracillariidae) in Serbia (Stojanović & Marković, Reference Stojanović and Marković2005). Therefore, parasitation rates of 35% recorded in our study indicate that the promotion of parasitoids could be a strategy not only to increase parasitation rates in the field, but also to limit horse chestnut leafminer population development (see discussion below). Moreover, the contribution of parasitoid species other than P. agraules could raise parasitation rates above 35%, if interspecific competition does not counterbalance the overall efficiency. To assess the overall impact of parasitoid species on horse chestnut leafminer population development more field studies are necessary.
An unexpected result was that the most abundant parasitoid species, i.e. M. frontalis, was not contributing to leafminer parasitation in our experiments. In the field, this species is normally one of the dominant species during the first larval leafminer generation and reaches parasitation rates comparable to Pnigalio agraules (Grabenweger & Lethmayer, Reference Grabenweger and Lethmayer1999; Freise, Reference Freise2001; Grabenweger, Reference Grabenweger2003b; Stojanović & Marković, Reference Stojanović and Marković2004). Two non-exclusive reasons might contribute to the observed results. (i) The parasitoid species M. frontalis is not a facultative hyperparasitoid as described by Freise (Reference Freise2001) and Hellrigl (Reference Hellrigl2001) but is an obligate hyperparasitoid. This would explain the complete failure of Minotetrastichus frontalis in our experiments, since parasitized leafminer larvae were not available. (ii) An alternative explanation for the failure of M. frontalis could be unfavourable experimental conditions. In particular, carbohydrate- and amino acid-containing food sources, which might be important for egg maturation and survival, were almost entirely missing in our experimental units. More detailed studies are needed to clarify the role of M. frontalis in the parasitoid complex.
More important to the public than increased parasitation rates per se is the impact of parasitoids on leafminer population development. Sustainable effects can only be achieved if the reduced number of surviving moth larvae results in lower number of leafminers on the horse chestnut trees in the next generation. The desirable effect should be that horse chestnut trees look healthy in terms of green leaf area and that trees will most likely not show early leaf fall. In our experiments, population increase in the control treatment from the first to the second larval leafminer generation was six-fold. Approximately 700 emerging adults of the first larval generation produced almost 4000 mines on five horse chestnut trees in the second larval generation. A parasitation rate of 1% was without any effect on leafminer population development, while 11% was enough for leafminer population to be reduced by approximately 40%. Since host feeding is a common phenomenon for leafminer parasitoids (Askew & Shaw, Reference Askew and Shaw1979; Casas, Reference Casas1989; Neale et al., Reference Neale, Smith, Beattie and Miles1995; Bernardo et al., Reference Bernardo, Pedata and Viggiani2006), we have to keep in mind that not only parasitised larvae but also killed larvae contributed to the lower number of emerging adults and, consequently, to the reduced mine density in the second leafminer generation.
Although approximately 11% of the first larval generation of the leafminer was parasitized by P. agraules, only a few horse chestnut leafminer larvae of the second generation were parasitized. Most likely, the asynchrony in the developmental times of the parasitoid and the host is responsible for this unexpected result. Compared to the horse chestnut leafminer, the parasitoid species P. agraules grows two times faster (personal observation) and, therefore, it is likely that most parasitoid species do not live long enough to parasitize hosts of the following leafminer generation. Even the fact that part of each horse chestnut leafminer generation enters diapause (Dimić et al., Reference Dimić, Mihajlovic, Vukca, Peric, Krnjajic and Cvetkovic2000; Freise, Reference Freise2001) and are, therefore, continuously available as hosts, did not lead to the increased or at least constant parasitation rates in our experiments. Diapausing horse chestnut pupae are less frequently parasitized then other developmental stages, including non-diapausing pupae (Freise, Reference Freise2001). Most likely, diapausing pupae are protected by a physical defence (Freise & Heitland, Reference Freise and Heitland2004), i.e. the silky cocoon and/or behavioural defences, i.e. host wriggling inside the mine (Meyhöfer et al., Reference Meyhöfer, Casas and Dorn1994; Bacher et al., Reference Bacher, Casas and Dorn1996). Finally, it is likely that the effect of host-parasitoid asynchrony on parasitation rates was enhanced by our semi-natural conditions, which do not allow immigration of parasitoids developing on other leafminer species in the habitat.
Although overlapping horse chestnut generations in the field might guarantee for continuous availability of horse chestnut larvae, the observations made so far indicate that parasitation only slightly increases from one to the other generation (Kehrli, Reference Kehrli2004). To which extent alternative host species in the field are responsible for the overall low parasitation rates should be investigated in the future.
Acknowledgement
The authors thank Dr Gitta Siekmann for comments on the manuscript. Kirsten Strauss and Michaela Erhard assisted in dissecting and counting leaf mines. The research was funded by the Federal German Ministry for Education and Research (BMBF, project no. 0313131).







