Introduction
At the core of ecological theory lays the concept of the relationship between the organism and its surrounding biotic and abiotic environment (Prosser et al., Reference Prosser, Bohannan, Curtis, Ellis, Firestone, Freckleton, Green, Green, Killham, Lennon, Osborn, Solan, Van Der Gast and Young2007). Ecology has traditionally dealt with natural and human modified environments, such as agroecosystems (Vandermeer, Reference Vandermeer2011) and urban ecosystems (Alberti et al., Reference Alberti, Marzluff, Shulenberger, Bradley, Ryan and Zumbrunnen2003). However, completely human-made ecosystems, such as insect larval diet, have seldom been approached from this perspective. The present study examines the dynamic processes observed in an artificial diet developed for the mass-rearing of a tephritid fruit fly species for future application in the Sterile Insect Technique (SIT). The study conceived the larval diet as a dynamic ecosystem in which fertilized fruit fly eggs develop for a period of several days until the mature larvae abandon the diet. During this process, the biotic environment of the diet, especially the microorganisms, and the abiotic conditions interact with the developing fruit fly in a way that determines the success or the failure of its development.
Area wide application of SIT against tephritid fruit flies requires mass production of the insects using artificial diets (Calkins & Parker, Reference Calkins, Parker, Dyck, Hendrichs and Robinson2005). Development of such diets is often a complex process (Tzanakakis, Reference Tzanakakis2003; Cohen, Reference Cohen2015) considering the balance between purchasing cost of and availability of nutritious feeding materials. This is essential for the production of competitive and high quality insects to be released in expensive and logistically complex control programs (Mello et al., Reference Mello, Wayadande, Yokomi and Fletcher2009). In addition, diets, especially for larvae, are rich in nutrients and are maintained for days in temperatures and relative humidity, which are optimal for microbial growth. As a consequence, larval diets are prone to spoilage, leading to economic losses and disruption of the production (Sørensen et al., Reference Sørensen, Addison and Terblanche2012). In order to avoid spoilage of diets and waste of valuable substrates that would increase the cost of production, most of the larval diets include antimicrobial agents and preservatives (Calkins & Parker, Reference Calkins, Parker, Dyck, Hendrichs and Robinson2005), which in turn may affect drastically the quality of the produced insects. As an example, in the larval diet developed for mass-rearing of the olive fruit fly (Bactrocera oleae), the antibiotic nipagine (Tzanakakis, Reference Tzanakakis2003) is added to control bacterial contamination. This chemical has been shown not only to affect the microbial community of the olive fly larva midgut, but is also acting as a selective force, altering the allelic frequencies of basic metabolic genes such as alcohol dehydrogenase in the reared olive fly population (Konstantopoulou et al., Reference Konstantopoulou, Economopoulos and Raptopoulos1999; Konstantopoulou & Raptopoulos, Reference Konstantopoulou and Raptopoulos2003). This, along with other major bottlenecks to the olive fly population during the first generations of the insects in the laboratory (domestication) resulted in flies with inferior quality (Estes et al., Reference Estes, Nestel, Belcari, Jessup, Rempoulakis and Economopoulos2012), unable to successfully compete with wild males in the field (Economopoulos & Zervas, Reference Economopoulos and Zervas1982).
In recent years we have been working on the development of mass rearing systems, irradiation doses (Rempoulakis et al., Reference Rempoulakis, Castro, Nemny-Lavy and Nestel2015) and quality assurance protocols for the Ethiopian fruit fly Dacus ciliatus (Nemny-Lavy et al., Reference Nemny-Lavy, Nestel and Rempoulakis2016). D. ciliatus (Loew) (Tephritidae) is an oligophagus fly and an A1 EPPO quarantine pest species (EPPO, 2011), attacking a variety of Cucurbitae plants (cucumbers, zucchinis etc.). This pest invaded the South of Israel approximately two decades ago (Maklakov et al., Reference Maklakov, Ishaaya, Freidberg, Yawetz, Horowitz and Yarom2001; Nestel et al., Reference Nestel, Nemny-Lavy, Zilberg, Weiss, Akiva and Gazit2004), and in order to contain its advancement the development and launch of an SIT control program was considered as a feasible measure (Rempoulakis et al., Reference Rempoulakis, Nemny-Lavy, Castro and Nestel2016). Towards this aim, we have been developing an artificial diet for the mass rearing of the fly. Throughout the development of the diet, one of the main problems was spoilage. Diet deterioration was not predictable, and batches of adequately yielding diets intercalated with batches of diet that decomposed rapidly, curtailing the larval development of seeded D. ciliatus eggs. In spite of the industrial importance of the larval diets, studies on the specific factors that lead to spoilage are relatively scarce. Since larval diets are critical for the production of sufficient numbers of competent flies and are of economic importance, the objective of this study was to determine the factors leading to spoilage of the diet. Towards this goal, we studied the microbial distribution of a variety of bacterial populations within the diet during larval development. It was our aim that knowledge derived from this case model will be utilized to enhance the quality of diets developed for other species of fruit flies for future SIT control projects.
