Introduction
Insecta comprises the most diverse animal group and occupies almost all environments on Earth. Recent estimates of the number of insect species range between 1.0 and 5.5 million (Mora et al., Reference Mora, Tittensor, Adl, Simpson and Worm2011; Stork, Reference Stork2018) and about 21–29% of these species remain to be discovered (Costello et al., Reference Costello, Wilson and Houlding2012). One of the reasons for this information gap could be the disproportionate research efforts focused on economically important or charismatic taxa, whereas other groups, such as many families of beetles, flies and parasitic wasps, have been almost completely ignored (Stork, Reference Stork2018).
One of the most abundant groups of insects is the megadiverse order Diptera comprises 157 recognized families (Wiegmann et al., Reference Wiegmann, Trautwein, Winkler, Barr, Kim, Lambkin, Bertone, Cassel, Bayless, Heimberg, Wheeler, Peterson, Pape, Sinclair, Skevington, Blagoderov, Caravas, Kutty, Schmidt-Ottm, Kampmeier, Thompson, Grimaldi, Beckenbach, Courtney, Friedrich, Meier and Yeates2011) including Tephritidae, whose frugivorous members are known as ‘true fruit flies’ and include almost 5000 currently recognized species (Norrbom, Reference Norrbom2004). Tephritid flies display a wide distribution all over the world, inhabiting tropical, subtropical and temperate areas. One-thousand and sixty tephritid species have been recorded from the American continent, of which 957 have been found in the Neotropical Region from Mexico to Chile and Argentina (Norrbom et al., Reference Norrbom, Carroll, Thompson, White, Freidberg and Thompson1999; Norrbom, Reference Norrbom2004; Hernández-Ortiz et al., Reference Hernández-Ortiz, Guillén-Aguilar, López, Montoya, Toledo and Hernández2011). Many fruit fly species belong to the subfamily Trypetinae and have relevance since they produce damage and economic losses in fruit production. Within this group, the tribe Carpomyini is composed of 140 species arranged in 12 genera (Smith and Bush, Reference Smith, Bush, Aluja and Norrbom1999; Norrbom, Reference Norrbom2004) that inhabit the Holartic and Neotropical Regions and of which, the genus Rhagoletis is the most recognized. In contrast, most of other genera have been poorly studied and there is scarce information about their natural history, biology, behaviour and phylogenetic relationships (Norrbom, Reference Norrbom1994; Smith and Bush, Reference Smith, Bush, Aluja and Norrbom1999; Rull et al., Reference Rull, Abraham, Schlisermann, Ordano and Ovruski2017).
Studies about the natural history of a species form the basis for a good understanding of the ecology and evolution of behavioural patterns. Some aspects, such as adult food, mating or egg laying sites, have been poorly investigated within the Carpomyini, except for several economically important Rhagoletis, Zonosemata and Carpomya species (Prokopy and Papaj, Reference Prokopy, Papaj, Aluja and Norrbom1999; Boucher et al., Reference Boucher, Ashley and Adams2005; Yee et al., Reference Yee, Hernández-Ortiz, Rull, Sinclair and Neven2014). Regarding sexual behaviour, research to date indicates that the mating system of Carpomyini can be categorized as male resource defence, where males are located near or above host fruit, awaiting females that are seeking egg-laying sites. Meanwhile, males defend such sites from intruding males and eventually copulate without any courtship display. Based on studies performed on Rhagoletis pomonella, and a few other species in the genus, both males and females are highly promiscuous, and the last male to copulate fertilizes most of the eggs laid by the female (Opp and Prokopy, Reference Opp and Prokopy2000). According to available information to date, with the exception of species in the walnut infesting R. suavis group, all other Rhagoletis females normally lay only a single egg per fruit (Prokopy and Papaj, Reference Prokopy, Papaj, Aluja and Norrbom1999; Nufio et al., Reference Nufio, Papaj and Alonso-Pimentel2000). Also, the majority of species of Carpomyini are monophagous, stenophagous or oligophagous, and their life cycle includes one full generation per year, exploiting hosts with discrete fruiting periods separated by adverse environmental conditions; consequently, such organisms become dormant during the pupal stage and emerge as adults during the following fruiting season of their host plant, however in some species, part of each generation diapauses for multiple years (Prokopy and Papaj, Reference Prokopy, Papaj, Aluja and Norrbom1999). After adult emergence from dormant pupae, the majority of adult Carpomyini need to acquire protein for gonadic development (Prokopy and Papaj, Reference Prokopy, Papaj, Aluja and Norrbom1999). However, there are some exceptions to this rule, such as Haywardina cuculi and Rhagoletis blanchardi which engage in sexual behaviour immediately after adult emergence (Rull et al., Reference Rull, Abraham, Schlisermann, Ordano and Ovruski2017; Rull, Reference Rull, Montoya, Toledo and Hernández2020), suggesting that sexual maturation occurred during metamorphosis using larval stores.
