Introduction
Trophic interactions are amongst the primary processes in natural and managed ecosystems, regulating community dynamics and ecosystem productivity and stability. Both natural enemies (top-down effects) and resources (bottom-up effects) may play important regulatory roles. Predatory interactions between natural enemies at the same trophic level, defined as intraguild interactions where the protagonists share a common prey, are among the top-down trophic forces that can directly or indirectly affect populations at lower trophic levels. Intraguild predation occurs widely in most ecosystems and has been recognized as functionally important (Sunderland et al., Reference Sunderland, Axelsen, Dromph, Freier, Hemptinne, Holst, Mols, Petersen, Powell, Ruggle, Triltsch and Winder1997; Rosenheim, Reference Rosenheim1998; Brodeur & Rosenheim, Reference Brodeur and Rosenheim2000). Experimental work suggests that interactions between biological control agents and their own natural enemies can disrupt effective control of herbivore populations (Snyder & Ives, Reference Snyder and Ives2001) or leave them unaffected (Colfer & Rosenheim, Reference Colfer and Rosenheim2001). Determination of the significance of intraguild interactions is needed for better quantification of the role such interactions play in food web dynamics and herbivore control. However, the challenges faced by those studying these often complex trophic interactions, under undisturbed natural conditions, are formidable (Sunderland et al., Reference Sunderland, Powell, Symondson and Jervis2005).
Molecular approaches offer exciting new approaches to the analysis of host-parasitoid and predator-prey interactions (Symondson, Reference Symondson2002; Greenstone, Reference Greenstone2006; Gariepy et al., Reference Gariepy, Kuhlmann, Gillott and Erlandson2007; King et al., Reference King, Read, Traugott and Symondson2008) and allow us to track trophic links which are inaccessible by conventional means, such as interactions in below-ground systems (Juen & Traugott, Reference Juen and Traugott2007) or among soil micro-arthropods (Read et al., Reference Read, Sheppard, Bruford, Glen and Symondson2006). In particular, DNA-based approaches have shown great potential in assessing trophic links in multi-species communities because new molecular markers and detection systems can be generated rapidly and markers can be used in combinations that permit many targets to be screened for within the same assay (Gariepy et al., Reference Gariepy, Kuhlmann, Haye, Gillott and Erlandson2005; Harper et al., Reference Harper, King, Dodd, Harwood, Glen, Bruford and Symondson2005; Traugott et al., Reference Traugott, Zangerl, Juen, Schallhart and Pfiffner2006). In addition, factors that can have significant effects on the interpretation of field-derived data, such as secondary predation (Sheppard et al., Reference Sheppard, Bell, Sunderland, Fenlon, Skervin and Symondson2005), predator feeding mode (Greenstone et al., Reference Greenstone, Rowley, Weber, Payton and Hawthorne2007), scavenging (Foltan et al., Reference Foltan, Sheppard, Konvicka and Symondson2005; Juen & Traugott, Reference Juen and Traugott2005) and temperature (von Berg et al., Reference von Berg, Traugott, Symondson and Scheu2008), have been addressed.
Here we assess, for the first time, the value of PCR to study intraguild predation using a model herbivore-parasitoid-predator system to track predation on parasitized prey. The model system used in the present study included the black bean aphid (Aphis fabae Scopoli; Homoptera: Aphididae) as the herbivore, the parasitoid Lysiphlebus testaceipes (Cresson) (Hymenoptera: Aphidiidae) and the predators Demetrias atricapillus L. (Coleoptera: Carabidae) and Erigone sp. (Araneae: Linyphiidae). Aphis fabae occurs throughout the temperate world on a variety of plants and is an important pest due to its transmission of viral diseases. Lysiphlebus testaceipes is a generalist parasitoid whose host range includes more than 100 aphid species on numerous plants (Pike et al., Reference Pike, Stary, Miller, Graf, Allison, Boydston and Miller2000). It was introduced from Central America to southern France in the 1970s, as a biocontrol agent against various aphid species, and spread rapidly over the Mediterranean region (Stary et al., Reference Stary, Lyon and Leclant1988; Lumbierres et al., Reference Lumbierres, Stary and Pons2007). It proved to be very effective at parasitizing aphids in experimental arenas (J. Rademacher, personal communication), which made it an ideal candidate for the experimental work carried out in the present study. Demetrias atricapillus is a small carabid beetle, which occurs throughout Europe. It has been found to be a significant aphid predator in cereal crops and is able to climb vegetation (Sunderland & Vickerman, Reference Sunderland and Vickerman1980). Linyphiid spiders within the genus Erigone also regularly occur in European farmland (Nyffeler & Sunderland, Reference Nyffeler and Sunderland2003) and are known to be significant predators of aphids (Harwood et al., Reference Harwood, Sunderland and Symondson2004).