Materials & methods
Insect colony
The eggs used for all the experiments were collected from a laboratory colony of D. ciliatus that was established at the Agricultural Research Organization of Israel (ARO) for more than 4 years before the beginning of the experiments (>45 Generation). The general rearing conditions and the propagation method of this colony have been described in details elsewhere (Rempoulakis et al., Reference Rempoulakis, Afshar, Osorio, Barajas-Aceves, Szular, Ahmad, Dammalage, Tomas, Nemny-Lavy, Salomon, Vreysen, Nestel and Missirlis2014a; Rempoulakis et al., Reference Rempoulakis, Castro, Nemny-Lavy and Nestel2015). To collect the eggs, cylindrical (9 cm height × 6 cm diameter) perforated (with ~200 holes of 1 mm diameter) plastic bottles were placed in large entomological cages containing 300–500 couples of mature and mated flies (~15 days after adult emergence). The bottles were lined in their interior with a double layer of filter paper soaked in zucchini juice to attract gravid females for egg laying. After 24 h the bottles were removed from the cages and the eggs were rinsed from the paper, sieved and their volume was measured with the use of a 1 ml syringe. They were then ready for seeding in the larval medium.
Larval diet and general rearing procedures
The artificial diet used for the experiments is the outcome of a series of experimental diets that started from a complex formulation that was gradually simplified to the current diet (unpublished results). The aim of the development was to provide a rearing substrate to accommodate larval development of D. ciliatus, that is an oligophagous species and has particular nutritional needs (Rempoulakis et al., Reference Rempoulakis, Afshar, Osorio, Barajas-Aceves, Szular, Ahmad, Dammalage, Tomas, Nemny-Lavy, Salomon, Vreysen, Nestel and Missirlis2014a). The original diet contained a large amount of antibiotics and nutritional sources (e.g., melon seed powder), which needed to be simplified to a working diet for mass rearing. Following a simplification process throughout 2 years, the final artificial diet composition consisted of sugar beet pellets (15.3%), dry brewer's yeast (2.8%), sugar (4%), potassium sorbate (0.1%), and water (77%). The pH of the diet is adjusted to 4.9 with the addition of few ml of 2 M HCl.
Larval rearing in all the experiments was conducted as follows: 600 g of freshly prepared artificial larval diet were poured into paper rectangular baking trays (15 × 5 × 5 cm3 deep). Fresh diet was then seeded with 0.22 ml (~8000) of 24 h old eggs. Eggs were spread evenly on the top of the diet substrate using a Pasteur pipette. At the fifth day of larval development, the medium containing the developing larvae was transferred from the deep rectangular trays into flat trays (20 cm × 15 cm × 3 cm3 depth) by gently mixing the contents, and the trays were placed into plastic pupation containers with 2 cm thick fine sand at their bottom. Diet samples for microbial characterization (see below) were taken just before egg seeding and continued every 48 h until the larvae hopped out from the diet and pupated. Six days after pupation, all pupae were collected and counted, and their weight was measured to estimate percentage of egg-pupa recovery and average pupal size.
Microbiological analysis of the larval diet
The characterization of microbial succession in the diet during larval development was based on the enumeration of total heterotrophic bacteria (total count), yeasts and molds, coliforms, and lactobacilli. For this aim, samples of larval diet were taken before and after egg seeding (at intervals of 2 days) and until most of the larvae abandoned the diet and pupated in the sand (ca. 12 days after seeding). Larval-diet samples consisted of 3 g of pooled substrate randomly collected from various depths and places of the rearing tray (ca. 1 g per location in tray). The diet sample was suspended in 27 g phosphate buffer saline (PBS). The suspension was vortexed (Vortex-Genie 2) for 1 min in high power to release the associated microorganisms, and aliquots of 2 ml were taken for microbiological determination. Samples were tenfold serially diluted by adding 200 µl aliquots to 1.8 ml PBS, and 100 µl from each of the dilutions were spread on Plate Count Agar (PCA; HiMedia), Violet Red Bile Agar (VRBA; HiMedia), and Potato Dextrose Agar (PDA; Merck) for the enumeration of total heterotrophic count (HPC), coliforms, and yeast and molds, respectively. The PCA and VRBA were incubated for 24 h at 37°C before reading, while the PDA plates were incubated for 48 h at 30°C. For enumeration of lactobacilli, aliquots of 1 ml serially diluted samples were mixed with 12 ml of warm Rogosa agar medium (HiMedia) and poured into sterile polystyrene plates. Colony Forming Units (CFU) were counted following incubation of 5 days at 30°C. Data are reported as mean (n = 3) Log10 CFU/g of diet substrate. In parallel to diet sampling for microbial characterization, the temperature and pH of the diet substrate was measured (in at least three different places and depths of the larval diet).
