Introduction
Intraspecific competition for host resources among immature parasitoids has a major influence on ecological and evolutionary processes of parasitoids (Godfray, Reference Godfray1994). In gregarious parasitoids, several larvae may develop and use host resources; but, if a second clutch of eggs is laid (i.e. superparasitism) and an excess of larvae develops on or in the host, the result can be competition for host resources (Jervis et al., Reference Jervis, Copland, Harvey and Jervis2005). This competition may end with the emergence of several small individuals with lower fitness and probability of survival or with the elimination of competitors through physiological suppression or physical conflict. In solitary parasitoids, only one individual can develop per host. When several eggs are laid, competition for host resources occurs principally through physical conflicts until only one individual is left and it can fully use the host's resources (Godfray, Reference Godfray1994). Combats occur during the first larval instar when sufficient host resources remain for the surviving larva to complete its development (Clausen, Reference Clausen1940; Salt, Reference Salt1961). In these cases, the first instar larva has morphological adaptations for fighting that it does not possess in the following instars, such as large mandibles and caudal appendages or setae that increase their mobility (Clausen, Reference Clausen1940; Salt, Reference Salt1961; van Baaren et al., Reference van Baaren, Boivin, Le Lannic and Nénon1997; Mayhew & van Alphen, Reference Mayhew and van Alphen1999).
Metaphycus flavus females (Howard) (Hymenoptera: Encyrtidae) normally lay a small, female-biased clutch of 2–3 eggs in immature brown soft scale Coccus hesperidum L. (Hemiptera: Coccidae) (Bernal et al., Reference Bernal, Luck and Morse1999a; Kapranas et al., Reference Kapranas, Pacheco, Forster, Morse and Luck2008; Tena et al., Reference Tena, Kapranas, Garcia-Marí and Luck2008). If a second female subsequently encounters this scale, she will lay an additional clutch of 2–3 eggs in them (=superparasitism), in excess of those that can develop successfully within the scale. Under these circumstances, the larvae normally engage in physical conflicts in which the supernumerary larvae are eliminated and consumed (Tena et al., Reference Tena, Kapranas, Garcia-Marí and Luck2009). Interestingly, in contrast to what would be expected given this behaviour, the larval stage of other species of Metaphycus that have been described lack the large, piercing mandibles typical of fighting species (Flanders, Reference Flanders1942; Bartlett & Ball, Reference Bartlett and Ball1964; Saakyan-Baranova, Reference Saakyan-Baranova1966); and, moreover, the movements of the larvae are restricted because they are attached at their posterior end to the host's cuticle via an aeroscopic plate which limits encounters between individuals (Maple, Reference Maple1954; Saakyan-Baranova, Reference Saakyan-Baranova1966). Consequently, Metaphycus larvae do not present the typical morphology that usually characterizes an aggressive endoparasitoid species. We, thus, describe the development of M. flavus larvae and their associated morphology within the host. We focus on their mandibles and their means of attachment to the host cuticle and how these characteristics may favour or restrict the physical conflicts that Tena et al. (Reference Tena, Kapranas, Garcia-Marí and Luck2009) previously described in this species. Lastly, we also describe the sensory organs found in the third and fourth larval instar.
Material and methods
Scale and parasitoid cultures
We established a brown soft scale culture using crawlers obtained from an infested pineapple guava plant, Feijoa sellowiana O. Berg (Myrtaceae), located at the University of California, Riverside, CA campus (UCR). The scales were reared on excised Yucca recurvifolia Salisbury (Agavaceae) leaves maintained hydroponically in the UCR insectary at 27–28°C, 60% R.H. with a 21L:3D photoperiod. We obtained the excised yucca leaves from plants grown at UCR Agricultural Operations.
The Metaphycus flavus colony used in this study was established in 1996 with individuals collected from citricola scale, Coccus pseudomagnoliarum Kuwana (Hemiptera: Coccidae), infesting citrus near Kozan, in south central Turkey (Bernal et al., Reference Bernal, Luck and Morse1999b). The colony has since been maintained in the UCR insectary by introducing mated females into 7.5 cm dia.×50 cm long plastic tubes, each containing one or two scale-infested yucca leaves with ca. 300 brown soft scales per leaf. We maintained the culture in the rearing tubes at a ratio of approximately one female parasitoid per ten scales. The rearing tubes were capped with plastic lids at both ends, which had holes that were covered with a fine nylon mesh to allow air circulation while preventing adult parasitoid escape or entry. Honey was streaked on the inside wall of the tubes as a carbohydrate source for the introduced or emerged parasitoids. The tubes were maintained at 25±1°C, 50–70% R.H. and a 14L:10D photoperiod.