By combining molecular detection of parasitoid DNA within hosts and detection of parasitized host DNA within predator gut contents, we aimed to determine how (i) PCR format (singleplex vs. multiplex), (ii) parasitoid developmental stage and (iii) predator type (spider vs. carabid beetle) affect parasitoid and host detection success.
Materials and methods
Insects
Starting cultures of A. fabae and L. testaceipes were obtained from Katz Biotech AG (Baruth, Germany). Aphids were maintained on young bean plants and kept inside fine mesh cages within a laboratory (~24±4°C, 18:6 h L:D). The cages prevented invasion of aphid colonies by other aphid species and parasitoids. Fresh plants were provided as needed. Parasitoids were maintained in fine mesh cages kept in a separate laboratory (~24±4°C, 18:6 h L:D). Pots of aphid-infested bean plants were provided every three to four days to ensure a continuous supply of parasitoids.
Adult D. atricapillus and Erigone sp. were collected within winter wheat fields at the Warwick HRI experimental farm, Wellesbourne, Warwickshire, UK (52012.18'N, 1036.00'W). As reliable identification of living spiders to species level was not possible (especially females), they were assigned to genus level only. Catches within a field at the same farm at the same time were dominated by Erigone atra (Blackwall), and this species probably represented most of the specimens used in the feeding experiments. Beetles and spiders were maintained in a climate chamber at 16°C on a 16:8 h L:D cycle and housed separately in 50×15 mm vented Petri dishes containing a damp plaster of Paris base to avoid desiccation of the animals. Prior to the start of the feeding experiments, carabids and spiders were fed for approximately one month on a diet of live grain aphids, Sitobion avenae (F.) (Homoptera: Aphididae) followed by a one- (carabids) or two-week (spiders) starvation period.
Parasitism and feeding experiments
Aphis fabae nymphs were individually parasitized with L. testaceipes by placing aphids in transparent 20 ml tubes containing one female parasitoid. After the parasitoid had been observed to have oviposited into the aphid, the aphid was transferred onto a fresh bean plant, which was housed in a separate fine mesh cage under laboratory conditions, as described above for aphids rearing.
To assess how early parasitoid DNA could be detected within the host, parasitized aphids were collected from the bean plants at 5 min, 6 h, 12 h, 24 h, 48 h, 72 h and 96 h post parasitism. Aphids were placed individually into 1.5 ml Eppendorf tubes and frozen at −80°C, with ten replicates frozen at each time point.
Parasitized aphids, for use as prey in subsequent feeding experiments, were frozen at two and five days post parasitism (dpp) (n=150 for each parasitoid developmental stage). Starved beetles and spiders (n=10) were frozen at −24°C as starved controls. The remaining predators, housed individually in Petri dishes, were fed with 1–2 freeze-killed parasitized A. fabae, which contained either two-day-old or five-day-old parasitoids. Predators were allowed to feed on their prey for 1 h and any non-feeding individuals were excluded from the experiment. For each feeding experiment, nine D. atricapillus and six Erigone sp. were killed by freezing at −24°C immediately after the feeding period. The remaining predators were moved to clean Petri dishes and kept in a climate chamber at 16°C (16:8 h L:D). Further predators were frozen at 2, 4, 8, 16, 24 and 32 h after feeding. Six predators (whether carabids or spiders) were frozen at each time point in all experiments, except where D. atricapillus were fed with aphids containing five-day-old parasitoids when nine replicates were taken.
