Introduction
Predator-prey interactions are an essential part of any understanding of the population dynamics of the species involved. The prey preferences of non-specialist generalist predators are determined by complex interactions between factors such as encounter rates, switching behaviour, relative densities of potential prey, functional responses, availability of refugia and the defensive capabilities of the prey (Begon et al., Reference Begon, Harper and Townsend2000; Symondson et al., Reference Symondson, Glen, Ives, Langdon and Wiltshire2002a). Analysis of predation in the field, where multiple prey species are available, can provide essential background information for evaluating potential biological control agents for pest species. Such analyses are particularly useful for identifying natural enemies that are capable of attacking alien species, such as the Iberian slug Arion lusitanicus Mabille (sensu Altena 1955) in Norway.
Arion lusitanicus (also recognised as A. vulgaris Moquin-Tandon 1855 (Anderson, Reference Anderson2005)) is spreading through large parts of northern Europe (von Proschwitz, Reference von Proschwitz1992, Reference von Proschwitz1994; Dolmen & Winge, Reference Dolmen and Winge1997; Grimm et al., Reference Grimm, Paill and Kaiser2000) and was first recorded in Norway in 1988 (von Proschwitz & Winge, Reference von Proschwitz and Winge1994). The species is causing major concerns for gardeners, horticulturalists and farmers (Hofsvang, Reference Hofsvang2003), causing damage to crops such as strawberries and devaluing grass fodder for cows. There is also a concern that these slugs may have negative effects on native slugs such as the closely related A. ater, by invading their habitats (von Proschwitz, Reference von Proschwitz1997). The success of alien pests is sometimes explained by the enemy release hypothesis (Keane & Crawley, Reference Keane and Crawley2002; Mitchell & Power, Reference Mitchell and Power2003; Torchin et al., Reference Torchin, Lafferty, Doubson, McKenzie and Kuris2003), in which the pests thrive when released from native natural enemies that have evolved to feed on them. Populations of A. lusitanicus are reaching plague proportions in some areas (von Proschwitz, Reference von Proschwitz1992), thus development of new pest management is necessary. Molluscicidal baits are the most commonly used control against gastropods, although formulations have sometimes been either too weak, so that gastropods do not ingest a lethal dose before they are sated, or too strong, which means they reject the baits immediately (Barker, Reference Barker2002). Furthermore, molluscicides cause collateral damage to other organisms, especially methiocarb, which is poisonous for mammals such as dogs and hedgehogs (Bailey, Reference Bailey and Barker2002). Thus, there is a need to identify native predators that are capable of killing and consuming these alien slugs. Only then can pest management strategies be devised that foster these potentially useful natural enemies.
Many carabid beetles are opportunistic generalist predators and have been shown to be beneficial to agriculture as predators of pest species (Thiele, Reference Thiele1977; Luff, Reference Luff1987; Lövei & Sunderland, Reference Lövei and Sunderland1996; Kromp, Reference Kromp1999; Holland, Reference Holland2002). Symondson (Reference Symondson and Barker2004) reviewed carabids and other beetle groups as natural enemies of molluscs, concluding that many polyphagous predators in these groups are important gastropod predators. Many studies have focused on the generalist Pterostichus melanarius (Ayre, Reference Ayre2001; McKemey et al., Reference McKemey, Symondson, Glen and Brain2001, Reference McKemey, Symondson and Glen2003, Reference McKemey, Glen and Symondson2004; Oberholzer & Frank, Reference Oberholzer and Frank2003; Langan et al., Reference Langan, Taylor and Wheater2004), which is a major predator of slugs in arable fields (Symondson et al., Reference Symondson, Glen, Wiltshire, Langdon and Liddell1996, Reference Symondson, Glen, Ives, Langdon and Wiltshire2002a; Bohan et al., Reference Bohan, Bohan, Glen, Symondson, Wiltshire and Hughes2000). Pterostichus melanarius has been shown to aggregate to areas of high slug densities (Bohan et al., Reference Bohan, Bohan, Glen, Symondson, Wiltshire and Hughes2000) and to be capable of developing a semi-coupled relationship with slugs between years (Symondson et al., Reference Symondson, Glen, Ives, Langdon and Wiltshire2002a). Most work to date has been on the field slug Deroceras reticulatum, while only a few studies have dealt with A. lusitanicus (Paill, Reference Paill, Brandmayr, Lövei, Brandmayr, Casale and Taglianti2000, Reference Paill2004; Paill et al., Reference Paill, Backeljau, Grimm, Kastberger and Kaiser2002; Oberholzer & Frank, Reference Oberholzer and Frank2003).