Rhagoletotrypeta Aczél is a small genus of Carpomyini of Nearctic and Neotropical distribution that comprises 12 species placed into two groups (Smith and Bush, Reference Smith, Bush, Aluja and Norrbom1999). The annulata group includes R. annulata Aczél, R. argentinensis Aczél, R. intermedia Norrbom, R. morgantei Norrbom, R. rohweri Foote and R. uniformis Steyskal while that xanthogastra group includes R. cubensis, R. parallela Norrbom, R. pastranai Aczél, R. xanthogastra Aczél, R. chapecensis Norrbom and R. gelabertae Norrbom (Ovruski et al., Reference Ovruski, Norrbom, Schliserman and Aluja2005; Norrbom et al., Reference Norrbom, Savaris and Marinoni2016). To our knowledge, four species have been found in Argentina associated with host plants belonging to the genus Celtis L. (family Cannabaceae) (Berg and Dahlberg, Reference Berg and Dahlberg2001) such as C. tala Gillies ex Planch. and C. iguanaea (Jacq.) Sarg. (Norrbom, Reference Norrbom1994; Ovruski et al., Reference Ovruski, Norrbom, Schliserman and Aluja2005). Both species are native to the Neotropical Region; and can be found in the Yungas and Chaco ecoregions, between 400 and 1200 m above sea level (Romanczuk and Del Pero de Martinez, Reference Romanczuk and Del Pero de Martínez1978). These plants are small trees (8–12 m) whose fruits are almost or entirely spherical drupes with fruiting taking place between late summer and early fall (Dottori and Hunziker, Reference Dottori, Hunziker and Hunziker1994). There are many unknown aspects of the biology and ecology of Rhagoletotrypeta, e.g. duration of life stages, patterns of sexual maturation, mating behaviour and adult longevity; therefore, this plant–insect affiliation constitutes a good opportunity to generate knowledge about a poorly studied genus of tephritid fruit flies. In the present study, we report data on distribution, host–plant associations, infestation and parasitism levels, regulation of dormancy, and demographic and behavioural observations on adults of R. pastranai in an effort to contribute to understanding the evolution of life history strategies in the Carpomyini.
Materials and methods
Study area
Biological material for this study was obtained from two sites in the Tucumán Province (NW Argentina), one of them with several sampling points along a section of provincial route RP 338 between Villa Nougués (26°51′21.16″S–65°21′52.64″W, elevation 1112 m a.s.l.) and San Pablo (26°52′4.28″S–65°20′48.77″W, elevation 664 m a.s.l.), both localities are situated in Lules department. The second site was located at El Cadillal (26°37′01″S–65°11′43″W, 615 m a.s.l.) in Tafi Viejo department, where sampling took place alongside the road circling a dam. The sampled sites belong to the southernmost end of the Yungas ecoregion (Brown et al., Reference Brown, Pacheco, Lomáscolo, Malizia, Brown, Martínez Ortiz, Acerbi and Corcuera2006) where mean annual temperature is 18 °C (Climate-Data.org, 2020) and on account of a strong altitudinal gradient, two different types of vegetation can be distinguished (Brown et al., Reference Brown, Grau, Malizia, Grau, Kappelle and Brown2001): pre-montane forest, elevation between 400 and 700 m with mean annual rainfall between 800 and 1500 mm and lower montane forest situated between 700 and 1500 m, with mean annual rainfall reaching 2000 mm. Overall, the climate in the study area can be categorized as Cwa according to Köppen and Geiger classification, i.e. subtropical warm-temperate climate with a dry season during the coldest months and rainfall recorded mainly from October to March.
