Introduction
The reproduction and abundance of insects are affected by the interaction between intrinsic life history traits and extrinsic factors, such as food quality, temperature, photoperiod and moisture (Awmack & Leather, Reference Awmack and Leather2002; Malaquias et al., Reference Malaquias, Ramalho, Fernandes, Nascimento Júnior, Correia and Zanuncio2010). Among the abiotic factors, temperature may be the most important (Hentz et al., Reference Hentz, Ellsworth, Naranjo and Watson1998; Sagarra et al., Reference Sagarra, Vincent, Peters and Stewart2000) because it directly affects reproductive parameters, such as the duration of oviposition period, total fecundity and egg viability (Kim & Lee, Reference Kim and Lee2003; Son & Lewis, Reference Son and Lewis2005; Ali & Rizvi, Reference Ali and Rizvi2008).
The diamondback moth, Plutella xylostella L. (Lepidoptera: Plutellidae), is the most important pest of brassicaceous crops worldwide (Talekar & Shelton, Reference Talekar and Shelton1993). It has a cosmopolitan distribution, occurring in regions with distinct climates ranging from the cold Himalayan Mountains (Mohan et al., Reference Mohan, Sushil, Selvakumar, Bhatt, Gujarb and Gupta2009) to the dry heat of Ethiopia (Ayalew & Ogol, Reference Ayalew and Ogol2006). Several studies investigated the influence of temperature on the development of P. xylostella (Shirai, Reference Shirai2000; Liu et al., Reference Liu, Chen and Zalucki2002; Golizadeh et al., Reference Golizadeh, Kamali, Fathipour and Abbasipour2007); however, the impact of temperature on reproduction has received little attention. Apart from the study of Golizadeh et al. (Reference Golizadeh, Kamali, Fathipour and Abbasipour2009), which investigated the effect of temperature on life table parameters of P. xylostella, no studies have provided quantitative information on age-specific survivorship and fecundity at different temperatures (Sarnthoy et al., Reference Sarnthoy, Keinmeesuke, Sinchaisri and Nakasuji1989; Wakisaka et al., Reference Wakisaka, Tsukuda and Nakasuji1992; Shirai, Reference Shirai2000), which are important factors influencing population growth (Birth, Reference Birth1948).
Understanding the effect of temperature on an insect's reproductive parameters is critical for predicting the timing of oviposition and other events related to insect population growth and pest status (Son & Lewis, Reference Son and Lewis2005). Such knowledge can also be useful in predicting the impact of global climate change on the abundance of various pest insects by estimating the lower and upper temperature thresholds for insect population growth. In this context, mathematical models are important analytical tools to predict and understand the population dynamics of a species (Sporleder et al., Reference Sporleder, Kroschel, Quispe and Lagnaoui2004). Oviposition models can be developed based on temperature-dependent components, such as total fecundity, age-specific oviposition rate and age-specific survival. Such an approach, using simple mathematical models to describe these reproductive parameters as a function of temperature, was undertaken in the example of black vine weevil, Otiorhynchus sulcatus (Son & Lewis, Reference Son and Lewis2005). In this study, the influence of temperature on reproduction and population growth of P. xylostella was evaluated in the laboratory; and, based on these data, an oviposition model was developed that may be used to predict both the timing and intensity of egg laying in the field.
Materials and methods
Laboratory rearing of P. xylostella
A colony of P. xylostella was established in the laboratory with ca. 200 larvae and pupae collected in commercial crops of broccoli (Brassica oleracea cv. italica) and cauliflower (B. oleracea cv. botrytis). Every week, 50 field-collected larvae were added to the laboratory colony to maintain the genetic diversity of the stock. The collection was made in the county of Colombo, in the state of Paraná, southern Brazil (25°17′S; 49°13′W). This is a transitional zone between the tropical and temperate climates characterised by cold and dry winters, and moderately warm and wet summers.