Lactobacilli inoculation experiments
Experiments were conducted where lactobacilli were added to the diet in order to test their effect on pupal production. A commercial strain of Lactobacillus plantarum MTD1 (NCIMB 40027, Volac), which is used as a supplement for the fermentation of silage (Ellis et al., Reference Ellis, Bannink, Hindrichsen, Kinley, Pellikaan, Milora and Dijkstra2016), was selected for these experiments. Three identical trays containing the larval diet were prepared with the methods described earlier. The diet in two of the trays was mixed (i.e., inoculated) with the L. plantarum before seeding the D. ciliatus eggs and the third tray was kept as a control (i.e., no artificial inoculation of L. plantarum). Each of the two inoculated trays contained different concentrations of L. plantarum (105 and 107 CFU g−1 per tray) to investigate the effects of different initial density of the lactobacilli population. That is, each experiment consisted of a control tray, with no artificially inoculated lactobacilli, a second tray with 105 CFU g−1 lactobacilli and a third tray with 107 CFU g−1 lactobacilli. The microbiological and physical characterizations of the diets were performed as described above. The inoculation experiments were repeated two times during different dates using egg collections from different cohorts of adult flies.
Statistical analysis
Data on pupal recovery from good and poorly yielding diets were analyzed using t-test after log10 transformation. The data on microbial counts in the diets were log10 transformed to homogenize the variance. Differences in microbial counts through time in ‘Good yield’ and ‘Low yield’ diets were analyzed using a Repeated Measures ANOVA. Differences in pH and temperatures were also analyzed with Repeated Measures ANOVA. Differences over time in microbial densities (lactobacilli and coliforms) in the larval diets trays inoculated with L. plantarum before egg-seeding, or kept as control trays without inoculation of lactobacilli, were analyzed with GLM. CFU were log10 transformed and time was used as covariate. Due to the similarities in contents and trends for the two trays inoculated with lactobacilli in each experiment, these were pooled together after statistically proving their similarity with GLM. Contrasts in the trends of lactobacilli and coliforms in each separate experiment were conducted between the average of the two trays inoculated with L. plantarum and control trays. Contrast was also conducted between the two control trays derived from the different experiments. Statistics were analyzed using Statistica version 10 software (StatSoft, 2011).
Results
Microbial composition in good and poorly yielding diets:
Of six trays of artificial larval diet seeded with D. ciliatus eggs, three trays showed a relatively high performance of the diet (i.e., egg-pupae efficacy of at least 3%, pupal weight above 8 mg and good adult emergence of more than 75%) and three trays showed a poor yield of the diet (i.e., egg-pupae efficacy of less than 1.5%, pupae weight 5 mg and less than 20% adult emergence) (fig. 1). Difference between the two groups of trays (good and poor yield) in egg-pupae efficacy was significant (t = −2.8, P < 0.05).