Sequencing and primer design
For the design of the species-specific primers, published sequences of Erigone dentipalpis (AY383538) and E. atra (AY383537) were used, and part of the cytochrome oxidase subunit I (COI) was sequenced from two individuals each of A. fabae (EU294096, EU294097), L. testaceipes (EU29410, EU294101) and D. atricapillus (EU294098, EU294099) using universal invertebrate primers LCO-1490 and HCO-2198 (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) (numbers in parentheses are the respective GenBank accession numbers). PCR was carried out in a GeneAmp 9700 thermocycler (Applied Biosystems) in 20 μl reaction volumes containing 3 μl of Chelex-extracted DNA (extraction protocol see below), 0.25 mM dNTPs (Invitrogen), 1 μM of each primer, 2 μl 10×buffer (Invitrogen), 3 mM MgCl2 and 0.75 U Taq DNA polymerase (Invitrogen). Initial denaturation was done at 94°C for 2 min, followed by 35 cycles at 94°C for 15 s, 50°C for 30 s, 72°C for 45 s and final elongation at 72°C for 2 min. PCR products were purified using ExoSAP-IT (USB), subjected to sequencing PCR using Big-Dye Terminator mix (version 1.3, Applied Biosystems) and sequenced in both forward and reverse directions. Sequences were aligned using BioEdit (Hall, Reference Hall1999) and corrected manually.
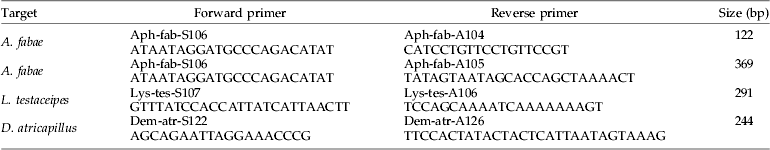
Primer pairs targeting A. fabae, L. testaceipes and D. atricapillus (table 1) were designed using PrimerPremier5 (Biosoft International), following the guidelines for allele-specific primer design given by Hawkins (Reference Hawkins1997). Primer pairs were tested for their specificity using DNA from the target species (ten individuals each) and all the other species included in our experiment (ten individuals per species). Optimisation of the PCR protocol included determination of optimum annealing temperatures by temperature gradient PCR, testing different concentrations of primers and adjusting cycling conditions.
Table 1. Primer pairs designed from COI mtDNA sequences of Aphis fabae, Lysiphlebus testaceipes and Demetrias atricapillus.
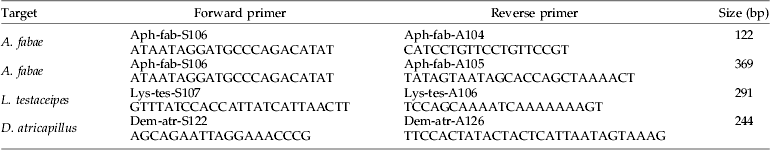
Columns show the primer targets, primer names, primer sequences (5′–3′) and the expected product size.
DNA extraction and PCR
DNA extraction from parasitized aphids was done using a Chelex-based protocol. Single aphids were homogenised in 1.5 ml Eppendorf tubes containing 20 μl phosphate buffered saline using a plastic pestle. Thereafter, 5 μl of Proteinase K (9.95 mg ml−1; Sigma Aldrich) and 200 μl 10% Chelex solution (BioRad) were added; samples were incubated overnight at 56°C before incubation at 94°C for 15 min and storage at −24°C.
The DNA of whole predators from the feeding experiments was extracted using DNeasy Blood & Tissue Kit (Qiagen GmbH, Hilden, Germany) following the manufacturer's instructions; 200 μl DNA extracts were stored at −24°C.
Within each batch of 24 DNA extractions, one control extraction (containing no DNA) was included and tested for DNA of the target species in singleplex PCR to check for DNA carry-over contamination.
Detection of parasitoid DNA in parasitized aphids, and detection of aphid and parasitoid DNA in predators, was done using multiplex PCR (MP-PCR). We compared the sensitivity of multiplex vs. singleplex PCR by parallel screening.