Larvae were reared on broccoli leaves until pupation in Petri dishes (18 cm diameter, 2 cm height). Newly hatched larvae were reared in groups of approximately 60 individuals; and, as they grew bigger, the number of individuals per dish was reduced. Pupae were kept in groups of five in polyethylene tubes (2 cm in diameter, 4 cm high) until adult emergence. Adults were reared in cylindrical polyethylene cages (10 cm in diameter, 20 cm high) and fed with 10% honey solution in a cotton ball. Females of P. xylostella laid eggs only in the presence of a host plant, and therefore a piece of broccoli leaf was used as a stimulant. This broccoli leaf (5×5 cm) was placed between two superposed lids, and the lower lid had an opening (4×4 cm) in the centre to allow adults to contact with the leaf inside the cage. The internal surface of the lower lid was lined with sulfite paper, and eggs were laid around the exposed broccoli leaf. Every three days the paper containing eggs was removed and the broccoli leaf was changed. The colony was maintained in a climatic chamber (FANEM® Ltd, model 347 CDG) at 20±1°C and a photoperiod of 12:12 h (light:dark).
Experimental procedure
The reproduction of P. xylostella was evaluated in environmental chambers regulated at constant temperatures of 10, 15, 20, 25, 30 and 32.5±0.5°C, and a photoperiod of 12:12 h (light:dark). Insects were reared from the first instar through adult emergence at each temperature. Twenty males and 20 females were paired, and each pair was kept in a cylindrical polyethylene cage (10 cm diameter, 10 cm high) lined with absorbent paper towel. Pieces of broccoli leaf were used to induce ovipositing behaviour, as described above. Adults were fed with 10% honey solution in a cotton ball placed on a round plastic lid (2 cm in diameter) at the base of the cage. Food was changed, and the number of eggs laid and adult longevity were recorded daily for each insect pair. Egg viability was assessed using eggs laid by females reared throughout the adult stage at each temperature. However, because none of the eggs deposited by females reared at 32.5°C gave rise to hatchlings, we tested whether the time of exposure to this temperature influenced hatchability. For this purpose, viability was assessed on eggs that were laid at 20°C and immediately transferred to 32.5°C. In this test, 30 replicates of 40 eggs were used. The following parameters of adult life history were measured: pre-oviposition and oviposition periods, longevity, survival, fecundity and egg viability.
Reproduction model
Pre-oviposition and oviposition period and longevity model
These parameters were modelled as a function of temperature using the three-parameter polynomial equation:
where Y is the mean pre-oviposition period, oviposition period or longevity at temperature, T (°C); and a, b and c are parameters to be estimated.
Normalized age
Cumulative probability distributions of oviposition and survival rate are displaced in time because of the strong influence of temperature on adult longevity. The timing of each measured distribution can be normalized, allowing the use of a single temperature-independent curve to describe the distributions at all temperatures (Wagner et al., Reference Wagner, Wu, Sharpe and Coulson1984). The cumulative oviposition and survival rates distributions were normalized by dividing the adult age by the mean longevity. This procedure was repeated for each temperature, and the values were plotted within the same graph (Skinner et al., Reference Skinner, Ragsdale, Hansen, Chandler and Moon2004).
Total fecundity and age-specific oviposition rate model
The relationship between total fecundity and temperature was described by the Gaussian equation (Taylor, Reference Taylor1981):
where F(T) is the total number of eggs laid per female during its lifetime in a given temperature, T; F max is the maximum reproductive capacity; T max is the temperature at which maximum reproduction is achieved; and k is a parameter to be estimated.
Age-specific cumulative oviposition rate is the proportion of the total lifetime reproductive potential during a period of time (Kim & Lee, Reference Kim and Lee2003). The four-parameter Weibull cumulative function was used to describe the cumulative oviposition rate against the normalized age:
where P(x) is the cumulative oviposition rate at the normalized age x, b is the normalized age at 50% cumulative oviposition, a, c and d are fitted parameters.
Age-specific survival rate
The age-specific survival rate is the proportion of individuals alive at a given age (Kim & Lee, Reference Kim and Lee2003). A sigmoid function was used to describe the age specific survival distribution of P. xylostella females, according to the equation:
where S(x) is percentage of live females at the normalized age x; b is the normalized age at 50% survival; and a and c are fitted parameters.