Fruit collection and infestation levels
Fruit sampling was done by collecting drupes of two Celtis species, C. tala and C. iguanaea (syn. Celtis pubescens (Kunth) Spreng.) during March and April 2018 which corresponds to the fruiting period for both plants. Six Celtis trees were surveyed in Villa Nougués – San Pablo and ten in El Cadillal. Ripe fruits were recovered on and under tree canopies and placed into separate plastic bags for each plant species and locality. Afterwards the same day, the entire fruit collection was taken to the Laboratorio de Investigaciones Ecoetológicas de Moscas de la Fruta y sus Enemigos Naturales (LIEMEN – PROIMI) in San Miguel de Tucumán (Tucumán Province, Argentina). There, collected fruits of each sample were counted and placed in plastic trays inside larger plastic containers (5000 ml) that were capped with a fine mesh-covered lid to allow air passage. Containers were kept under controlled environmental conditions at 25 ± 1 °C, 60 ± 10% R.H. and a 13/11 h L/D photoperiod and inspected daily to record numbers of larvae and pupae. Pupae/larvae were transferred to 200 ml plastic cups with a mesh-covered lid containing sterilized moist vermiculite and held under controlled environmental conditions (described above) until the emergence of adult flies and/or parasitoids. The time from collection of infested fruit to the emergence of larvae and pupae was recorded to estimate the duration of immature life stages.
To estimate infestation levels for both Celtis species, per cent of infested fruit and the number of larvae per kg of fruit were calculated. For this purpose, a subset of 35 fruits of C. tala and 36 fruits of C. iguanaea were weighted and placed individually in small covered 25 ml plastic cups which, in turn, were placed into large plastic containers described above. Individual fruit was also kept under controlled environmental conditions and inspected daily to record the number of larvae and pupae emerged from each fruit.
Emergence of adult flies and parasitoids
Pupae in 200 ml plastic cups were observed periodically to record adult fly and parasitoid emergence. The time from pupation to adult emergence was used to estimate the duration of development and/or dormancy during the pupal stage. Once emerged, adult flies and parasitoids were kept in plastic cages (12.5 × 12.5 × 13.5 cm) with free access to water and food for 4 days to allow full wing pigmentation and were preserved in 100% ethyl alcohol for further identification. Adult fruit fly diet was prepared following Jaldo et al. (Reference Jaldo, Gramajo and Willink2001) and consisted of sugar (57.9%) (Ledesma S.A., Jujuy, Argentina), hydrolysed yeast (14.5%) (Yeast Hydrolysed Enzymatic, MP Biomedicals ®), hydrolysed corn (27.3%) (Gluten Meal, ARCOR® Tucumán, Argentina) and vitamin E (0.3%) (Parafarm®, Buenos Aires, Argentina) (w/w).
Effect of winter length on development and/or dormancy in the pupal stage
In order to assess the effect of winter length on the duration of development and/or dormancy, pupae recovered from infested fruits of C. tala were placed in 200 ml plastic cups as described above. Subsequently, pupae were submitted to a pre-winter period with control of temperature (25 ± 1 °C) in the laboratory for 10 days and then subjected to artificial winter periods taking account of the mean temperature in Tucumán during the coldest months is 12.7 °C with values that rarely reach 0 °C (Climate-Data.org, 2020). Pupae were separated in 12 cups, two cups per treatment were placed in a conventional refrigerator at 8 ± 1 °C for 2, 4, 6, 8 and 10 weeks (w) and two cups were left under constant laboratory conditions (25 ± 1 °C). The sample size for treatment varied according to the number of pupae recovered during inspection of fruit; 2 w (N = 100 and N = 32), 4 w (N = 24 and N = 87), 6 w (N = 22 and N = 32), 8 w (N = 49 and N = 29), 10 w (N = 80 and N = 34) and control (N = 164 and N = 167). Vermiculite was moistened with 2% (w/v) sodium benzoate solution once a week during the entire experimental period; at the end of artificial winter periods, cups were returned to pre-winter conditions inside the laboratory and emergence of adult flies and parasitoids was recorded for all treatments. One year and two months after fruit collection, uneclosed pupae remaining in cups were separated from vermiculite, counted and inspected to record numbers of live and dead (dry) pupae. The total overwintering mortality per treatment was calculated by adding the number of emerged adult flies and parasitoids and the number of live pupae and subtracting this sum from the total number of larvae recovered after fruit collection.