The versatility of PCR-based methods for detecting predator-prey interactions is now well recognised and is the most practical approach where prey are soft bodied, leaving no recognisable hard parts in the guts or faeces of predators (Symondson, Reference Symondson2002). These techniques are highly sensitive and have been used to detect predation on a wide range of species (Zaidi et al., Reference Zaidi, Jaal, Hawkes, Hemingway and Symondson1999; Agusti et al., Reference Agusti, Shayler, Harwood, Vaughan, Sunderland and Symondson2003; Harper et al., Reference Harper, King, Dodd, Harwood, Glen, Bruford and Symondson2005; Traugott & Symondson, Reference Traugott and Symondson2008). Slugs such as D. reticulatum and A. hortensis have been found to be detectable after approximately 30 h in the gut of the carabid beetle P. melanarius (Harper et al., Reference Harper, King, Dodd, Harwood, Glen, Bruford and Symondson2005). Multiplex PCRs, in particular, provide an efficient means of disentangle trophic interactions by detecting many species at the same time in each predator gut sample (Harper et al., Reference Harper, King, Dodd, Harwood, Glen, Bruford and Symondson2005; King et al. Reference King, Moreno-Ripoll, Agustí, Shayler, Bell, Bohan and Symondson2010a,Reference King, Vaughan, Bell, Bohan and Symondsonb).
Here, our aim was to design and optimize multiplex PCRs to detect predation on arionid slugs, including the invasive Iberian slug A. lusitanicus, and to measuring rates of predation in the field. Symondson (Reference Symondson and Barker2004) emphasized the need to identify key predator species that are numerous enough to affect prey populations. Carabus species are generally regarded as oligophagous specialists on earthworms and gastropods, with mouthparts adapted for killing and feeding on such prey (Hengeveld, Reference Hengeveld1980a,Reference Hengeveldb; Evans & Forsythe, Reference Evans and Forsythe1985). In many parts of Europe, including Scandinavia, Carabus nemoralis Müller is widespread and common in agricultural landscapes (Lindroth, Reference Lindroth1985; Turin et al., Reference Turin, Penev, Casale, Arndt, Assmann, Makarov, Mossakowski, Szél, Weber, Turin, Penev and Casale2003). It is also active during the spring (Lindroth, Reference Lindroth1985) when individuals of A. lusitanicus are small enough for the beetles to be able to prey upon them (Hatteland et al., Reference Hatteland, Grutle, Mong, Skartveit, Symondson and Solhøy2010). Laboratory experiments have found that C. nemoralis is capable of killing and consuming A. lusitanicus up to 1.3 g, with a preference for slugs <1 g (Hatteland et al., Reference Hatteland, Grutle, Mong, Skartveit, Symondson and Solhøy2010). These experiments found no preference for D. reticulatum over the more recently introduced A. lusitanicus. We, therefore, hypothesised that C. nemoralis has no specific preferences for any slug species within the Arion and Deroceras genera and that rates of predation, detected using PCR, would simply reflect relative population densities of different slug prey.
Materials and methods
Field site
The carabid beetles C. nemoralis, P. melanarius and P. niger were sampled at two sites, a meadow in Bergen (60°38′N, 5°34′E) and a site with strawberry patches surrounded by set-aside land in Askøy (60°28′N, 5°12′E), western Norway. The molluscicide Ferramol® and the herbicide Roundup® were used in the latter field by the farmer. Temperature was measured hourly in the field by data logger (Dickson HT100) during the sampling periods.
Beetles collected for gut content analysis
Sampling was by dry pitfall traps arranged in arrays of eight traps at each sample point. Beetles trapped in any of the eight traps were pooled as one sample. In the meadow, the traps were arranged in a total of 21 circles randomly spread out across the field, where each circle of traps represented one sample with minimum distance of three meters between circles. However, at the site with strawberry patches, we used nine transects from surrounding set-aside land into the strawberry patches. Each transect consisted of 4–8 sampling points, depending on the size of the respective strawberry patch, with four meters between each sampling point (fig. 1). Half of the sample points were set up in the set-aside (meadow or grassland) and half were set up within a strawberry patch. A total of 57 sample points were used at this site, each comprised of eight traps in double lines (456 pitfall traps in total).