Daily egg production
The daily egg production can be described by the product of the temperature-dependent total fecundity [f(T)], age-specific cumulative oviposition rate [p(Px i)], and the age-specific survival rate [s(Px i)]. According to Kim & Lee (Reference Kim and Lee2003), the number of eggs laid by a female during the interval between x i and x i+1 is calculated as:
Life table calculations
Life tables were constructed for each temperature, except for 32.5°C, at which all the eggs failed to hatch. The reproductive parameters calculated were the net reproductive rate (R 0), the rate of increase (r m), the finite rate of increase (λ) and the mean generation time (T), according to Carey (Reference Carey1993). To obtain the sex ratio (females/males+females) at each temperature, 60 newly hatched larvae were reared on broccoli leaves until adult emergence. The sex ratios used in the construction of life tables were 0.45, 0.55, 0.43, 0.50 and 0.53 at 10, 15, 20, 25 and 30°C, respectively. The relationship between net reproductive rate and temperature was described by the Gaussian model. The model proposed by Briere et al. (Reference Briere, Pracos, Le Roux and Pierre1999) was used to describe the relationship between temperature and the intrinsic rate of increase. All parameters employed in the mathematical models were estimated with the software Table Curve 2D (Systat Inc., 2002).
Statistical analysis
The effect of temperature on reproductive parameters was evaluated using analysis of variance (ANOVA). To compare longevity between males and females, a factorial ANOVA was performed using sex and temperature as factors. When differences were detected (P<0.05), Tukey's HSD test was applied to classify the means. The Kaplan-Meier analysis was performed to compare survival rates of males and females between temperatures (Kaplan & Meier, Reference Kaplan and Meier1958). The analyses were performed with the software Statistica (Statsoft Inc., 2001).
Results
Pre-oviposition and oviposition periods and longevity
The lengths of pre-oviposition (F (5,105)=5.99, P<0.001) and oviposition periods (F (5,115)=31.81, P<0.001) were significantly affected by temperature. These traits decrease linearly with an increase in temperature in a range from 10° to 30°C (table 1). Adult longevity was significantly affected by temperature (F (5,205)=39.94; P<0.001), sex (F (1,205)=23212.77; P<0.001) and the interaction between these two factors (F (5,205)=861.64; P<0.001). Males lived longer than females at all temperatures evaluated (table 1). The three-parameter polynomial function provided a good fit to describe the relationship between temperature and pre-oviposition period (table 2, fig. 1A), oviposition period (table 2, fig. 1B) and female longevity (table 2, fig. 1C).

Fig. 1. (A) Temperature-dependent pre-oviposition period (B) oviposition period and (C) longevity of Plutella xylostella (●, observed; ![]() , estimated).
, estimated).
Table 1. Mean length of pre-oviposition and oviposition periods, longevity, fecundity and fertility of Plutella xylostella at six constant temperatures.

Means followed by the same lower case letter in rows and upper case letters in columns are not significantly different from each other according to ANOVA, Tukey's HSD test (P>0.05).
Table 2. Estimated parameters for the model employed to describe the relationship between temperature and adult life history traits of Plutella xylostella.
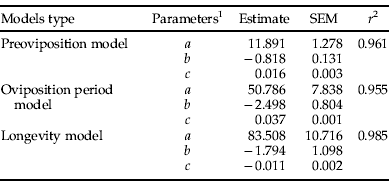
1 a, b and c are fitted parameters; r 2, coefficient of determination.
Total fecundity
Total fecundity of P. xylostella was significantly influenced by temperature (F (5,105)=14.91, P<0.001), and the maximum fecundity observed was 333.3 eggs at 20°C. At temperatures below and above 20°C, fecundity decreased and, at 32.5°C, only 79 eggs were laid on average (table 1). The relationship between temperature and total fecundity was suitably described by the Gaussian model (table 3, fig. 2A), which predicted a maximum reproductive output of 332.5 eggs per female at an optimum temperature of 19.3°C.