Parasitized A. fabae were screened for DNA of L. testaceipes in SP-PCR using 20 μl reactions containing primers S107 and A106 at 1 μM each, 0.2 mM dNTPs, 1×PCR buffer (Invitrogen), 3 mM MgCl2, 1 μl bovine serum albumin (BSA; 5 mg ml−1), 3 μl of extracted DNA and 0.75 U Taq DNA polymerase (Invitrogen). As detection success of parasitoid DNA was equally sensitive in 10 μl compared to 20 μl reactions (serial dilution experiments, data not shown), all further SP-PCRs were conducted using 10 μl reactions (see below). The thermal cycling profile was 3 min at 94°C, 40 cycles of 30 s at 94°C, 30 s at 62°C, 60 s at 72°C and final elongation for 5 min at 72°C.
Primer mix 1 (primers S107, A106, S106 and A105 each at 2 μM) was used to amplify DNA from the parasitoid and its aphid host simultaneously in MP-PCR. Each 10 μl PCR contained 1.5 μl of extracted DNA, 1×Multiplex PCR master mix (QIAGEN), 1 μl of primer mix, 1 μl BSA (5 mg ml−1) and PCR-water (QIAGEN). Cycling conditions were 15 min at 95°C, 40 cycles of 30 s at 94°C, 3 min at 62°C, 60 s at 72°C and final elongation for 10 min at 72°C.
MP-PCR detection of aphid and parasitoid DNA in D. atricapillus and Erigone sp. was done using primer mix 2 (primers S107, A106, S122 and A126 at 10 μM; primers S106 and A105 at 2 μM; primer A104 at 4 μM) and primer mix 3 (primers S107, A106, S106 and A105 at 2 μM, primer A104 at 8 μM), respectively. Note that primer pair S122/A126 served as an internal control, targeting DNA of D. atricapillus, whereas no such control was included in the spider samples. Each 10 μl PCR contained 1.5 μl of extracted DNA, 1×Multiplex PCR master mix (QIAGEN), 1 μl of primer mix and PCR-water (QIAGEN). Cycling conditions were 15 min at 95°C, 30 and 40 cycles (for beetles and spiders, respectively), of 30 s at 94°C, 3 min at 62°C, 60 s at 72°C and final elongation for 10 min at 72°C.
Primer pairs S106/A104, S106/A105 and S107/A106 were used in SP-PCR to detect DNA of A. fabae and L. testaceipes, respectively. Each 10 μl PCR contained 0.2 mM dNTPs, 1×PCR buffer (Invitrogen), 3 mM MgCl2, 1 mM of each primer, 1.5 μl DNA extract and 0.375 U Taq DNA polymerase (Invitrogen). Cycling conditions were 3 min at 94°C, 40 cycles of 30 s at 94°C, 30 s at 62°C, 60 s at 72°C and final elongation for 5 min at 72°C.
Spider samples from which no prey DNA was successfully amplified were tested with the general primers LCO-1490 and HCO-2198, using the PCR cocktail and cycling conditions described above for sequencing, to verify the presence of amplifiable DNA and exclude false negative results. PCR water and DNA from A. fabae, L. testaceipes, D. atricapillus and Erigone sp. were run within each PCR assay to test for DNA carry-over contamination, false-negative and false-positive amplifications. Amplified DNA fragments were separated by their length and visualised using ethidium bromide-stained 2.5% agarose gels.
Statistical analysis
Differences in amplification success of prey DNA between PCR type, primer pairs, predator species or prey type along all seven time points post feeding were tested for their significance by the G-test (Dytham, Reference Dytham2003) using MS Excel. Chi2-tests were performed with SPSS 12.0 to detect significant differences in prey detection rates at specific time points after feeding.
Results
Newly designed primers
The newly designed primers proved to be species-specific within the investigated aphid-parasitoid-predator system, as no amplification products were observed when DNA of non-target species was tested in either singleplex or multiplex assays.