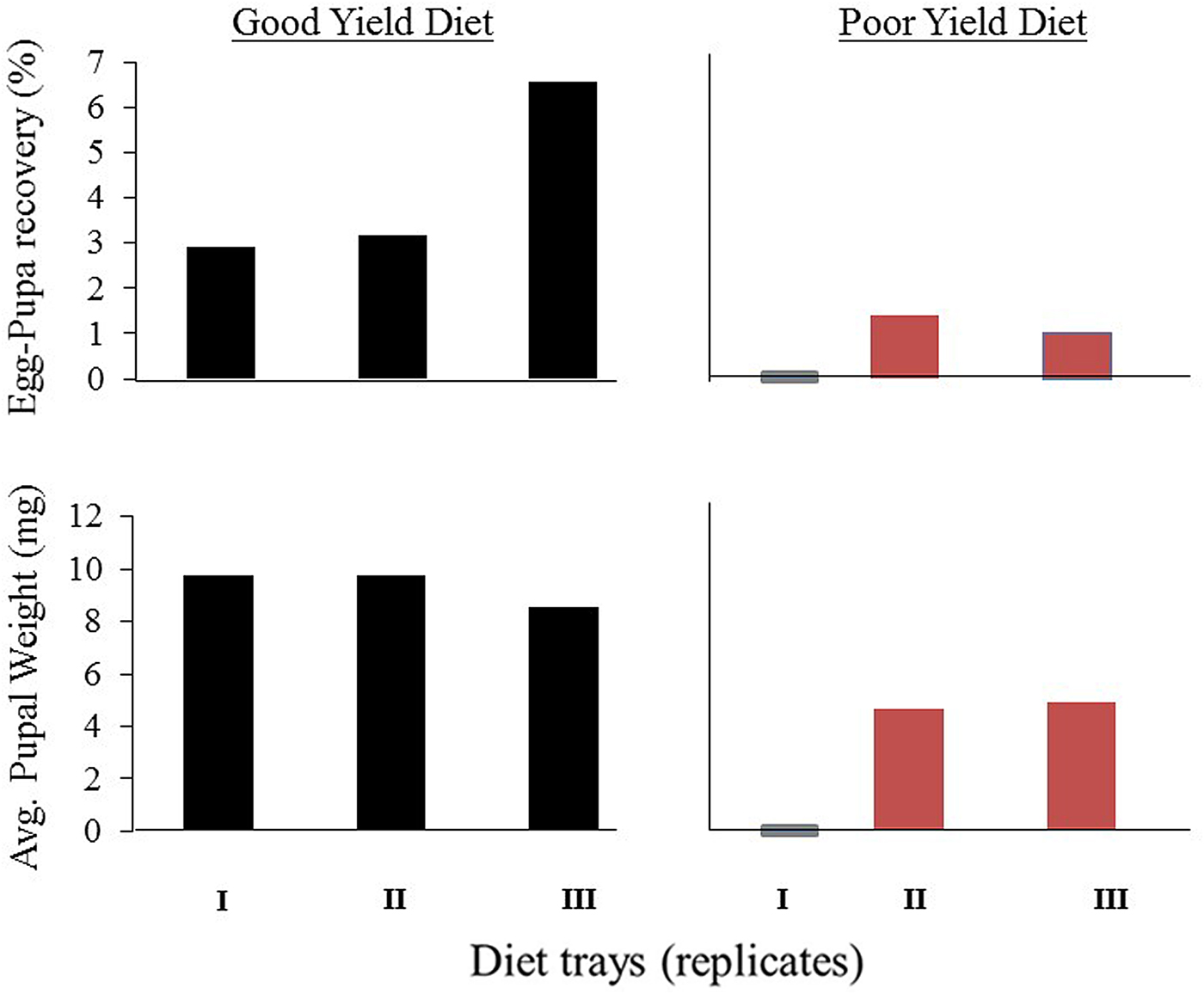
Fig. 1. Percent egg-pupa recovery and average pupal weight in six trays of artificial food seeded with Dacus ciliatus eggs. Trays performance differed between ‘good yield diets’ (trays having an egg-pupae efficacy above 3% and pupae weighting more than 8 mg) and ‘poor yield diets’ in which egg-pupal efficacy was below 1% and produced pupae were below 5 mg.
The dynamic of the microbial populations during larval development in the good and poorly yielding diets is shown in fig. 2. For both diets, total counts of heterotrophic bacteria and yeast and mold counts showed similar patterns: average CFU significantly increased during the whole period of larval development (total count, F 6,24 = 19.9, P < 0.01; yeast and mold counts, F 6,39 = 14.9, P < 0.01). No significant differences were found in CFU between good and poorly yielding diets (total count, F 1,24 = 0.12, P = 0.7; yeast and mold, F 6,39 = 0.86, P = 0.4), and no significant interactions were observed between treatment and sampling date (total count, F 6,24 = 1.9, P = 0.12; yeast and mold, F 6,39 = 1.95, P = 0.1). In contrast, coliform bacteria and lactobacilli showed differential patterns in the two types of diets (fig. 2). Good yield diets were characterized by a significant increase in coliforms over time (F 6,24 = 4.2, P < 0.01) and no detectable lactobacilli; in contrast, poorly yielding diets showed an initial increase in the CFU of coliforms until day 6 followed by a collapse of the population. In addition, the numbers of lactobacilli in the poorly yielding diets sharply increased by day 2 after seeding, staying at this level until the end of the larval-developmental period (F 6,24 = 15.8, P < 0.01) (fig. 2). Coliform and lactobacilli CFU significantly differed between good and poorly yielding diets (coliforms F 1,24 = 13.7, P < 0.05; lactobacilli F 1,24 = 236.8, P < 0.01). Both coliform and lactobacilli abundance showed significant interactions between treatment and sampling date (coliforms F 6,24 = 9.4, P < 0.01; lactobacilli F 6,24 = 15.8, P < 0.01).