Fig. 1. Map of the strawberry patches surrounded by set-aside land at the Askøy site outside Bergen. The strawberry patches are represented as squares while the sampled transects are represented as rectangles. A schematic view of a transect is given below the map with pitfall traps represented as circles and searching plots for gastropods represented as rectangles. The line in the middle of the transect represents the edge of the strawberry patch.
Traps were plastic cups, 7-cm wide×9.5-cm deep, buried to the rim and covered by a metal roof ca. 2 cm above the rim. Each trap had a 2.4 mm mesh insert through which smaller prey could fall, reducing potential predation by the beetles after being caught. The traps were emptied twice per day, in the morning and in the evening, to minimise digestion times and possible predation within the traps. In the meadow, pitfall trapping was performed from 13 May to 30 June and 4 September to 7 October 2006, plus a short supplementary period from 21 June to 28 June in 2007. The strawberry site in Askøy was sampled from 24 April to 1 May and from 18 September to 27 September 2007. Beetles were transported to the laboratory and killed at −20°C in spacious plastic boxes with ca. 2 cm of sphagnum moss peat to reduce regurgitation due to stress. The beetles were then removed from the boxes into individual plastic tubes and stored at −80°C. Since pitfall trapping is not an absolute measure of density (Thiele, Reference Thiele1977; Luff, Reference Luff1982, Reference Luff and Holland2002), we use the term ‘activity-density’ thorough this paper when referring to the abundance of beetles.
Molluscs sampled for field density analyses
The field density of slugs and snails were determined in 109 quadrats associated with each cluster of pitfall traps. In addition, eggs of A. lusitanicus were counted in the field. Eleven, ten, ten and 21 quadrats were sampled in the meadow site at 11 May 2006, 8 June 2006, 12 October 2006 and 1 July 2007, respectively. All the quadrats used in the meadow site were 50×50 cm. Furthermore, 57 quadrats, each 20×125 cm, were sampled in the strawberry site, including set-aside land. Each quadrat was situated 50 cm from each set of eight pitfall traps representing one sample point. The vegetation within the quadrat was thoroughly searched for molluscs and was then cut ca. 2 cm above ground level and the ground searched. Finally, the remaining moss was stripped away, and we searched carefully around any plant bases remaining. We made an attempt at the strawberry site to locate molluscs lying below the soil surface by taking one circular soil sample of 10 cm depth and 10 cm in diameter from each quadrat, which was then hand sorted. All molluscs were counted and weighed to the nearest 0.1 g except slugs <0.1 g, which were denoted as 0.05 g. The slugs were released after weighing at the points where they were collected to avoid locally reducing populations.
Feeding experiments
Deroceras reticulatum slugs and juvenile slugs of A. lusitanicus, as well as C. nemoralis beetles, were sampled along the perimeter of the meadow field. Slugs were maintained in plastic bags with ca. 2 cm of sphagnum moss peat at 4−6°C prior to the feeding experiments. The beetles were kept at 14°C in a climate chamber (RUMED® Rubarth Apparate GmbH), simulating the light conditions from the field following a light intensity regime of 50% from 6:00 to 8:00, 100% from 8:00 to 20:00 and 50% from 20:00 to 22:00 followed by darkness from 22:00 to 6:00. The beetles were maintained on earthworms (Lumbricus rubellus) then starved for ten days prior to the feeding experiments. Feeding experiments were performed in Petri dishes containing moistened filter paper with two live slugs per dish under the same climate conditions as the starvation period. Two slugs were chosen to increase the probability of predation within a 2-h feeding period. Beetles were killed in batches of eight beetles (five males and three females) at 10 h, 20 h, 40 h and 60 h post feeding, and stored at −80°C. Unfed beetles were kept as negative controls and non-feeding beetles were discarded from the experiment.
Beetle dissections
The beetles were defrosted then dissected. The foreguts were removed, weighed and stored in microfuge tubes at −80°C prior to DNA extraction. Forceps and scalpel was sterilized between beetles by cleaning in 96% ethanol and open flame.
DNA extractions
DNA was extracted from beetle foreguts and non-target organisms for the cross-amplification test using the DNeasy Blood & Tissue Kit (Qiagen), following the manufacturer's instructions. Extraction negatives (no tissue) were included for all sets of extractions to test for possible contamination during the extraction process. Extractions were stored in elution buffer at −80°C. DNA was extracted from mollusc foot fringes using the E.Z.N.A. tissue kit (Omega Bio-Tek) following manufacturer's instructions and stored in elution buffer at 4°C.