Age of sexual maturity
Newly emerged R. pastranai adults were sorted by sex and placed in plastic cages (12.5 × 12.5 × 13.5 cm) with free access to water and food. As treatment, two types of diet were provided: (1) adult diet as previously described and (2) sucrose diet. Five 1-, 5-, 10- and 15-day-old males and females of both treatments were individually frozen at −18 °C for later dissection under a Leica EZ4-D stereoscopic microscope. For males, testes width and length were measured, and in the case of females, size of ovarioles and the number of mature eggs in each ovariole were counted. Additionally, measures of head width were obtained as a control for relative adult size.
Behavioural observations and demography
On the day of emergence, R. pastranai adults were sorted by sex and placed in plastic cages (12.5 × 12.5 × 13.5 cm) with free access to water and food. In total, 40 adults between 13 and 17 days old (sexually mature) were separated for these studies.
For behavioural and mating observations, three glass cages (30 × 30 × 60 cm) were set with two Citrus aurantium twigs and four oviposition devices hung on the ceiling of each cage. Oviposition devices consisted of agar spheres (12 g agar in 250 ml water) with 30 g sucrose and 3–4 drops of orange edible dye. Spheres were wrapped with Parafilm®, Chicago, IL, USA, in order to mimic host fruits (fig. 1). On the day before beginning observations, five males and five females were marked on the dorsum of the thorax with a small dot of distinctive acrylic paint to facilitate identification and tracking of individuals. Then, five pairs of marked flies were released in one of the glass cages. Observations consisted of periodical scanning of cages (c.a. every 15 min) recording behavioural activities. Scanning encompassed a daily time period from 9 am until 7 pm during 14 days. In addition, five couples were separately placed in 250 ml plastic cups covered with organdi cloth in order to record mating events and copula duration during a period of time from 2 pm until 7 pm for 7 days. Descriptions of sexual behaviour were based on notes taken during observations.

Figure 1. Adult male 13–17 days old Rhagoletotrypeta pastranai used for behavioural observations in an experimental 30 × 30 × 60 cm glass cage with four artificial fruit mimics and five sexually mature R. pastranai couples.
After observations, males and females in the three cages provided with water and food were submitted to a demographic study. Cages were inspected daily and the date and sex of dead adult flies were recorded.
Statistical analysis
The relationship between fruit weight and number of larvae per fruit was explored using a simple regression. The proportion of adults emerging from pupae exposed to low temperatures for different time periods was compared among treatments using a χ2 test of observed proportions vs. proportions expected from a uniform distribution. The proportions of dead (dry) pupae, live pupae (in prolonged dormancy), disintegrated pupae and total overwintering mortality were also compared among treatments using a χ2 test of observed proportions vs. proportions expected from a uniform distribution. The duration of dormancy (from pupation to adult emergence) in days corrected for winter length (artificial winter period subtracted) was compared among treatments with a GLM with duration as the dependent variable and sex and treatment as factors and replicate as a random factor. The age at sexual maturity was established with ANCOVA where size (head width) as a covariate and diet (sugar/sugar + protein) and ovary length, or ovary width, or number of mature eggs in the ovaries as dependent variables for females and testes width or length for males. Adult longevity was compared between sexes with a GLM with longevity in days as the dependent variable, sex as a categorical factor and replicate as a random factor.