Detection of parasitoid DNA in aphid hosts
Using singleplex PCR, DNA from L. testaceipes could be amplified from all samples of parasitized A. fabae. Even aphids that were frozen 5 min after they were parasitized yielded strong bands of the expected length (fig. 1a). When the same samples were tested using multiplex PCR (for parasitoid and host DNA), parasitoid detection was possible in all aphids collected between 5 min and 48 h post parasitism, except for one aphid each frozen at 12 and 24 h post parasitism. Multiplex PCR, however, yielded relatively weak parasitoid amplicons in aphids collected between 5 min and 12 h post parasitism (fig. 1b), whereas much stronger bands of the parasitoid amplicon could be visualized on agarose gels from 24 h post parasitism onwards (fig. 1c).

Fig. 1. Detection of the parasitoid Lysiphlebus testaceipes in its host Aphis fabae using (a) singleplex- and (b, c) multiplex PCR. Amplicons for L. testaceipes and A. fabae were 291 bp and 369 bp in length, respectively. Parasitoids developed in aphids for 5 min (lanes 1–8 in (a) and (b)) or 24 h (lanes 1–8 in (c)) after observed attack. Samples and sample order is the same in (a) and (b); note that parasitoid amplicons in (b) are very faint. Lane 9 is 100 bp DNA ladder, arrows indicate 300 bp fragments.
Detection of parasitized prey in predator gut contents
Carabids and spiders fed with parasitized aphid prey were screened by multiplex PCR for host and parasitoid DNA. In the carabid feeding trial, an additional primer pair targeting D. atricapillus was included in the multiplex assay to serve as an internal amplification control (fig. 2).

Fig. 2. Multiplex PCR detection of Aphis fabae parasitized with Lysiphlebus testaceipes within the gut contents of the carabid beetle, Demetrias atricapillus. Amplicon size for L. testaceipes and D. atricapillus are 291 bp and 369 bp, respectively; DNA of A. fabae was targeted using two primer pairs amplifying 122 bp and 369 bp fragments. Lanes 1, 2, 3, 4, 5 and 6 represent negative control, D. atricapillus fed with A. fabae containing five-day-old parasitoids at 2 h post feeding, adult L. testaceipes, A. fabae, starved D. atricapillus and 100 bp DNA ladder, respectively.
Aphid and parasitoid DNA could be amplified from carabids and spiders fed with parasitized aphids up to 32 h post feeding (figs 3 and 4). In both D. atricapillus and Erigone sp. fed with aphids containing five-day-old parasitoids, detection rates for the aphid prey and the parasitoid within it were not significantly different (table 2). However, parasitoid detection rates decreased rapidly in predators fed with aphids containing two-day-old parasitoids (figs 3a, b and 4a); parasitoid DNA could only be amplified up to 8 h and 0 h post feeding in spiders and carabids, respectively. SP-PCR proved to be more efficient in amplifying DNA from two-day-old parasitoids compared to MP-PCR (e.g. 0 h post feeding, MP-PCR vs. SP-PCR: D. atricapillus, 0% vs. 22%; Erigone sp., 34% vs. 67%; figs 3a, b and 4a).

Fig. 3. Singleplex and multiplex PCR detection of Lysiphlebus testaceipes-parasitized Aphis fabae fed to carabid beetles Demetrias atricapillus. Beetles fed with aphids (a, b) two days post parasitism (pp) or (c, d) 5 days pp and assayed with (a, c) singleplex or (b, d) multiplex PCR. Amplicon length for parasitoid DNA was 291 bp and for aphid DNA was 122 bp and 369 bp (—●—, Aphid 396 bp;...○..., Aphid 122 bp; –▼–, Parasitoid 291 bp).

Fig. 4. Detection of Lysiphlebus testaceipes-parasitized Aphis fabae fed to spiders Erigone sp. Spiders fed with aphids (a) two days post parasitism (pp) or (b) 5 days pp. Prey detection was done using multiplex PCR; singleplex PCR was used to amplify parasitoid DNA in feeding experiment with aphids two days pp only. Amplicon length for parasitoid DNA was 291 bp and for aphid DNA was 122 bp and 369 bp (—●—, Aphid 396 bp;...○..., Aphid 122 bp; –▼–, Parasitoid 291 bp (MP); –△–, Parasitoid 291 bp (SP)).