Primer design
The universal invertebrate primers LCO1490 and HCO2198 designed by Folmer et al. (Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) were used to amplify the cytochrome c oxidase subunit 1 (cox1) gene of A. lusitanicus, A. ater and A. rufus. Each PCR was conducted in 25 μl, containing 12.5 μl Promega PCR Mastermix (Promega), 1.0 μl of each primer (10 μM), 2.0 μl DNA (10:1 diluted) and 5.5 μl distilled H2O. PCRs were carried out in a MJ Research PTC220 Peltier thermal cycler, with cycling conditions of 94°C for 2 min, followed by five cycles of 92°C for 60 s, 45°C for 1.5 min, 72°C for 1.5 min, then 30 cycles of 92°C for 30 s, 50°C for 60 s and 72°C for 50 s and a final cycle of 72°C for 7 min. PCR products were checked and visualized on 1% agarose gels following standard procedures, before purification with ExoSap-IT (GE Healthcare) and sequenced with version 3.1 Big Dye terminator chemistry on an ABI3700 capillary sequencer (Applied Biosystems). The sequences were aligned using the software BioEdit, including the multiple alignment function of CLUSTAL W (Hall, Reference Hall1999). Species-specific primers were designed for A. lusitanicus, A. ater and A. rufus (table 1) by using the software PRIMER 3 (Rozen & Saletsky, Reference Rozen, Saletsky and Krawetz1996–1998).
Table 1. Primers (5′–3′) for detection of slugs in carabid beetles foreguts.

FAM, fluorescent label.
PCR amplification and optimization
To avoid false negatives, extractions were tested by PCR for the presence of amplifiable DNA using general invertebrate primers for a 710 bp fragment of the mitochondrial cox1 gene (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994). PCRs were performed in 12.5 μl, containing 6.25 μl GoTaq (Promega) Mastermix, 0.5 μl of each primer (10 μM), 0.125 μl BSA, 1.0 μl DNA and 4.125 μl dH2O. PCRs were carried out in a MJ Research PTC220 Peltier thermal cycler, with cycling conditions of 94°C for 1.5 min, followed by five cycles of 94°C for 30 s, 45°C for 1.5 min, 72°C for 1 min, then 35 cycles of 94°C for 30 s, 51°C for 1.5 min and 72°C for 1 min and a final cycle of 72°C for 5 min. Any samples that failed to amplify were excluded from further analyses.
Two diagnostic multiplex PCRs were used to simultaneously screen each predator gut sample for the presence of multiple slug species. The first multiplex PCR (cox1 multiplex PCR) amplified short fragments of the cox1 gene from A. lusitanicus, A. ater and A. rufus. The assay was optimized for gut content analysis by adding the PCR facilitator bovine serum albumin (BSA), which has been found to overcome PCR-inhibition in gut-content samples (Juen & Traugott, Reference Juen and Traugott2006). The second multiplex PCR targeting the small mitochondrial ribosomal gene (12S rRNA multiplex PCR) and contained primers for D. reticulatum and an Arion genus-specific primer pair (Dodd, Reference Dodd2004: table 1). The latter primer pair amplifies different sized fragments for each Arion species (Dodd, Reference Dodd2004; Harper et al., Reference Harper, King, Dodd, Harwood, Glen, Bruford and Symondson2005). PCRs were conducted in 12.5 μl volumes, containing 6.25 μl GoTaq (Promega) or Qiagen Multiplex PCR Mastermix, 0.25 μl of each primer (10 μM), 0.125 μl BSA, 1.0 μl DNA and 3.875 μl dH2O. Cycling conditions for the cox1 multiplex PCR (table 1) were 94°C for 2 min, followed by 30 cycles of 92°C for 30 s, 51°C for 1 min and 72°C for 50 s. A final cycle of 68°C for 5 min was used when applying Qiagen Multiplex PCR Mastermix, while denaturing for 2 min, annealing for 30 s, and extension at 70°C was carried out when using the GoTaq PCR Mastermix. Cycling conditions for the 12S rRNA multiplex PCR were 94°C for 15 min, followed by 35 cycles of 94°C for 30 s, 53°C for 30 s and 72°C for 1 min, and a final cycle of 72°C for 10 min. All PCRs included positive (target prey) and negative controls consisting of PCR reagents with distilled water as substitute for DNA. PCR products were visualized on 1–2% agarose gels following standard procedures. In some cases, we checked the absolute amplicon size by using Hi-Di formamide and 0.25 μl GESESCAN 350 (ROX) size standard (both Applied Biosystems) and then separated using a 3130xl Genetic Analyzer (Applied Biosystems). The software Genemapper 4.0 (Applied Biosystems) was used to score electropherograms.