Procedure for obtaining parasitoids for morphological examination
We obtained the adult parasitoids for our studies by removing 100–200 scales containing parasitoid pupae (=‘mummies’) from the yucca leaves and placing them in a 2.5 cm dia.×9.5 cm long glass vials. Each vial was then sealed with a plastic cap that had a central ventilation hole covered with fine nylon mesh. The developing wasps were allowed to emerge from these scales. These wasps were collected daily and confined as a mixed-sexed group within a second, 2.5 cm dia.×9.5 cm long vial held at 25±1°C, 50–70% R.H. and 14L:10D photoperiod for two days. This allowed the females to mate and mature their eggs. All of the vials contained a streak of honey on their inside walls as a carbohydrate source for the parasitoids. Prior to each experiment, we isolated two-day-old females from these vials by placing each female in a 1 cm dia. glass vial with a drop of honey on its inside wall and sealing the vial with a cotton plug.
Larvae for morphological examination were taken from superparasitised scales using the same procedures and experimental methods described by Tena et al. (Reference Tena, Kapranas, Garcia-Marí and Luck2009). This allowed us to compare our results from this study with those from this previous study. To obtain these larvae, we confined a single, mated, 3-day-old female with a section of yucca leaf having a 23–28-day-old scale, 1.8±0.05 mm wide by 2.5±0.10 mm long. Each yucca leaf section with its scale was confined in a 4 cm dia.×1.5 cm high glass Petri dish, which formed an observation arena. Using a cool fiber optic light and a dissecting microscope at 10–50× magnification, we observed and noted the behaviour of each female wasp continuously in this arena until she had laid her initial egg clutch in the scale. Four hours after the initial female was removed, we exposed the scale to a second M. flavus female following the same procedure. We used the protruding egg stalk associated with each M. flavus oviposition to confirm the deposition of an egg (Maple, Reference Maple1954; Tena et al., Reference Tena, Kapranas, Garcia-Marí and Luck2008). A total of 130 superparasitised scales were available for examination in this study. The superparasitised scales, along with their associated yucca leaf section, were maintained in an incubator at 25±1°C and 14 h of light, followed by 18±1°C and ten hours of darkness in 50–70% R.H. to allow normal parasitoid development.
On days one through nine post oviposition, we detached the superparasitised scales from the leaves daily and dissected them in saline solution (1% NaCl) under a dissecting microscope to determine whether the larval parasitoids were attached to the scale cuticle. If so, we detached each larva within the scale from the host cuticle and transferred and fixed it to a glass slide using a thin film of saline solution. We then measured the length and width of each larva using an ocular micrometer mounted in the eyepiece of a compound microscope. We also measured the length and width of the tentorium and mandibles of each larva within the scale by placing a glass cover slip over the larva and gently pressing on it to flatten it against the slide. We then counted the number of spiracles present in each of the parasitoid larvae within the scale and measured length and width of their tentorium and mandibles. We present these measurements as an average±standard deviation, along with the number of observations (table 1, col. 4). A total of 221 eggs and larvae were measured. We also prepared specimens for scanning electron microscopy (SEM) or for slide mounting, clearing and photography.
Table 1. Morphological characteristics of Metaphycus flavus larvae.
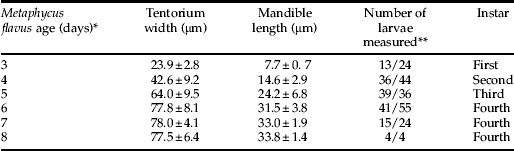
* days after oviposition.
** The numbers represents the number of tentoria and mandibles measured respectively.
The specimens for scanning electron microscopy (SEM) were dehydrated using a series of increasing ethanol concentrations which ended with 100% ethanol. The ethanol was then replaced with three changes of hexamethyldisilizane/hexamethyldisiloxane (HMDS-Polysciences, Warrington, PA, USA), and the specimens were then air dried in a fume hood (Heraty & Hawks, Reference Heraty and Hawks1998). These dried specimens were mounted on aluminum stubs with carbon conductive adhesive tabs (Pelco Tabs® Ted Pella Inc., Redding, CA, USA), sputter coated with gold/palladium alloy and examined with a Phillips XL30-FEG scanning electron microscope (FEI Co., Hillsboro, OR, USA).