Results
Infestation levels
A total of 1376 larvae were recovered from 807 Celtis tala drupes. Up to five larvae were recovered from individual fruit with an average (±s.d.) of 0.85 ± 0.84 larvae per fruit. Larval recovery began 6 days after fruit collection and the longest time recorded was of 20 days. There was no significant relationship between fruit size and number of larvae per fruit (R 2 = 0.08, P = 0.08). A total of 426 adult flies (263 females and 163 males) and nine parasitoids emerged from recovered larvae over a year. Only four flies emerged without becoming dormant (<40 days after pupation), whereas all but one parasitoid emerged within 40 days after pupation. Parasitoids were identified as an undescribed species of Utetes close to U. anastrephae (Viereck). Non-dormant parasitoids emerged 19.75 ± 2.37 days after pupation, while one individual female emerged 172 days after pupation. In the case of Celtis iguanea, only 11 pupae were recovered from 945 drupes out of which a single adult emerged 208 days after fruit collection.
Effect of winter length of survival and duration of dormancy
Of 326 adults recorded during observations on winter length, very few (N = 3) emerged from pupae without becoming dormant (<40 days). Winter length had no effect on survival (per cent adult emergence) of R. pastranai pupae exposed to 8 °C for different time periods (χ2 = 7.02, df = 5, P = 0.21). A small proportion of flies entered in prolonged (>year) dormancy (1.56 ± 2.14%) but there were no differences in the proportions according to treatment (χ2 = 7.02, df = 5, P = 0.21). Large proportions of uneclosed pupae were dead (dry) after a year (23.7 ± 10.2%) (χ2 = 21.8, df = 5, P = 0.005) with pupae exposed to 4, and 6 w winters having larger proportions of dead pupae than expected from a uniform distribution. Large proportions of pupae were not found after a year in overwintering cups (34.25 ± 13.15%) (χ2 = 24.8, df = 5, P < 0.001) with pupae exposed to 0 and 2 w winters having larger proportions of unaccounted pupae than expected from a uniform distribution. Overall overwintering mortality was high (58 ± 6.48%) with no significant difference among treatments (χ2 = 4.67, df = 5, P = 0.45) (fig. 2). Duration of dormancy was not affected by winter length (F 5,325 = 0.679, P = 0.65) (fig. 3). Average duration of dormancy was 144.9 ± 3.9 days for females and 143.2 ± 3.38 days for males which was not statistically different (F 5,325 = 0.43, P = 0.63).

Figure 2. Per cent emerged adults, live (in prolonged dormancy) and dry (dead) pupae a year after collection, and disintegrated pupae.

Figure 3. Average number of days from pupation to adult eclosion corrected for artificial winter length (by subtracting the time spent in the refrigerator under 8°C) for Rhagoletotrypeta pastranai pupae subjected to artificial winters of different duration (0, 2, 4, 6, 8, and 10 weeks).
Age at sexual maturity
There was no significant effect of diet (F 1,18 = 0.55, P = 0.46), age (F 3,18 = 0.139, P = 0.93) or the diet×age interaction (F 3,18 = 0.138, P = 0.93) on testes width. There was no significant effect of diet on testes length (F 1,18 = 0.58, P = 0.45). By contrast, there was a significant effect of age on testes length (F 3,18 = 3.39, P = 0.04) with older males having longer testes, while the interaction diet×age was not significant (F 3,18 = 0.58; P = 0.63) (fig. 4). In the case of females, there was a significant effect of diet (F 1,18 = 9.24, P = 0.007) and age (F 3,18 = 5.87, P = 0.006) on ovary width while the interaction diet×age (F 3,18 = 0.14, P = 0.26) was not significant. Females fed a protein diet and older females had wider ovaries than sugar-fed flies and younger flies, respectively. Similarly, there was a significant effect of diet (F 1,18 = 6.63, P = 0.019) and age (F 3,18 = 4.49, P = 0.016) on ovary length, while the interaction between diet×age (F 3,18 = 1.11; P = 0.37) was not significant (fig. 5). Finally, there was a significant effect of diet (F 1,18 = 13.10, P < 0.001), age (F 3,18 = 21.96, P < 0.001) and their interaction diet×age (F 3,18 = 8.90, P = 0.001) on the number of mature eggs in the ovaries. Older female flies fed a protein-rich diet had progressively more eggs in the ovaries while sugar-fed flies had few eggs irrespective of age (fig. 6).

Figure 4. Average testes length according to age in days for five Rhagoletotrypeta pastranai males. Different letters above bars represent significant differences (P < 0.05).