Cross-amplification tests on non-target organisms
All primers were tested for cross-amplification using DNA extracted from 54 potential prey taxa plus the three carabid species analysed in this study (table 2). Most of the taxa were common in the field where the beetles were sampled. Others were selected in order to increase the diversity and breadth of taxa. The non-target organisms were tested individually with one multiplex PCR at a time. At least one specimen was tested for each taxon.
Table 2. Cross-amplification tests on non-target prey, target prey and predators using each of the two multiplex PCRs (cox1 and 12S rRNA) separately.

–, not tested; N, no amplification.
Statistical analysis
All statistical analyses were performed using R version 2.8.0 (R Development Core Team, 2008). Generalized linear models (GLMs) were used to analyze the data from the controlled feeding experiments. As the data consisted of PCR-negatives and PCR-positives, a binomial distribution was used in the models. Median detection times (the time at which 50% of beetles tested positive, equivalent to the detectability half-life of Chen et al., Reference Chen, Giles, Payton and Greenstone2000) were calculated from the binomial regression equations. GLMs were also applied to test if the detection of slug DNA in beetle foreguts were significantly different between species of slugs and between sexes of beetles. The latter analyses were carried out using presence and absence of DNA as the response variables and slug species and beetle sex as explanatory variables.
GLM was also used to analyse predation in the field by using a binomial distribution of the presence-absence data of slug DNA in field-caught beetles. The proportions of slug-positive beetles were used as the response variable, while the abundance of A. lusitanicus (defined as the abundance of A. lusitanicus as a proportion of the total abundance of all target slug species: A. lusitanicus, A. distinctus, A. ater×A. rufus and D. reticulatum) was used as the explanatory variable.
Finally, generalised linear mixed-effect models (GLMMs) were used to test the differences in density of slugs and beetles in strawberry patches versus set-aside land. This was done by using the function ‘glmmPQL’ available in the package ‘MASS’ in R. The different transects were used as a random factor. GLMM approximation for analysing non-normal data such as counts has recently been reviewed by Bolker et al. (Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2008). The quasipoisson distribution was used due to over-dispersion in the count data, as suggested by diagnostic plots (leverage, normal Q-Q, fitted and scale location), and by comparing the residual deviance with degrees of freedom.
Results
Primer specificity and sensitivity
None of the 57 non-target prey and predator species were amplified using either the cox1 or 12S rRNA multiplex PCRs (table 2). The 12S rRNA multiplex PCR yielded 221 bp and 109 bp amplicons for the target species A. distinctus and D. reticulatum, respectively, when using fluorescent primers, which are the same as for British specimens (Harper et al., Reference Harper, King, Dodd, Harwood, Glen, Bruford and Symondson2005). However, no amplification of other small arions like A. silvaticus was achieved. Furthermore, the general Arion primers in the 12S rRNA multiplex PCR could not accurately separate the large Arion species; hence, the design and inclusion of the cox1 multiplex PCR. The latter provided clear separation of the three closely related, large arionids, A. ater, A. rufus and A. lusitanicus.
Feeding trials with Arion lusitanicus and Deroceras reticulatum
The maximum detection period of A. lusitanicus and D. reticulatum DNA in the foreguts of C. nemoralis was up to 40 h post-feeding with median detection periods of 22.4 and 19.7 h, respectively (fig. 2). Detectability of the two slug species over time was not significantly different (P=0.995, binomial GLM); neither was there a difference between male and female beetles (P=0.455, binomial GLM).

Fig. 2. Detection period of prey DNA in the foreguts of Carabus nemoralis fed with Arion lusitanicus and Deroceras reticulatum using the cox1 and 12S rRNA multiplex PCR, respectively. The solid line represents the binomial model, while the dotted lines represent the upper and lower 95% confidence limits. The vertical lines represent the replicates and one line may in some cases consist of more than one replicate. (a) The detection of A. lusitanicus (median detection period=22.4 h). (b) The detection of D. reticulatum (median detection period=19.7 h).