Fig. 2. (A) Temperature-dependent total fecundity curve (●, observed; ![]() , predicted), (B) age-specific cumulative oviposition rate (●, 10°C; ■, 15°C; ⧫, 20°C; ▴, 25°C; ○, 30°C; □, 32.5°C;
, predicted), (B) age-specific cumulative oviposition rate (●, 10°C; ■, 15°C; ⧫, 20°C; ▴, 25°C; ○, 30°C; □, 32.5°C; ![]() , predicted) and (C) age-specific survival rate of Plutella xylostella (●, 10°C; ■, 15°C; ⧫, 20°C; ▴, 25°C; ○, 30°C; □, 32.5°C;
, predicted) and (C) age-specific survival rate of Plutella xylostella (●, 10°C; ■, 15°C; ⧫, 20°C; ▴, 25°C; ○, 30°C; □, 32.5°C; ![]() , predicted).
, predicted).
Table 3. Estimated parameters for temperature-dependent models of Plutella xylostella reproduction.
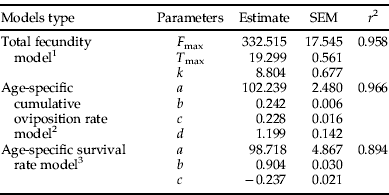
r 2, coefficient of determination;
1 F max is the maximum reproductive capacity; T max is the temperature at which maximum egg production is achieved; k is a fitted parameter.
2 b is the normalized age at 50% cumulative oviposition; a, c and d are fitted parameters.
3 b is the normalized age at 50% survival; a and c are fitted parameters.
The variation in cumulative egg production among different temperatures was reduced using the normalized age, and is well described by the four-parameter Weibull function (table 3, fig. 2B). According to the Weilbull function, 50% of the eggs were laid at 0.24 normalized age. Egg viability was also affected by temperature (F (5, 105)=18.49, P<0.001) and, at 32.5°C, all eggs laid were infertile (table 1). However, when eggs were laid at 20°C and immediately transferred to 32.5°C, 62% of them hatched. No significant differences were detected in hatchability at the other temperatures.
Survival of diamondback moth adults
The Kaplan-Meier survival analysis showed that temperature affected the survival of both females (χ2(5)=70.24, P<0.001) and males (χ2(5)=70.64, P<0.001). The survival curve exhibited low rates of mortality at early and late ages, and a linear decrease in survival during mid-ages (fig. 2C). According to the sigmoid function, 50% mortality was reached at normalized age of 0.90 (table 3).
Daily egg production
The predicted reproductive density curve in relation to temperature and female cohort age showed that both egg production and oviposition period decreased at temperatures below 15°C and above 25°C. At the extreme temperatures, the daily egg production was drastically reduced (fig. 3).

Fig. 3. Predicted oviposition density curves of Plutella xylostella in relation to adult age and temperature.
Life table parameters
The finite rate of increase (λ) was greater at higher temperatures (table 4), whereas the mean generation time (T) decreased as temperature increased. The net reproductive rate (R 0) increased between 10°C and 15°C and, above this range, decreased linearly with an increase in temperature. The relationship between R 0 and temperature was well described by the Gaussian model (table 5, fig. 4A), which estimated the highest R 0 of 74.6 at 18.4°C. The intrinsic rate of increase (r m) increased proportionally with temperature in a range from 10°C to 25°C. This pattern was well described by the nonlinear model, which estimates lower and upper limits of 2.6°C and 35.4°C, respectively, and an optimum temperature of 28.6°C (table 5, fig. 4B).

Fig. 4. Temperature-dependent net reproductive rate (R 0, A) and intrinsic rate of increase (r m, B) of Plutella xylostella (●, observed; ![]() , estimated).
, estimated).
Table 4. Estimated life table parameters of Plutella xylostella at five constant temperatures.
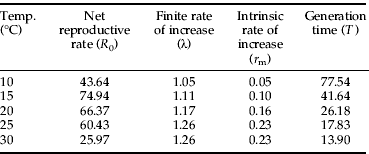
Table 5. Estimated parameters for the models employed to describe the relationship between temperature and life table parameters of Plutella xylostella.