The specimens that were slide mounted and photographed using light microscopy were prepared by killing the parasitoid in ethanol and mounting each specimen on a microscope slide using Hoyer's mounting medium (20 parts chloral hydrate, 5 parts water, 3 parts gum Arabic, 2 parts glycerin). The specimens were then covered with a glass cover slip and placed on a slide warmer (ca. 30–35°C) for about one week to allow each specimen to clear. Digital photographs of these specimens were obtained using a Zeiss Axioskop 2 compound microscope (Carl Zeiss Inc., Oberkochen, Germany) with a JVC 3-CCD digital camera (Model KY-F7O) using Auto-Montage software (Syncroscopy, Cambridge, UK).
Results
Measurements of the mandibles, tentoria and larvae (table 1, fig. 1) indicated that M. flavus has four larval instars. Under our rearing conditions, the larvae hatched from their eggs three days after oviposition and they pupated five days later. The first instar larvae were encyrtiform (Clausen, Reference Clausen1940) (fig. 2a) and almost spherical, measuring 0.17±0.023 mm long by 0.12±0.013 mm wide (n=24). The anterior part of the larval head contained the tentorium and a pair of mandibles. These latter were shaped like minute hooks (see table 1 for length measurements) and they appeared to be weakly sclerotized (fig. 3a). The posterior end of the first instar (fig. 2a) was surrounded by the chorion, which allowed the two pairs of open spiracles to protrude through the host cuticle. This provided a pathway for the respiratory gases to enter the posterior spiracles (see Discussion). These posterior spiracles were connected to two longitudinal, lateral trunks with simple ramifications, and they constituted the first instar's metapneustic tracheal system. The larvae were attached to the host's cuticle via the egg chorion, which restricted the larvae from moving within the scale. At ecdysis, the larval exuvium was gradually sloughed off from the anterior to posterior, but the exuvium remained attached to the egg chorion.

Fig. 1. Metaphycus flavus egg and larval development. Length and width of the egg and larvae of M. flavus at different time intervals after oviposition (⧫, larval length; ![]() , larval width).
, larval width).

Fig. 2. Lateral view of different larval instars of Metaphycus flavus. (a) First instar partially covered by the egg shell. (b–c) Second and third instars attached to the host cuticle. (d) Fourth instar showing the open spiracles. (e) Fourth instar spiracles on abdominal segments 2–4. (f) Detailed view of spiracle on abdominal segment 3. (Figs. 2a–e anterior is to the right and posterior to the left).

Fig. 3. Mandibles of four larval instars of Metaphycus flavus: (a) first instar; (b) second instar; (c) third instar; (d) fourth instar.
The second and third larval instars were also metapneustic and encyrtiform (fig. 2b, c). The second instar began on day four post oviposition and measured 0.29±0.042 mm long by 0.20±0.037 mm wide (n=43). The third instar appeared on day five and measured 0.46±0.1 mm long by 0.31±0.067 wide (n=47). The head of each instar was easily distinguished (fig. 2b, c). The mandibles became progressively larger with each instar (table 1). They still appeared to be weakly sclerotized through the second instar (fig. 3b), but the anterior part of the mandibles of the third instar became sclerotized (fig. 3c). The posterior end of the larvae remained attached to the chorion and the exuvia from the earlier instars, which surrounded the two pairs of posterior spiracles. The tracheal system was similar to that of the first instar, and the larvae were tethered to the host cuticle.
The respiratory system changed drastically when the larva molted to the fourth instar. It became peripneustic and manifested nine lateral pairs of open spiracles (fig. 2d–f). Under our rearing conditions, the fourth instar larvae occurred on days six through eight post oviposition. On day six, the larvae averaged 0.87±0.24 mm long by 0.48±0.095 mm wide (n=60) and reached its maximum size on day seven (1.23±0.23 mm long, 0.59±0.08 mm wide) (n=24) (fig. 1). At the beginning of the fourth instar, the larvae were still attached to the chorion and host cuticle, but they subsequently became detached from the chorion during this instar and were able to move freely within the host. Its mandibles also underwent a major transformation during the fourth instar. They became large, hook-like and well-sclerotized (fig. 3d). The head of the fourth instar larvae was also easily distinguished from the rest of the larva (fig. 2d). Once the fourth instar larvae had consumed the remaining scale contents, leaving only the scale's cuticle, the larvae excreted their meconia within the scale, which caused the larvae to shrink in size (fig. 1, day 8). The larvae then became white just prior to pupation.