Slug densities
The most common slug species in the present study were A. silvaticus, A. lusitanicus, A. distinctus and D. reticulatum (table 3). Other molluscs found in the study sites were Arion ater introgressed with A. rufus (based on a combination of morphology and the cox1 multiplex PCR Hatteland et al., in preparation), A. circumscriptus, A. fasciatus, A. intermedius, Deroceras panormitanum, D. laeve, Boettgerilla pallens, Arianta arbustorum, Cepaea hortensis, Cochlicopa lubrica, Nesovitrea hammonis, Trochulus hispidus and Discus rotundatus. In the meadow, D. reticulatum was the most numerous species, but A. silvaticus and A. lusitanicus were also common. On the other hand, A. lusitanicus was more numerous compared with other species at the strawberry site, co-occurring with C. nemoralis in higher numbers within the surrounding set-aside land (fig. 3). However, only the density of A. lusitanicus was significantly higher in the set-asides compared with the strawberry patches (P<0.001, GLMM). These findings are mainly based on sampling the vegetation down to the soil surface. We only found six slugs in the subsurface soil samples at the strawberry site; three specimens of B. pallens plus single specimens of A. lusitanicus, A. silvaticus and D. reticulatum.

Fig. 3. Densities of slugs and beetles in nine transects from surrounding set-asides to the strawberry patches in spring 2007. The plots along transects were 4 m apart and were sampled once in late April. (a) Density of Arion lusitanicus per 0.25 m2. (b) Activity-density of Carabus nemoralis per sample point (eight traps for one week). The vertical line represents the edge of the strawberry patch. Error bars are given as standard errors.
Table 3. Predation in both fields by Carabus nemoralis (percentages of PCR-positives) compared with densities and mass of Arion lusitanicus, A. ater introgressed with A. rufus, A. distinctus and Deroceras reticulatum.

–, no data available; ±, standard errors.
Predation in the field
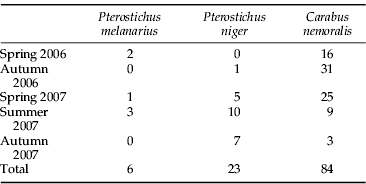
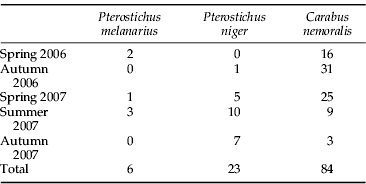
In total, we collected 113 adult carabids, of which C. nemoralis was the most numerous (table 4). The mean foregut weights of 40 field-collected C. nemoralis were 12±2 mg and 19±4 mg for males and females, respectively.
Table 4. The total number of adult carabid beetles used for screening of slug DNA.
Predation rates by C. nemoralis in all the fields together ranged from 16% to 39% slug-positive beetles in spring, of which 7% contained DNA from two slug species. In addition, predation by P. niger ranged from 10% to 20%. Furthermore, predation by C. nemoralis was density related with beetles feeding on the most abundant slug species (GLM, P=0.0340; fig. 4), which was often A. lusitanicus (table 3). In autumn 2006, only 3% of C. nemoralis were positive for A. lusitanicus and D. reticulatum, even though both species were present in high numbers as eggs (82±40 per m2 s.e.) and newly hatched juveniles.

Fig. 4. Predation by Carabus nemoralis on Arion lusitanicus in response to the proportion of A. lusitanicus compared with the total abundance of all target slug species (Arion distinctus, A. lusitanicus, A. ater, A. rufus and Deroceras reticulatum) across all dates and both field sites. The solid line represents the binomial model, while the dotted lines represent the 95% confidence limit. The vertical ticks represent the replicates (number of positives and negatives for each dataset), and one line may in some cases consist of more than one replicate.
Discussion
The main finding was that C. nemoralis was a consumer of the invasive A. lusitanicus, the ‘plague’ slug that is causing severe damage to crops through much of northern Europe. Importantly, the data showed that alien species were not avoided and that the different slug species were eaten seemingly in proportion to their density, with little discrimination between species.