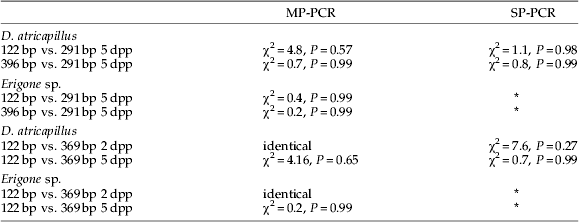
Table 2. Comparison of prey DNA detection success in Demetrias atricapillus and Erigone sp. fed with Aphis fabae parasitized by Lysiphlebus testaceipes using G-test.
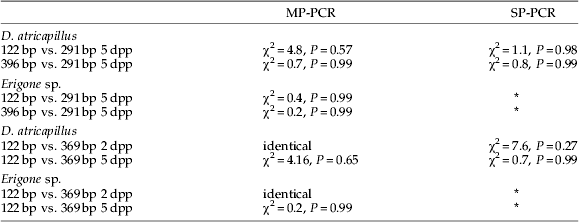
Presence of aphid DNA was indicated by 122 bp and 396 bp products; parasitoid DNA yielded a 291 bp fragment. For all tests, df=6; MP- and SP- refer to multiplex and singleplex assays, respectively; 2 dpp and 5 dpp refer to aphids containing two-day-old and five-day-old parasitoids, respectively.
* No SP-PCR assays were run for these spider feeding experiments. No significant differences were found between tested groups.
Aphid prey DNA detection rates in D. atricapillus appeared higher in SP-PCR than in MP-PCR (fig. 3); however, G-test results indicated that these differences were not statistically significant (SP-PCR vs. MP-PCR for 122 bp and 369 bp fragment was χ2=11.76, P=0.07 and χ2=11.39, P=0.08, respectively; df=6 for all tests, data from 2 dpp and 5 dpp feeding trials combined). The same was true for detection rates of DNA from five-day-old parasitoids (G-test SP-PCR vs. MP-PCR χ2=6.66, df=6, P=0.35; fig. 4).
When detection of parasitized aphids (5 dpp) was compared between spiders and carabids, significantly higher prey detection rates 24 and 32 h post feeding were found in spiders (Chi2-test: aphid DNA 24 h and 32 h post feeding χ2=25.0, P<0.001 and χ2=26.8, P<0.001, respectively (data for both aphid fragments and feeding experiments combined); parasitoid DNA 24 h and 32 h post feeding χ2=5.0, P<0.001 and χ2=15.6, P<0.001, respectively).
Detection of aphid DNA with primer pairs that amplify 122 bp or 369 bp fragments was identical in carabids and spiders fed with aphids containing two-day-old parasitoids using MP-PCR (figs 3b and 4a). No significant differences in prey DNA detection success using these two different-sized amplicons were found in any other feeding trial (table 2).
Discussion
In the present study, we clearly show that the detection of parasitoid DNA in aphids is possible (in 100% of parasitized hosts) as early as 5 min after insertion of the egg by the female wasp. To our knowledge, there is currently no study demonstrating this level of sensitivity. Within brown citrus aphids Toxoptera citricida, parasitized by L. testaceipes, parasitoid DNA was detectable in 8, 66, 94 and 100% of hosts frozen at 2, 24, 48 and 72 h after oviposition, respectively (Weathersbee et al., Reference Weathersbee, Shufran, Panchal, Dang and Evans2004). Similarly, in brown citrus aphids, Persad et al. (Reference Persad, Jeyaprakash and Hoy2004) were able to detect DNA of the parasitoids L. testaceipes and Lipolexis oregmae 6 h post parasitism in 34 and 46% of aphids, respectively. Jones et al. (Reference Jones, Giles, Chen and Shufran2005) were able to detect DNA of L. testaceipes in aphids 48 h after oviposition, but no amplification of parasitoid DNA was possible from aphids frozen at 0 h and 24 h post parasitism. Parasitoid DNA was detected in 100% (n=11) of Lygus hesperus nymphs, which were frozen, 20 h following oviposition by female Peristenus stygicus (Zhu et al., Reference Zhu, Riddick, Williams, Schotzko, Logarzo and Jackson2004). Using a multiplex PCR assay, Gariepy et al. (Reference Gariepy, Kuhlmann, Haye, Gillott and Erlandson2005) were able to detect faint parasitoid-specific bands when screening Lygus rugulipennis nymphs placed in ethanol immediately after they were stung by Peristenus wasps.