We examined the sensilla present on the last two larval instars and found numerous sensilla on the head of the third and fourth instar. A pair of spherical, multiporous sensilla occurred on the cranium, lateral to the mouthparts (fig. 4a, c), and two pairs of spherical, multiporous sensilla occurred on the maxillary-labial complex (fig. 4b, d). We also noted a single pair of coeloconic sensilla on the maxillary-labial complex. These later had the form of a raised torus (‘doughnut’) within a shallow depression in which a short, grooved peg protruded from the center of the torus (fig. 4b, d, e). Additionally, we also noted two other sensilla types that were associated with the mouthparts. Three pairs of short peg sensilla occurred on the clypeo-labrum (fig. 4b), which had the form of a papiliform peg sunk within a depression (fig. 4b, f) and that resembled basiconic or styloconic sensilla (Keil, Reference Keil and Hansson1999). We also found three pairs of sensilla, one pair occurred laterally on the clypeo-labrum and two pairs occurred on the maxillary-labial complex (fig. 4b, d). The morphology of these sensilla did not match closely the traditional classifications of sensilla. Based on their shape, we referred to them as mamilliform sensilla. In addition to these sensory organs, we also noted two pairs of deep pits that were located dorsally on the cranium (fig. 4a, g).

Fig. 4. Head of Metaphycus flavus. (a) Anterior view of entire head; black arrowheads, spherical multiporous sensilla (detail in c); white arrowheads, pits (detail in g). (b) Anterior-ventral view of mouth region (above the mouth opening, mo, is the clypeo-labrum and below is the maxillary-labial complex). (c) Spherical olfactory sensillum on the cranium. (d) Three ventral-most sensilla on the maxillary-labial complex. (e) Detail of central peg of a coeloconic sensillum on the maxillary-labial complex. (f) Peg sensilla on the clypeo-labrum. (g) Deep pit on the cranium. cl, clypeo-labrum; cr, cranium; cs, coeloconic sensillum; mlc, maxillary-labial complex; mo, mouth opening; ms, mamilliform sensillum; ps, peg sensillum; pt, prothorax; smps, spherical multiporous sensillum.
Discussion
The role of host attachment in larval cannibalism
Metaphycus flavus larvae are endoparasitoids that are attached initially to their host's cuticle via the egg chorion until the fourth instar. This type of attachment restricts larval movement within the scale. Also, the first three larval instars are metapneustic and obtain their oxygen via two pairs of posterior spiracles that attach each larva to its egg chorion via an aeroscopic plate. This attachment is similar to that reported for its congeners, M. helvolus (Compere) and M. luteolus (Timberlake), both of which also possess two pairs of posterior spiracles and an aeroscopic plate (Flanders, Reference Flanders1942; Saakyan-Baranova, Reference Saakyan-Baranova1966). Thus, this pattern of attachment appears to be characteristic of the genus. According to Maple (Reference Maple1954), encyrtid larvae maintain contact between their posterior spiracles and the atmosphere by aeroscopic plates on the egg, which project externally through the host cuticle. In the fourth instar, the respiratory system becomes peripneustic manifesting nine pairs of open spiracles that are distributed laterally on each side of the larva. Initially, this larval stadium remains attached to the host via the chorion; but, as the host contents become consumed, the larva detaches from the chorion and moves freely within the host. During this instar, air is obtained directly via the nine larval spiracles, and the remaining contents within mummified scale are consumed.
Tena et al. (Reference Tena, Kapranas, Garcia-Marí and Luck2009) have recently documented that supernumerary M. flavus larvae engage in physical conflicts that result in the consumption of the losing larvae (i.e. cannibalism). These conflicts only occurred when the larvae within a scale were six or more days old. The developmental conditions of the larvae in that experiment were similar to this study (environmental conditions; host conditions, host instar and size; superparasitism within four hours), which indicates that larvae were in the fourth instar when they were six days old. Thus, physical conflicts among M. flavus larvae appear to occur only during the fourth instar, probably after the larvae have severed their attachment to the host cuticle and they are able to move freely within the scale. However, host attachment in other Metaphycus species does not interfere with larval encounters and subsequent larval conflict. Metaphycus luteolus, another brown soft scale parasitoid, engages in physical conflicts during the second and third instar (Bartlett & Ball, Reference Bartlett and Ball1964) while the larvae are still attached to the host (Saakyan-Baranova, Reference Saakyan-Baranova1966). Interestingly, both M. flavus and M. luteolus allocate clutches of similar size (Tena et al., Reference Tena, Kapranas, Garcia-Marí and Luck2008; Kapranas et al., Reference Kapranas, Wanjberg and Luck2009), and the eggs of both species are attached internally to the host cuticle (Saakyan-Baranova, Reference Saakyan-Baranova1966). However, these species differ in the way they distribute their eggs within the host scale. Metaphycus flavus deposits each egg individually within the host, at different points around the scale's periphery. In contrast, M. luteolus deposits its eggs in a single location within the scale (A.Tena, personal observations). This species-specific difference in egg distribution may affect the likelihood and timing of larval encounters within the host. For example, the encounters among the M. luteolus larvae within a host scale may occur earlier during larval development than at the encounters among M. flavus larvae because of the clustered nature of the M. luteolus larvae. The more dispersed distribution of M. flavus eggs appears to prevent the larvae from contacting one another with their mandibles until they become detached from their aeroscopic plate during the fourth instar.