Previous studies have shown that many factors affect detection times of prey DNA in the guts of predators. The fragments amplified by primers used in the present study were about 300 bp in length, which is set as a preferred upper limit, since larger fragments degrades faster than shorter fragments (Symondson, Reference Symondson2002; King et al., Reference King, Read, Traugott and Symondson2008). The choice of predator taxa has also been found to significantly influence the detectability (Chen et al., Reference Chen, Giles, Payton and Greenstone2000; Ma et al., Reference Ma, Li, Keller, Schmidt and Feng2005; Read et al., Reference Read, Sheppard, Bruford, Glen and Symondson2006; Greenstone et al., Reference Greenstone, Rowley, Weber, Payton and Hawthorne2007; Harwood et al., Reference Harwood, Desneux, Yoo, Rowley, Greenstone, Obrycki and O'Neil2007; Traugott & Symondson, Reference Traugott and Symondson2008), even in related species within the Carabidae family (von Berg et al., Reference von Berg, Traugott, Symondson and Scheu2008a). However, the detection period of D. reticulatum in C. nemoralis in the present study was similar to the results obtained in other studies (Dodd, Reference Dodd2004; Harper et al., Reference Harper, King, Dodd, Harwood, Glen, Bruford and Symondson2005) when using P. melanarius. Many other factors also influence the detection of prey DNA, such as temperature (Hoogendoorn & Heimpel, Reference Hoogendoorn and Heimpel2001; von Berg et al., Reference von Berg, Traugott, Symondson and Scheu2008a), weather (von Berg et al., Reference von Berg, Traugott, Symondson and Scheu2008b), combination of prey DNA (Harper et al., Reference Harper, King, Dodd, Harwood, Glen, Bruford and Symondson2005) and the amount of prey DNA ingested (de Leon et al., Reference de Leon, Fournier, Hagler and Daane2006; King et al., Reference King, Vaughan, Bell, Bohan and Symondson2010b). Our calibrating feeding experiment with A. lusitanicus and D. reticulatum makes it possible to compare predation on these species in the field directly without adjustment since detection times were not significantly different. Furthermore, the detection time in male and female C. nemoralis was not significantly different; thus, predation by field-caught beetles may be interpreted without considering sex. This is in accordance with Dodd (Reference Dodd2004), who did not find any significant differences in detection of D. reticulatum in male and female P. melanarius.
The method we applied for sampling of slugs does not give absolute numbers, since only the vegetation down to the soil surface was sampled. Thus, the accuracy of the methods is probably affected by the vegetation and soil structure. There are reasons to assume that slugs will be found deeper down in the soil in arable fields than in meadows (lower vegetation cover and coarser soil particles in arable fields as compared to meadows). However, very few slugs were found in the analysed soil samples in the present study, leading us to conclude that most slugs were located on the soil surface or in the vegetation, at least in the fields included in our study. As the soil at the study sites was compacted, we believe that sampling down to the soil surface recovered most slugs in the area, at least during the part of the year when they are active.
The present study is the first to demonstrate that C. nemoralis and P. niger feed on the invasive Iberian slug, A. lusitanicus, as well as D. reticulatum in the field. Carabus nemoralis fed on slugs with no obvious prey preference, and predation on A. lusitanicus occurred mainly in spring when juveniles were present. Hence, C. nemoralis has potential in conservation biological control since this carabid is most active in spring (Lindroth, Reference Lindroth1985). In addition, P. niger was found to be a predator of A. lusitanicus, as also suggested by laboratory experiments (Hatteland et al., Reference Hatteland, Grutle, Mong, Skartveit, Symondson and Solhøy2010). Paill (Reference Paill, Brandmayr, Lövei, Brandmayr, Casale and Taglianti2000, Reference Paill2004) found the same to be true for C. violaceus and P. melanarius, respectively, feeding on A. lusitanicus in June and in early autumn. However, Pterostichus species are more restricted in the size of prey taken compared to larger and more specialised predators, such as Carabus spp. (Hatteland et al., Reference Hatteland, Grutle, Mong, Skartveit, Symondson and Solhøy2010). The threshold of slug size for P. niger and P. melanarius seems to be approximately 100 mg, based on laboratory experiments (McKemey et al., Reference McKemey, Symondson, Glen and Brain2001; Hatteland et al., Reference Hatteland, Grutle, Mong, Skartveit, Symondson and Solhøy2010) and field studies (Paill, Reference Paill2004), while C. nemoralis consumes slugs of up to 1 g (Hatteland et al., Reference Hatteland, Grutle, Mong, Skartveit, Symondson and Solhøy2010). Surprisingly, we did not find many positive beetles in autumn, although slugs were abundant as newly hatched juveniles or eggs. The explanation might be that the beetles find it difficult to find newly hatched slugs, and their eggs (reviewed in Symondson, Reference Symondson and Barker2004), and/or alternative prey, may have been abundant at this time.