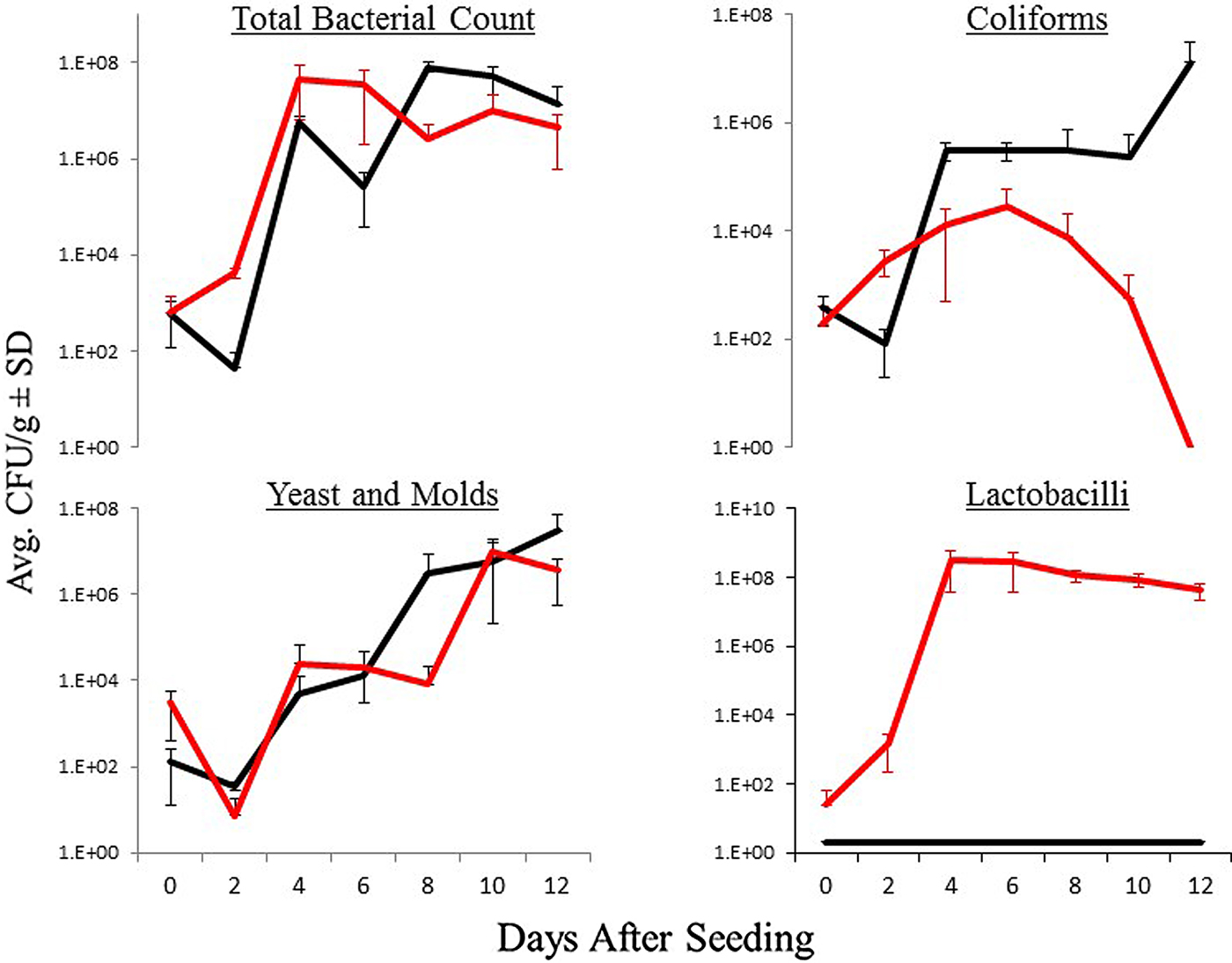
Fig. 2. Patterns of microbial populations during Dacus ciliatus larval development in ‘good yield diets’ (see fig. 1) (black line) and ‘poor yield diets’ (red line). The figure provides the average and standard deviation of Colony Forming Units (CFU) during the 12 days of D. ciliatus developmental period (from egg to pupae).
Although pH significantly decreased in the two types of diets by about 1 unit during the 14 days of measurements (from pH 5 to 4) (F 7,28 = 24.4, P < 0.01), no significant differences in pH patterns were found between the two diets (F 1,28 = 0.01, P = 0.9). Temperature in the two diets was between 24 and 26°C throughout larval development. Poorly yielding diets showed a significant difference of approximately 1°C (25–26°C) throughout larval development (F 1,28 = 199, P < 0.01).