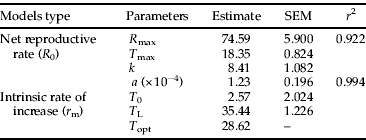
r 2, coefficient of determination; R max, maximum net reproductive rate; T max, temperature at which maximum net reproductive rate is achieved; k, fitted parameter; a, fitted parameter; T0 and TL, lower and upper temperature thresholds for intrinsic rate of increase; Topt, temperature at which maximum intrinsic rate of increase is achieved.
Discussion
The effect of temperature on insect development has been studied extensively, but comparatively little is known about its influence on reproduction. The diamondback moth is a good example of this. In this study, we evaluated the effects of constant temperatures on reproduction of P. xylostella. Although insects rarely live in a stable environment without temperature fluctuation in the field, the results of studies under constant temperatures are still useful in understanding the population dynamics of various insects (Summers et al., Reference Summers, Coviello and Gutierrez1984).
The results show that temperature plays a critical role in the reproduction of P. xylostella, as indicated by its effect on various reproductive parameters. The diamondback moth is able to survive and reproduce in a wide range of temperatures, unsurprising given its cosmopolitan distribution including regions of different climatic conditions. However, fecundity and egg viability are negatively affected by the extreme temperatures evaluated. Mean fecundity was particularly low at 10, 30 and 32.5°C. Furthermore, all eggs laid by females reared throughout the immature and adult stages at 32.5°C failed to hatch. At this temperature, eggs gave rise to hatchlings only when they were laid at 20°C and then transferred to 32.5°C, indicating that the exposure time to temperatures below or above the optimum range is an important factor influencing hatchability.
Only a few studies have investigated the effect of temperature on reproduction of P. xylostella (Sarnthoy et al., Reference Sarnthoy, Keinmeesuke, Sinchaisri and Nakasuji1989; Wakisaka et al., Reference Wakisaka, Tsukuda and Nakasuji1992; Shirai, Reference Shirai2000; Golizadeh et al., Reference Golizadeh, Kamali, Fathipour and Abbasipour2009), and two of these provided some information about fecundity and other reproductive parameters in a wide range of temperatures (Shirai, Reference Shirai2000; Golizadeh et al., Reference Golizadeh, Kamali, Fathipour and Abbasipour2009). The results obtained in our study showed some differences and similarities when compared with those studies. Wakisaka et al. (Reference Wakisaka, Tsukuda and Nakasuji1992) recorded an inverse relationship between temperature and fecundity between 25 and 33°C. As found in our study, Sarnthoy et al. (Reference Sarnthoy, Keinmeesuke, Sinchaisri and Nakasuji1989) observed a bell-shaped relationship between the two variables, with the highest egg production at 23°C. However, Shirai (Reference Shirai2000), using nine populations of P. xylostella, reported that fecundity decreased as temperature increased within a range from 15°C to 35°C. Likewise, Golizadeh et al. (Reference Golizadeh, Kamali, Fathipour and Abbasipour2009) investigated the influence of temperature on reproduction of P. xylostella and obtained results quite different from those of the present study, especially at low temperatures. Female longevity (30 days on cauliflower and 24 days on cabbage) and oviposition period (18 days on cauliflower and 13 days on cabbage) reported by the authors at 10°C were almost half of the values recorded in our study (table 1).
Comparison between studies should be conducted carefully because differences in experimental methods, such as larval food, temperature schedule and geographical origin of the insect, can affect biological parameters. It is known that larval host plants also influence reproduction of P. xylostella (Ayalew et al., Reference Ayalew, Löhr, Ogol and Baumgärtner2006), which may explain, at least in part, the differences among studies. Furthermore, in the present study, individuals were maintained in the tested temperatures from egg hatching; whereas, in the experiment of Shirai (Reference Shirai2000), all larvae were reared at 26°C before adults were tested at different temperatures. Thermal tolerance of insects may vary among different geographical populations (Honék, Reference Honék1996; Addo-Bediako et al., Reference Addo-Bediako, Chown and Gaston2000; Kimura, Reference Kimura2004), indicating that life history parameters may be adapted to the prevailing climatic conditions of the habitat of each population.