Temperature can also affect rates of predation on slugs (Ayre, Reference Ayre2001). The activity threshold of C. nemoralis has been found to be 4°C, and activity is greater when temperatures rise in spring, while activity is not correlated with temperature later in the season (Weber & Heimbach, Reference Weber and Heimbach2001). In our study area, the diel mean temperature at ground level was 10.6±0.2°C (s.e.) in the spring and 14.2±0.2°C in the summer of 2007 as measured by a data logger, which suggests sufficient temperatures for beetle activity. The activity-density of beetles was low at the strawberry site compared with the meadow, which might reflect the different densities of these carabids in the two fields surveyed. However, many factors tend to affect pitfall catches which make it hard to compare different results (Adis, Reference Adis1979; Spence & Niemelä, Reference Spence and Niemelä1994; Luff, Reference Luff and Holland2002). Activity of beetles may often be related to satiation levels; thus, pitfall catches can be used as a measure of prey availability (Szyszko et al., Reference Szyszko, Gryuntal and Schwerk2004). Satiation, however, is not always linked to predation. Pterostichus melanarius has been found to even kill prey when satiated (Hagley et al., Reference Hagley, Holliday and Barber1982). In addition, pesticides were used in our strawberry site. This, as well as a much higher degree of disturbance in the field compared to the meadow, may explain the lower numbers of beetles in the strawberry patches and the surrounding set-asides.
Unfortunately, our method of detecting prey remains in the predators does not discriminate between predation and scavenging (Foltan et al., Reference Foltan, Sheppard, Konvicka and Symondson2005). Pterostichus melanarius has been found to prefer dead slugs over live slugs, although preference changed to living prey the longer the decaying process progressed (Calder et al., Reference Calder, Harwood and Symondson2005; Foltan et al., Reference Foltan, Sheppard, Konvicka and Symondson2005). Another finding indicating carrion preference was that P. melanarius seems to orientate towards slugs by responding to volatiles produced by the decay process (McKemey et al., Reference McKemey, Glen and Symondson2004), although P. melanarius has been found to orientate towards fresh slugs as well as following mucus trails (McKemey et al., Reference McKemey, Glen and Symondson2004). Carabus nemoralis has also been found to follow mucus of D. reticulatum (Ayre, Reference Ayre1995). Furthermore, the detection period for DNA from decaying slug in the gut of a predator was very similar compared to live slugs (Foltan et al., Reference Foltan, Sheppard, Konvicka and Symondson2005). This means that the beetles testing positive for prey consumption in the present study may be from feeding on dead as well as live slugs. However, as Sunderland (Reference Sunderland, Symondson and Liddell1996) and Juen & Traugott (Reference Juen and Traugott2005) state, carrion may attract predators and may even signal that living prey is also available (Griffith et al., Reference Griffith, Wratten and Vickerman1985; Chiverton, Reference Chiverton1988; Winder et al., Reference Winder, Hirst, Carter, Wratten and Sopp1994). Furthermore, slug-positive beetles in the present study were clearly linked with the density of juvenile slugs in the spring, which suggests that predation is probably the main explanation for these results.
Future work should focus more on spatial rather than temporal data to further test the hypothesis that carabid beetles like C. nemoralis feed on slugs in proportion to numbers and biomass present. It may be possible to enhance the effectiveness of carabids as slug control agents by habitat management (Pickett & Bugg, Reference Pickett and Bugg1998). Provision of shelters and overwintering sites, lowering the amount of indiscriminate pesticides used as well as manipulating carabid activity by semiochemicals, may all have potential (Altieri et al., Reference Altieri, Hagen, Trujillo and Caltagirone1982; Kromp, Reference Kromp1999; Halaj et al., Reference Halaj, Cady and Uetz2000; Symondson et al., Reference Symondson, Sunderland and Greenstone2002b). However, predator-prey interactions are complex and affected by additional factors, such as microhabitat structure, feeding history, cannibalism, fertilizers, crop, season and alternative prey (Symondson et al., Reference Symondson, Sunderland and Greenstone2002b). On the other hand, native predators, including carabids, are clearly able to suppress or reduce indigenous and exotic pests (Sunderland, Reference Sunderland and Holland2002; Symondson et al., Reference Symondson, Sunderland and Greenstone2002b), and future studies should explore the efficiency of biological control of slugs.
Acknowledgements
We are very grateful to Heike Baldeweg, Stine Beate Balevik, Kathrin Bockmühl and Robin Corrià for assisting in sampling of beetles and slugs. Sincere thanks to Raul Ramirez for dissecting most of the beetles. We also thank Knut Helge Jensen for help with the statistics, and many thanks to Daniel Read and Michael Traugott, who gave valuable comments and suggestions. This study was partly funded by the University of Bergen and the Norwegian Research Council.