Effect of inoculated lactobacilli on the performance of the larval diet
In neither of the two independent experiments, did any of the four artificially inoculated L. plantarum trays produce any viable pupae. Control trays, however, had variable results. In one of the two experiments, the non-inoculated control tray was able to produce pupae (approximately a 1% egg-pupae efficacy with 9 mg pupae in average) with viable adults. This experiment is labelled as ‘good yield control tray’ in fig. 3. In contrast, in the 2nd experiment the control tray did not produce any pupae. This 2nd experiment is labelled as ‘poor yield control tray’ in fig. 3.

Fig. 3. Patterns of lactobacilli and coliforms bacterial abundance during development of Dacus ciliatus in high yield and low yield diet trays. Prior to egg-seeding, trays were either artificially inoculated with Lactobacillus plantarum solutions at 105 or 107 CFU g−1, or non-inoculated. Mean CFU/g are shown for Lactobacilli (a and b) and Coliforms (c and d) in inoculated (red lines) and non-inoculated (black lines) diet trays. High performance tray corresponds to the experiment where the non-inoculated tray yielded pupae with weight above 8 mg and good adult emergence of more than 75%. Low performance tray corresponds to the experiment yielding poor pupal production (<1%), with low weight (<5 mg) and less than 20% adult emergence. The CFU of lactobacilli and coliforms in the artificially inoculated diets corresponds to the average and Standard Deviation of two trays (red lines), while the CFU of the non-inoculated tray (black lines) represents the pattern in a single tray.
Figure 3 shows the patterns of lactobacilli and coliforms in the two experiments. In the two experiments, the four L. plantarum artificially inoculated trays showed a high (above 108) average lactobacilli CFU (red line in fig. 3) since the onset of the experiment (i.e., from the egg-seeding stage). Lactobacilli populations in the two control trays (black line in fig. 3), in contrast, were below detection levels at the time of egg-seeding. However, lactobacilli counts from indigenous occurring populations in the diet developed during the experiment: in the ‘good yield control tray’, lactobacilli started to increase after 2 days, reaching a maximal level of 106 CFU g−1 of diet by day 4 and onwards. In contrast, in the ‘poor yield control tray’ indigenous lactobacilli count rapidly increase to high loads (108 CFU g−1) in a few days, reaching similar levels to those of artificially inoculated trays by day 6 after egg-seeding (fig. 3). Lactobacilli average contents (i.e., during 12 days) in L. plantarum inoculated and non-inoculated trays in the ‘good yield control tray’ experiment significantly differed (F 1,13 = 25.2, P < 0.01). In contrast, lactobacilli average contents in the ‘poor yield control tray’ experiment did not significantly differ between L. plantarum inoculated trays and control (F 1,11 = 4.0, P = 0.08). Differences in the indigenous lactobacilli CFU trends between the two control trays significantly differed between them (F 1,12 = 7.29, P < 0.05).
Coliform trends differed in the two experiments. Coliform CFU in the ‘good yield control tray’ experiment increased to 1010 in the first 4 days, while the coliform population in the L. plantarum-inoculated trays increase only by 2 logs (104–106 CFU g−1) after 2 days and remained constant later on (F 1,13 = 12.1, P < 0.01) (fig. 3). In the ‘poor yield control tray’ experiment, coliform trends in lactobacilli inoculated trays and control trays behaved very similar (F 1,11 = 0.15, P = 0.70) (fig. 3). Coliform patterns in the control trays of the two experiments, however, significantly differed in their average CFU contents (F 1,12 = 14.4, P < 0.01).
Temperature patterns inside the developing trays vary according to the ambient temperatures. Although room-incubation temperatures were controlled, these oscillated a few degrees (between 23 and 27°C). Measured temperature patterns in all experimental diets did not show any specific dynamics (data not shown).
pH measurements of good yield and poor yield diets (from the above experiments and additional trays where we only followed pH and pupal production), in contrast, provided interesting results. The average pH in trays showing high populations of lactobacilli since the first days of diet incubation and no pupal production dropped from 4.9 to less than 4 in approximately 2 days. In contrast, trays with low, or undetected, indigenous lactobacilli, and other additional trays with successful production of pupae, showed an average pH above 4.5 for at least the first 6 days (fig. 4). At day 6 of tray incubation, we expect larvae to be molting from 2nd to 3rd instar. At day 8 of development, however, pH in all these trays reached the observed levels of L. plantarum inoculated trays.