Jones et al. (Reference Jones, Giles, Chen and Shufran2005) mention that parasitoid eggs have a tough, yet flexible, egg chorion, which might be hard to penetrate during DNA extraction. Moreover, parasitoid eggs are typical alecithal (yolk poor) and, upon immersion in host haemolymph, further embryonic development occurs and the egg expands greatly in size (Jervis & Copland, Reference Jervis, Copland, Jervis and Kidd1996). This indicates that, besides using a highly sensitive PCR assay, it is important to use DNA extraction methods which allow the ‘trapped’ DNA to be released from inside the parasitoid egg. Perhaps the high sensitivity of the PCR assay observed in the present study was a result of the overnight incubation step during DNA extraction, providing ample time to release DNA from the parasitoid eggs. Interestingly, SP-PCR proved to be more effective in amplifying DNA from 24-hour-old and younger parasitoid eggs than MP-PCR. Clearly, the aphid primers would be expected to out-compete the parasitoid primer pair for the available reagents at low levels of parasitoid DNA within the PCR. However, a more developed parasitoid embryo would provide more parasitoid DNA template and, thus, enhance parasitoid DNA amplification success in MP-PCR. Parasitoid developmental stage was also found to affect parasitoid detection by enzyme-electrophoresis in earlier studies (Walton et al., Reference Walton, Loxdale and Williams1990a,Reference Walton, Powell, Loxdale and Williamsb).
In this study, SP-PCR proved to be more sensitive than MP-PCR, both for amplifying DNA from early developmental stages of the parasitoid and for detecting prey in predators. Thus, further optimization of the multiplex assay to increase sensitivity is necessary when one of the target species is present in low template concentrations. Moreover, the sensitivity in detecting specific amplicons does not depend only on pre-PCR procedures, but is also influenced by the technique applied to separate and visualize PCR products. In this study, conventional agarose gel electrophoresis, employing ethidium bromide-stained 2.5% gels, were used to separate and visualize the PCR products. Highly sensitive systems, such as multi-channel capillary gel electrophoresis systems (von Berg et al., Reference von Berg, Traugott, Symondson and Scheu2008) or fragment analysis on a sequencer (Harper et al., Reference Harper, King, Dodd, Harwood, Glen, Bruford and Symondson2005), are more likely to allow clear identification of those PCR products which were only visible as faint bands on agarose gels.
When A. fabae containing five-day-old L. testaceipes was consumed by spiders and carabids, both aphid and parasitoid DNA was detectable within the gut contents of the predators for extended times post feeding. This demonstrates that PCR-based techniques of prey detection offer an effective approach to analyze predation on parasitized prey. However, when aphids containing two-day-old parasitoids were fed to the predators, hardly any parasitoid DNA was detectable in the predators' guts, even in predators which were frozen immediately after they had finished their meal. This markedly decreased detection success is unexpected because amplification of parasitoid DNA was readily possible in freshly parasitized aphids. At the temperatures used in the present study, it takes ~2 days for the L. testaceipes egg to hatch in its aphid host (Hardee et al., Reference Hardee, O'Brian, Elzen and Snodgrass1990). This means that aphids containing fully grown embryos, or first instar larvae, were fed to the predators in the two days post-parasitism feeding experiment. The parasitoid prey probably contained insufficient DNA to survive extended periods of time post feeding and/or was degraded too quickly during digestion. For the interpretation of field-derived predation rates on hosts and parasitoids, this finding has significant implications, as it highlights the fact that parasitoid DNA detection success in predators is strongly influenced by parasitoid developmental stage. Further work is needed to investigate how parasitoid developmental stage affects DNA detection rates in predators.