Figure 5. Average ovary length according to age in days for five Rhagoletotrypeta pastranai females fed a sugar (grey line) and a protein (black line) diet.

Figure 6. Average number of mature eggs in the ovaries according to age in days for five Rhagoletotrypeta pastranai females fed a sugar (grey line) and a protein (black line) diet.
Behaviour and demography
Sexual activity of R. pastranai in the laboratory was sparse with only four copulations recorded over 58 h of observation. All copulations occurred in the afternoon between 4 and 7 pm and had an average duration of 80 min. No apparent male courtship behaviour (e.g. pheromone emission, wing fanning or tactile approach) was observed. Males and females were occasionally observed visiting artificial fruit and when facing each other or an individual of their own sex, they exhibited a brief boxing event. Several copulation attempts were observed with males jumping on the female back but were most often resisted by females shaking and flying away and occasionally dropping to the cage floor. Copulations began on cage walls, ceiling or floor and in all cases, males that were seeking to mate, jumped and mounted on females apparently forcing them to copulate. Once intromission was achieved, females tended to hold their wings outward, positioning them in parallel to their bodies. Females exhibited host marking behaviour (dragging of the ovipositor) after egg laying. Eggs were laid singly in the artificial host with a small globular structure sticking out (fig. 7). Females fed a protein diet in the laboratory lived an average of 51.13 ± 3.06 days while males lived an average of 48.08 ± 3.76 days and there was no significant difference in longevity between males and females (F 1,35 = 0.78, P = 0.43).
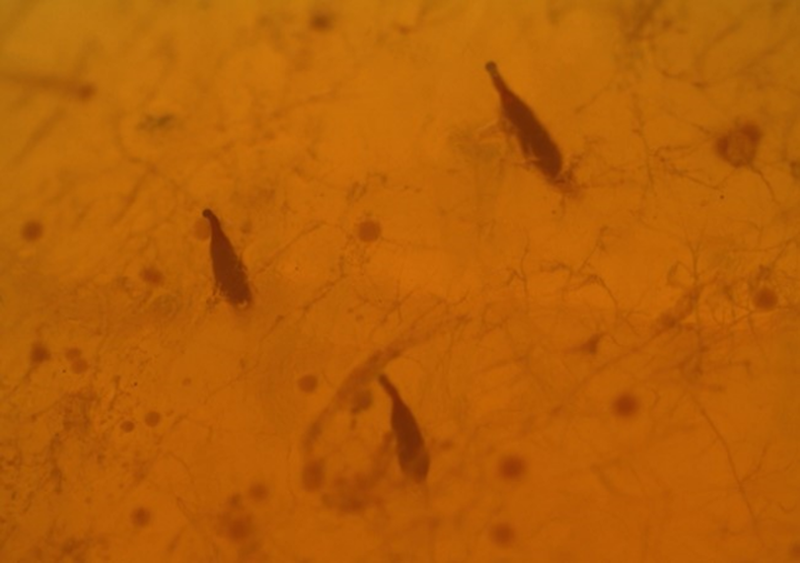
Figure 7. Eggs deposited in artificial agar egg laying spheres by Rhagoletotrypeta pastranai females.