Fig. 4. Average pH patterns during Dacus ciliatus larval development in trays with no pupal production (with and without artificial Lactobacilli inoculation, n = 5) (red line) and in trays showing successful production of pupae (black line, n = 2).
Discussion
Microbial spoilage of the diet has a significant effect on the performance of the diet as measured by the production of healthy and competent fruit fly pupae. Industrial production of suitable flies requires standardization of the diet and the growth conditions, as well as a rigorous quality control. In the present study we have unveiled an association between the microbial composition of the diet and its ability to produce high yield. Good yielding diets showed relatively low egg to pupa recovery (around 3% to 7%), but good pupal weight that developed into adults. The D. ciliatus egg-pupal efficacy, although relatively low, is comparable with the production of other tephritid fruit flies for which the development of diets has been particularly difficult, such as olive fly, Bactrocera oleae (Dimou et al., Reference Dimou, Rempoulakis and Economopoulos2010; Rempoulakis et al., Reference Rempoulakis, Dimou, Chrysargyris and Economopoulos2014b), and is probably related to the oligophagous nature of this fly (Rempoulakis et al., Reference Rempoulakis, Afshar, Osorio, Barajas-Aceves, Szular, Ahmad, Dammalage, Tomas, Nemny-Lavy, Salomon, Vreysen, Nestel and Missirlis2014a). A future research task will be of course to increase the yield of the diet and the observed egg-pupa recovery for a viable mass rearing system towards SIT application against D. ciliatus.
Bacteria, yeasts and molds are the main factors responsible for spoiling food and feed including insect diets, with every category of product being sensitive to different microorganisms (Inglis & Cohen, Reference Inglis and Cohen2004; Cohen, Reference Cohen2015). The development of acid producing bacteria may lower the pH and enable the multiplication of lactobacilli, which further reduce the pH to intolerable levels that may be detrimental to the insect development (Chan & Jang, Reference Chan and Jang1995). More recently, a study using bacterial isolates from the midgut of Bactrocera dorsalis, found similar detrimental effects upon its development when Lactococcus lacti was used as additive in the larval diet (Khaeso et al., Reference Khaeso, Andongma, Soulyianonh, Zhu, Krutmuang and Niu2017). Coliform bacteria, on the other hand, which are usually present in the gut of mammals (Savino et al., Reference Savino, Cordisco, Tarasco, Locatelli, Di Gioia, Oggero and Matteuzzi2011), have been reported as beneficial endosymbionts of fruit flies (Augustinos et al., Reference Augustinos, Kyritsis, Papadopoulos, Abd-Alla, Cáceres and Bourtzis2015). For example, Augustinos et al. (Reference Augustinos, Kyritsis, Papadopoulos, Abd-Alla, Cáceres and Bourtzis2015) have demonstrated that Medfly larval and pupal development was enhanced in the presence of Enterobacter sp. Similarly, Ben Ami et al. (Reference Ben Ami, Yuval and Jurkevitch2010) found that Enterobacteriaceae, especially Klebsiella oxitoca, greatly enhanced the performance of sterilized Medflies.
In the present study, we have examined the dynamic of four groups of microorganisms (total heterotrophic count, yeasts and molds, coliforms and lactobacilli), which commonly serve as simple and reliable indicators of microbial quality of food and feed (Belda et al., Reference Belda, Pedrola, Peretó, Martínez-Blanch, Montagud, Navarro, Urchueguía, Ramón, Moya and Porcar2011; Cohen, Reference Cohen2015). The levels of the microorganisms generally change during larval production and it is difficult to interpret whether these changes affect the performance of the diet. Based on the data presented in fig. 2, lactobacilli bacterial loads and coliform loads seem to behave in a contrasting way; i.e., when coliform loads increase and are relatively high, lactobacilli numbers tend to be low or undetectable. This type of behaviour resulted in the successful development of the D. ciliatus larvae and it is a characteristic of ‘good yield’ diets. In contrast, if lactobacilli numbers increase, the coliform population tends to be low or to collapse. When lactobacilli CFU are relatively high, D. ciliatus seems to be unable to develop. This seems to be a characteristic of ‘poor yield’ diets. This observation prompted us to conduct controlled experiments with the artificial inoculation of L. plantarum, as a way to test the hypothesis that increase in lactobacilli is associated with the failure of the diet to produce pupae. Our results show that high numbers of lactobacilli, either inoculated or indigenous, reduces the performance of the diet. A potential mechanism that may explain the effect of lactobacilli is the drop in pH. When L. plantarum was added to the diet, the pH tends to significantly drop (fig. 4). If pH drastically drops below 4 early during larval development (fig. 4), pupal production may be affected and the diet may completely fail to produce pupae.