Another factor, which has to be accounted for when analyzing predation rates on parasitized prey, is how parasitoid and host DNA detection success is affected by predator type. Here, we found that, within the spiders, prey DNA detection periods were significantly longer than in the carabids. Similar results were obtained in previous studies on prey DNA detection rates in linyphiid spiders and carabid beetles (Sheppard et al., Reference Sheppard, Bell, Sunderland, Fenlon, Skervin and Symondson2005). It has been suggested that the long prey detection times in spiders are caused by their resting metabolic rates that are 50–75% lower than those of other invertebrates, and the fact that spiders can considerably reduce their metabolic rates in response to starvation (Anderson, Reference Anderson1970; Greenstone & Bennett, Reference Greenstone and Bennett1980). Extended prey DNA detection was also found in other predators with extra-oral digestion, such as pentatomid bugs, whose mean prey DNA detection times were ~7 times longer than in coccinellid beetle larvae (Greenstone et al., Reference Greenstone, Rowley, Weber, Payton and Hawthorne2007). Perhaps the extended prey DNA detection times in these predators is also related to the lower quantity or activity of enzymes cleaving prey DNA, a hypothesis which needs to be tested in future studies.
Another interesting finding of the present study is the impact of prey DNA fragment length on detection success. Most previous studies employing DNA-based prey detection showed that shorter product length (<300 bp) leads to longer post-feeding detection times. In our study, in contrast, no significant differences in prey detection rates between 122 bp and 369 bp fragments were found in either spiders or carabids. This demonstrates that, although targeting short-fragmented prey DNA templates is essential for successful gut content or faecal analysis (Deagle et al., Reference Deagle, Eveson and Jarman2006), other factors such as primer efficiency, PCR reagents and/or cycling conditions have, not surprisingly, significant effects on prey DNA detection rates as well (Juen & Traugott, Reference Juen and Traugott2006; King et al., Reference King, Read, Traugott and Symondson2008).
So far, trophic links between predators and parasitized prey have mostly been inferred indirectly by manipulating predator and/or parasitoid abundance in field experiments and the subsequent recording of changes in host and parasitoid densities, parasitism rates and/or plant yield (Rosenheim, Reference Rosenheim1998; Colfer & Rosenheim, Reference Colfer and Rosenheim2001; Snyder & Ives, Reference Snyder and Ives2001). However, such field experiments are restricted to systems where density manipulation (while difficult in its execution) is actually feasible. This approach often relies mainly upon population effects, with little knowledge of the mechanistic links, giving rise to uncertainty about how, in particular, experimental outcomes arose. Apart from manipulative experiments, there are few alternative ways of tracking predation events on parasitized prey (Sunderland et al., Reference Sunderland, Powell, Symondson and Jervis2005). Video imaging has been used to directly observe parasitoid-predator interactions (Meyhofer, Reference Meyhofer2001) and can provide useful insight into behaviour and feeding relationships between predators and parasitized prey under natural conditions. But video observations are technically challenging and restricted to a limited, fixed area of observation, with few opportunities to gather quantitative data. Furthermore, both manipulative field experiments and video imaging (when used directly in the field) to some extent disturb the system under study. In contrast, our study reveals that DNA-based techniques for insect parasitoid-insect-host detection have great potential. Trophic links can be determined at a species-specific level under undisturbed natural conditions, a large number of links can be assessed and the strength of any trophic links estimated by measuring predation frequency.
The newly designed primers proved to be species-specific within the model aphid-parasitoid-predator system and might be used as molecular markers in field studies to assess predation on A. fabae, L. testaceipes and/or parasitized aphids. We recommend that the primers and the PCR protocol should be subjected to extensive non-target testing before screening field-collected predators, to minimize the risk of false positive results caused by primer cross amplification (Admassu et al., Reference Admassu, Juen and Traugott2006; King et al., Reference King, Read, Traugott and Symondson2008).
Acknowledgements
This study was funded by a Marie Curie Intra European Fellowship (no. 515216 P-P INTERACTIONS) granted to MT and held at Cardiff University with WOCS. We thank Jörg Rademacher (Katz Bitoch AG) for advice on parasitoid rearing and handling, James Bell for spider identification and two anonymous reviewers for their valuable comments.