Discussion
Rhagoletotrypeta pastranai was found infesting fruits of Celtis tala and Celtis iguanaea trees in two sites located at 600–700 m a.s.l. in the Yungas ecoregion in Tucuman Province, consistent with records of the genus reported by Ovruski et al. (Reference Ovruski, Norrbom, Schliserman and Aluja2005) in northwestern Argentina. On average, only one larva developed per fruit as is common in other species of Carpomyini (Averill and Prokopy, Reference Averill and Prokopy1987; Ovruski et al., Reference Ovruski, Norrbom, Schliserman and Aluja2005; Rull et al., Reference Rull, Abraham, Tadeo and Rodriguez2016, Reference Rull, Abraham, Schlisermann, Ordano and Ovruski2017) and there was no correlation between fruit size and number of larvae per fruit, perhaps owing to the fact that host fruits are small, abundant and very similar to each other with an average weight of 1.68 ± 0.30 g. A total of 426 adult flies emerged from 1376 recovered larvae from C. tala fruits over a year, of which only four individuals appeared without becoming dormant and the largest proportion of the population emerged after 40 days following pupation. Parasitism level was low with nine wasps emerged over a year of which only one became dormant and emerged 172 days after pupation. All parasitoids were identified as a species of Utetes genus close to U. anastrephae. Likewise, Leonel et al. (Reference Leonel, Zucchi and Wharton1995, Reference Leonel, Zucchi and Canal Daza1996), Ovruski et al. (Reference Ovruski, Norrbom, Schliserman and Aluja2005) and more recently Palacio et al. (Reference Palacio, Lacoretz, Perez and Ordano2019) reported that this species and other braconids such as Doryctobracon areolatus (Szepligeti), D. brasiliensis (Szepligeti) and Opius bellus (Gahan) were found associated with R. pastranai in Brazil and Argentina. For C. iguanaea, only 11 pupae were recovered from 945 collected drupes and only a single adult emerged after 208 days. There was no parasitoid emergence in this association.
Based on results of this work, R. pastranai can be considered a univoltine monophagous species with host specialization and a life cycle adapted to Celtis tala fruiting phenology; therefore, a large number of individuals undergo pupal dormancy and synchronize their reproductive stage with the main fruiting period of the host. According to Koštál (Reference Koštál2006), dormancy is considered as a state of suppressed development that evolved among animals to cope with long periods of adverse conditions. In the case of R. pastranai, low temperatures in the winter in subtropical Tucumán rarely reach levels low enough to cause pupal mortality. Host scarcity is more likely to be the driving factor in the evolution of dormancy, and seasonal humidity and day-length (photoperiod) remain to be tested as factors of importance in the regulation and fine tuning of dormancy for this species. Although flies in the genera Anastrepha and Bactrocera are generally multivoltine, dormancy has also been reported in the subtropical species Anastrepha tehuacana (Norrbom et al., Reference Norrbom, Castillo-Meza, García-Chávez, Aluja and Rull2014) and Bactrocera minax (Dong et al., Reference Dong, Wang, Clarke, Pereira, Desneux and Niu2013). As R. pastranai, both of these subtropical flies are highly specialized and may not only face seasonal periods of environmental hardship but also long periods of host fruit scarcity, both of which may have influenced the evolution of dormancy.
Results on the effect of winter length on development and/or dormancy in the pupal stage of R. pastranai revealed that winter length has no effect on survival of pupae, of which only a small proportion entered prolonged dormancy, with a large quantity of dead uneclosed pupae after a year. Likewise, there was a considerable number of missing pupae in overwintering cups, possibly owing to the action of some microorganisms that had grown in the vermiculite substrate. Factors of diapause/dormancy regulation and their overwintering mechanisms are poorly understood in subtropical species (Danks, Reference Danks2007), especially in members of the Carpomyini (Rull et al., Reference Rull, Abraham, Schlisermann, Ordano and Ovruski2017). Some factors, e.g. day length, pre-winter temperatures and winter duration, are less variable in tropical and subtropical regions than at higher latitudes (Denlinger, Reference Denlinger1986; Feder et al., Reference Feder, Powell, Filchak and Leung2010); therefore, other environmental cues, such as rainfall and humidity fluctuations, might have more effect on diapause/dormancy duration, as reported by Araujo-Diniz et al. (Reference Araujo-Diniz, Ribeiro de Albuquerque, Oliveira Oliva, Varjal de Melo-Santos and Junqueira Ayres2017) for tropical mosquitoes species.
As mentioned above, the majority of individuals became dormant and emerged after about 145 days after pupation under laboratory conditions. Nevertheless, Ovruski et al. (Reference Ovruski, Norrbom, Schliserman and Aluja2005) and Palacio et al. (Reference Palacio, Lacoretz, Perez and Ordano2019) reported that the pupal stage could last up to 12 months under uncontrolled conditions. Furthermore, dissections of males and females revealed that a short period of sexual maturation is necessary for both sexes and that they require feeding on protein sources to reach full development of sexual organs; R. pastranai is therefore, as most species of frugivorous Tephritidae (Drew and Yuval, Reference Drew, Yuval, Aluja and Norrbom1999), an anautogenous species. Adult male and female longevity was similar, with an average duration of 51 and 48 days, respectively. This constitutes the first report of lifetime duration of the adult stage for a species of Rhagoletotrypeta.