Artificial diet or host fruit acidity has been reported as a limiting factor to the development of many Tephritidae larvae. Vargas et al. (Reference Vargas, Williamson, Chang and Komura1984) reported on the detrimental effects of either too high or too low (below 4) pH of larval diet upon the development of Medfly. Similarly, Jang & Chan (Reference Jang and Chan1993) and Chan & Jang (Reference Chan and Jang1995) reported that when pH is above 6.2 the development of Medflies is severely hampered with poor pupal production. These authors also demonstrated that low pH (<4) severely increases larval developmental time and leads to poor Medfly pupal production. These authors proposed an initial pH value of 5 as the optimal pH for Medfly larval development and good pupal weight. Based on our observation with D. ciliatus during the development of the artificial diet for this species, we found that the optimal initial pH for larval development is between 4.5 and 5.0 (data not shown). No data regarding the dynamics of pH ensuring D. ciliatus larval development, however, was obtained during the development of this artificial diet. Based on the current study, it seems that the reduction of the pH below 4 at early development stage of the larvae results in decreased pupal recovery and poor larval development. These data support the notion that modulation of the diet pH might be the actual mechanism by which lactobacilli affect D. ciliatus larval recovery and development.
The relationship between coliforms and lactobacilli in the diet is not presently clear. The fact that populations of the latter increase as the coliform declines suggest some sort of interaction between the two populations. This interaction is not limited to the D. ciliatus larval diet that was observed in this study, but has already been reported in other systems, e.g., the human digestive tract (Savino et al., Reference Sørensen, Addison and Terblanche2011). This observed interaction between the two bacterial populations in the diet calls for the possible manipulation of the diet to reduce the development of lactobacilli. For example, since lactobacilli requires anaerobic conditions for growth, it would be interesting to investigate the effect of aeration of the diet as a mean to reduce lactobacilli development. Since the microbial ecology of the diet is far more complicated than the interactions observed in this study, however, a more comprehensive study on the diet microbiome is required.
The presence of large populations of coliform bacteria in the diet seem to also favour development of D. ciliatus larvae, notwithstanding the concurrent increase in populations of yeast and molds (fig. 2). Others have also reported on the beneficial role of Enterobacteriaceae, which includes genera that are generally categorized as coliforms, such as Escherichia and Klebsiella, and which comprises the major group in the midgut of tephritids like C. capitata (Ben Ami et al., Reference Ben Ami, Yuval and Jurkevitch2010; Augustinos et al., Reference Augustinos, Kyritsis, Papadopoulos, Abd-Alla, Cáceres and Bourtzis2015). Enterobacteriacae have been already proposed and used as probiotics in mass rearing medium with beneficial effects for larval development (Augustinos et al., Reference Augustinos, Kyritsis, Papadopoulos, Abd-Alla, Cáceres and Bourtzis2015). Although we did not proceed to isolate and characterize the specific genera that were part of the coliform group found in the diet, it is possible that they will play a positive role in the successful larval development when present in the larval medium.
To the best of our knowledge the present study is the first to conceive the artificial larval diet as the studied ecosystem, where biotic and abiotic factors dynamically interact changing and affecting each other. Understanding the diet's ecology may help in the design of more efficient insect diets that will successfully produce the required insect quality. In addition, the importance of the study relies on understanding the role of the insect microbiome, which is critical in the development of any organism (Mueller & Sachs, Reference Mueller and Sachs2015); therefore, studying the dynamics of D. ciliatus diet's microbiome hold promise for the development of optimal diets that will yield healthy mature flies that can successfully compete with wild flies during SIT application. Microbiological manipulation of Tephritidae fruit flies’ diet that may be able to limit the growth of spoilage microorganisms, may also help remove anti-microbial chemicals from the diets that are known to affect the insect symbiotic biota and population genetic constitution (Konstantopoulou & Raptopoulos, Reference Konstantopoulou and Raptopoulos2003), thus affecting the quality and effectiveness of the produced fly.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485317000943.
Acknowledgements
To Prof. Zwi G. Weinberg, for providing the L. plantarum strain and for fruitful discussions. This study was partially financed by a grant from the IAEA Technical Cooperation (ISR 5018) and by the Chief Scientist Fund of the Ministry of Agriculture and Rural Development, Israel (Grant no. 131-1676). S. Sela (Saldinger) was a member of the EU COST Action FA1202: A European Network for Mitigating Bacterial Colonisation and Persistence on Foods and Food Processing Environments (http://www.bacfoodnet.org/) and acknowledge this action for facilitating collaborative networking.
Disclosure
The authors have no conflicts of interest.