Models are important analytical tools for predicting, evaluating and understanding the dynamics of populations under a variety of environmental conditions and management practices (Sporleder et al., Reference Sporleder, Kroschel, Quispe and Lagnaoui2004). Our oviposition model allows the estimation of the daily egg production of P. xylostella in the field and shows that high egg production is expected at temperatures between 15°C and 25°C, with a significant reduction at temperatures below and above this range. This model can be incorporated into a population dynamics model and help to predict P. xylostella seasonal abundance. Comparison of the model outputs with field observations will provide a better understanding of the egg production of P. xylostella and indicate whether modifications will be necessary to improve the model (Kim & Lee, Reference Kim and Lee2003).
Other life history parameters, such as survival and development time of immature stages, influence insect population dynamics and are also important in determining the effects of temperature on population growth in the field. This effect can be quantified by the intrinsic rate of increase (r m), considered a key demographic parameter in the prediction of population growth potential under a given environmental conditions (Andrewartha & Birch, Reference Andrewartha and Birch1954). The r m estimated for each evaluated temperature demonstrates that a difference of 5°C in mean temperature can have a great impact on P. xylostella population growth. This might explain the situation in the counties of Brasilia and Colombo, Midwest and southern Brazil, respectively. Field surveys carried out in Brasilia, with an annual average temperature of 21.4°C, reported a population peak of 110 P. xylostella larvae per plant (Guilloux et al., Reference Guilloux, Monnerat, Castelo-Branco, Kirk and Bordat2003), a value significantly higher than the peak of five larvae per plant found in southern Brazil, where the mean temperature is 16.5°C (Marchioro, Reference Marchioro2011). The insects were collected on cabbage in Brasilia and on broccoli in Colombo, and in both areas other vegetable crops are grown along the year. In Brasilia and Colombo, a similar parasitism rate of ca. 23% was recorded.
The quantification of the impact of temperature on population growth is important in the context of global climate change. The third Intergovernmental Panel on Climate Change (IPCC) report predicted that global average surface temperature would further increase by 1.4±5.8°C by 2100 (Climate Change Impact Review Group, 1996; Houghton et al., Reference Houghton, Ding, Griggs, Noguer, van der Linden, Xiaosu, Maskell and Johnson2001). Increases of this magnitude in global average temperature can modify the pattern of distribution and abundance of P. xylostella worldwide. Using mathematical models, we estimated the lower and upper temperature thresholds, as well as the optimum temperature for population growth of the diamondback moth. The model allows a prediction of potential range limits of occurrence of P. xylostella imposed by temperatures. If global warming continues, it is possible that in the next decades P. xylostella might become less common in some regions where it is presently abundant (e.g. midwest Brazil) and become more abundant in others (e.g. southeastern Brazil).
In conclusion, our study demonstrates that temperature has a significant impact on reproduction of P. xylostella and provides quantitative information on age-specific survivorship and fecundity at different constant temperatures. Based on these data, our oviposition model may facilitate prediction of egg production at different temperatures in the field. This might lead to localised pest control recommendations based on meteorological data. Further studies should investigate reproduction under alternating temperatures, because our results showed that females of P. xylostella are able to lay viable eggs at unfavourable temperatures if they have grown at favourable temperatures as immature stages. Ultimately, studies using bioclimatic models could prove useful to predict changes in the distribution and abundance of P. xylostella, and other major pests, in the context of global climate change.
Acknowledgements
The authors are grateful to the MSc Flavia da Silva Krechemer for technical support and to Dr Sônia Maria Noemberg Lazzari formerly at UFPR and Dr Robert M. Perrin formerly at Syngenta Jealott's Hill, UK for reviewing the English language. The Brazilian National Research Council (CNPq) and Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) provided scholarships and financial support to the project.