Behavioural observations were carried out on sexually mature males and females under laboratory conditions. Sexual activity was scarce with only four copulations occurring during the entire observational period, all in the afternoon. This temporal pattern might have evolved in response to natural conditions during the fruiting season of C. tala where temperatures at that time of the day might be similar to those we applied in the laboratory. According to our observations, the mating system can be classified as resource defence by males, which is common among the Carpomyini group (Prokopy and Papaj, Reference Prokopy, Papaj, Aluja and Norrbom1999; Rull et al., Reference Rull, Abraham, Schlisermann, Ordano and Ovruski2017). However, because of the low number of copulations observed under laboratory conditions, and considering the fact that most species of Carpomyini whose sexual behaviour has been observed are highly promiscuous (Prokopy and Papaj, Reference Prokopy, Papaj, Aluja and Norrbom1999; Opp and Prokopy, Reference Opp and Prokopy2000; Rull et al., Reference Rull, Abraham, Schlisermann, Ordano and Ovruski2017), we believe that behavioural observations in the field are necessary to conclusively characterize the mating system of this species. Host derived stimuli have been found to influence ovarian development and sexual behaviour of Rhagoletis juglandis (Lachman and Papaj, Reference Lachmann and Papaj2001) another species of Carpomyini. Plant volatiles also enhance mating behaviour of the Mediterranean fruit fly Ceratitis capitata (Shelly et al., Reference Shelly, McInnis, Pahio and Edu2004), and play an important role in the sexual behaviour of several species of Bactrocera (Shelly, Reference Shelly2010). In the case of R. pastranai, the presence of suitable host fruit, and the volatiles they release might be stimuli triggering sexual behaviour or female receptivity. The lack of host-derived stimuli during our observations could therefore explain the low numbers of observed copulations.
Females laid a single egg per egg-laying bout and dragged their ovipositor to mark artificial host fruit. Such behaviour has also been observed for several species in the genera Anastrepha, Ceratitis and Rhagoletis but does not occur among Bactrocera species (Díaz-Fleischer et al., Reference Díaz-Fleischer, Papaj, Prokopy, Norrbom, Aluja and Norrbom1999). In the case of R. pomonella where females mark fruits with a contact pheromone to dissuade other females from laying eggs in a fruit in which a larva is already developing. Consequently, this chemical communication might reduce intraspecific competition among larvae that exploit an ephemeral host (Averill and Prokopy, Reference Averill and Prokopy1987). In general, with the exception of walnut-infesting species, for flies in the genus Rhagoletis, a single larva develops per fruit and the oldest larva apparently kills later arrivals (Averill and Prokopy, Reference Averill and Prokopy1987). In the case of Rhagoletotrypeta, we found that up to five larvae could develop to pupae in a single fruit. It would be interesting to examine if multiple larvae in a single fruit are siblings or if the progeny of different females can coexist.
Our work constitutes the first study on the natural history of R. pastranai. It describes interesting aspects of life history and mating behaviour of this species and contributes to the knowledge of the biology of non-economically important groups within the Tephritidae, particularly in the Carpomyini. It also highlights the value of investigating such species, considering that only 5% of the nearly 5000 described tephritids are considered pestiferous (Aluja, Reference Aluja1999; Norrbom, Reference Norrbom2004). Nevertheless, further research including observations of diel activity patterns in the field emphasizing sexual behaviour before, during and after mating, and experimental studies that explore the effects of different environmental factors on dormancy/diapause regulation, are necessary to broaden our comprehension and understanding of the evolution of overwintering mechanisms in subtropical insects and the evolution of mating systems and life history strategies.
Acknowledgement
A. Moyano thanks Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) for a granted scholarship. This study was supported by the Fund for Scientific and Technological Research (grants PICT 2013–0604, PICT 2015–2631 and PICT 2015–0191, Argentina).