The fourth instar as a fighting instar
The first instar larvae of many solitary endoparasitoid species possess morphological features that are used to eliminate supernumerary competitors through physical conflicts (Clausen, Reference Clausen1940). These features are usually lost or reduced after the initial molt, and the subsequent instars are often unable to fight (Hagen, Reference Hagen and DeBach1964). The morphological characteristics of an aggressive first instar larva typically include a large head with large, piercing, well-developed mandibles; a set of dorsal spines on the thorax and abdomen; and a large caudal segment and/or a large caudal spike (Clausen, Reference Clausen1940; Laing & Corrigan, Reference Laing and Corrigan1987; van Baaren et al., Reference van Baaren, Boivin, Le Lannic and Nénon1997). The spines and large caudal segment (developed ‘tail’) are responsible for the mobility of first instar larvae within a host (Clausen, Reference Clausen1940; van Baaren et al., Reference van Baaren, Boivin, Le Lannic and Nénon1997). However, first instar M. flavus larvae, as well as other Metaphycus species (Flanders, Reference Flanders1942; van Baaren et al., Reference van Baaren, Boivin, Le Lannic and Nénon1997), lack these morphological characteristics. Thus, movements of the first instar larvae are not only constrained by their attachment to their host but also by their lack of dorsal spines and a developed tail.
The large and well-developed piercing mandibles and the caudal spike found in species where the first instar is the fighting instar are used to attack other larvae within the host, both conspecifics and individuals of other species (Clausen, Reference Clausen1940; Laing & Corrigan, Reference Laing and Corrigan1987). In contrast to this pattern, the mandibles of first instar M. flavus are at their smallest, and they increase in size with each successive instar. The mandibles are at their largest and most heavily sclerotized during the fourth instar and are double the size of those in the second instar (fig. 3). These mandibles are likely capable of killing and consuming other competing conspecific larvae even though they are not the large and piercing mandibles typical of the first instar described in other parasitoid species.
Sensory organs and possible secretory glands in the third and fourth instar
The head and mouthparts of the third and fourth instar larvae are well equipped with multiple types of sensory organs. They have coeloconic sensilla (fig. 4e), which are chemosensory and usually olfactory in function (Keil, Reference Keil and Hansson1999). The spherical, multiporous sensilla (fig. 4c) are almost certainly olfactory, as the presence of the many pores is characteristic of insect olfactory sense organs (Keil, Reference Keil and Hansson1999). However, the occurrence of olfactory organs in the third and fourth instars is surprising since they live within a host, presumably in an aqueous environment. Gustatory sensilla would be more suitable for chemoreception in this type of environment. The peg sensilla on the clypeo-labrum (fig. 4f) bear similarities to both basiconic and styloconic sensilla; the former are usually chemosensory while the latter often function as temperature and/or humidity sensors (Keil, Reference Keil and Hansson1999). However, the senses provided by the peg sensilla is unknown. Similarly, the function of the mammiliform sensilla is unknown (fig. 4d). Transmission electron microscopy and electrophysiology would be required to determine unambiguously the functions of these sensilla. Finally, the pits on the cranium appear to be openings of secretory glands, but as noted for the sensilla, confirmation of their function requires transmission electron microscopy. If they are secretory, it raises intriguing questions: what are they secreting, and what is the effect of these secretions on the host and/or parasitoid competitors?
Acknowledgements
We thank Lisa D. Foster, Porfirio Pacheco and Robert Trautman for providing the scales, host plants and parasitoids to conduct these experiments. This research was supported in part by an USDA National Research Initiative grant (USDA-NRI 2005-01006) awarded to RFL and Jocelyn Millar and by a California Citrus Research Board grant (CRB 5500–159) awarded to Joseph Morse and RFL.







