Introduction
The use of generalist mite predators is widespread in integrated pest management programs (Hoy et al., Reference Hoy, Cunningham and Knutson1983; Johnson & Wilson, Reference Johnson, Wilson, Nechols, Andres, Beardsley, Goeden and Jackson1995; Flint & Dreistadt, Reference Flint and Dreistadt1998) despite a number of challenges (Gerson et al., Reference Gerson, Smile and Ochoa2003). The great body of predatory mite research has focused on the use of Phytoseiidae mites of the subfamilies Typhlodrominae, Phytoseiinae and Amblyseiinae for control of spider mites (Tetranychidae) (McMurtry & Croft, Reference McMurtry and Croft1997), but acarologists have long proposed the utility of lesser studied predatory mite families for control of insect and mite pests (Laing & Knop, Reference Laing, Knop, Hoy, Cunningham and Knutson1983). Blattisocius is a genus of mites (Acari) in the family Ascidae and is largely comprised of predatory taxa (Halliday et al., Reference Halliday, Walter and Lindquist1998). A few species have been noted for their potential as biological control agents of stored product pests (Hughes, Reference Hughes1976), but the biology of most Blattisocius remains poorly investigated. Several cosmopolitan species are widely associated with moths and beetles, as evidenced by their collection from preserved specimens and infestations of laboratory colonies (Treat, Reference Treat1975). One species, Blattisocius patagiorum Treat, is a known parasite of adult noctuids (Treat, Reference Treat1966).
Several laboratory studies have investigated predation by Blattisocius tarsalis Berlese, Blattisocius dendriticus Berlese and Blattisocius keegani Fox for their biological control potential. Blattisocius tarsalis is the best studied of these three and has been recorded on the moth genera Anagasta (=Ephestia), Plodia and Sitotroga (Hughes, Reference Hughes1976). This mite will feed and develop on eggs of the pyralid moths Plodia interpunctella Hübner (Darst & King, Reference Darst and King1969) and Ephestia cautella Walker (Haines, Reference Haines1981), as well as a noctuid, Epizeuxis (=Idia) aemula Hübner (Treat, Reference Treat1969). Haines (Reference Haines1981) observed that B. tarsalis preferred moth eggs over eggs of the beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). More recent studies suggest this mite may prove suitable as an effective biological control agent for eggs of Ephestia kuehniella Zeller (Nielsen, Reference Nielsen1999, Reference Nielsen2001, Reference Nielsen2003) and the flour mite, Acarus siro L. (Acari: Acaridae) (Thind & Ford, Reference Thind and Ford2006), in stored grains. Blattisocius dentriticus also has potential for biological control of pest mites, including eriophyids (Waite & Gerson, Reference Waite and Gerson1994), and will feed on all life stages of the mold mite, Tyrophagus putrescentiae Schrank (Acari; Acaridae) (Rivard, Reference Rivard1960, Reference Rivard1962a,Reference Rivardb).
Blattisocius keegani is a species similar in appearance to B. tarsalis and differing only by two morphological characters – the length of the cheliceral digits and peritremes (Chant, Reference Chant1963). It is associated with a diversity of habitats, from stored products to rat and bird nests (Chant, Reference Chant1963; Treat, Reference Treat1975; Halliday et al., Reference Halliday, Walter and Lindquist1998). Two laboratory studies suggest potential of B. keegani for biological control of stored grain beetles and some pest mites. Barker (Reference Barker1967) found that B. keegani fed on eggs of Cryptolestes, Tribolium, Trogoderma, Oryzaephilus and the mites, Glycyphagus domesticus De Geer and Aëroglyphus robustus Banks (Acari: Glycophagidae). However, they would not feed on Tyrophagus putrescentiae. Beavers et al. (Reference Beavers, Selhime and Denmark1972) further studied predation by B. keegani on eggs of Diaprepes abbreviatus L.
We investigate performance of B. keegani on eggs of navel orangeworm, Amyelois transitella Walker (Lepidoptera: Pyralidae), a key pest of almonds and pistachios in California orchards (Mullen et al., Reference Mullen, Alston, Sumner, Kreith and Kuminoff2005; Parra-Pedrazzoli & Leal, Reference Parra-Pedrazzoli and Leal2006). The current recommended integrated pest management program for A. transitella focuses on cultural controls with some biological control using parasitoids (Zalom et al., Reference Zalom, Barnett and Weakley1984, Reference Zalom, Pickel, Bentley, Haviland and Van Steenwyk2009; Meals & Caltagirone, Reference Meals, Caltagirone, Nechols, Andres, Beardsley, Goeden and Jackson1995) and could benefit from the addition of an effective egg predator. Blattisocius keegani has been collected from live and preserved moth specimens, but the nature of their relationship to moths remains ambiguous (Treat, Reference Treat1975). Their biology on moth eggs or other Lepidopteran life stages has not been previously studied in the laboratory. To address the possibility of mass rearing B. keegani in an insectary for biological control purposes, we also measured fecundity of B. keegani when reared on eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae).
Methods
Blattisocius keegani colonies
Blattisocius keegani mites were obtained and identified from a serendipitous infestation of a navel orangeworm colony in December, 2008. From this infestation, a primary B. keegani colony was established and fed on fresh A. transitella eggs. Colonies were maintained in petri dishes surrounded by a water moat. The A. transitella laid eggs onto filter paper: eggs were cut from this and placed on top of squares of paper toweling in the mite colonies. Subsequent colonies were established under the following conditions: (i) fed frozen A. transitella eggs (ii) provisioned with adult moth bodies in addition to eggs (iii) under high heat conditions, provisioned with eggs alone or eggs and adult moth bodies, and (iv) fed E. kuehniella eggs. Ephestia kuehniella eggs are laid free of a substrate and were transferred directly to the paper towels using a dry paint brush. Almonds were placed in mite colonies that were fed fresh eggs to reduce predation by A. transitella larvae on unhatched eggs (Finney & Brinkman, Reference Finney and Brinkman1967), and the moth larvae were removed regularly. There was no evidence for intraspecific predation by B. keegani. The high heat colonies were held at 32.2°C and 30% relative humidity (r.h.), conditions that were based on mean temperature and humidity present at the University of California's Kearney Agricultural Station (Parlier, CA) located in the San Joaquin Valley where most almonds and pistachios are grown. The high heat colonies were fed only frozen A. transitella eggs for feasibility of colony maintenance, to avoid excessive hatch of A. transitella. All other colonies were held at 25°C and 50–60% r.h.; all colonies were reared at 16:8 (L:D).
Egg predation
Maximum egg consumption was established by providing individual female mites with 1, 3 or 5 A. transitella eggs. Female mites were 1–3 days old from their emergence as adults. To investigate the relationship between consumption of navel orangeworm eggs and mite egg-laying, 2–5-day-old mated female mites from the fresh egg colony were provided with ten fresh eggs of A. transitella over a 72-h interval. The number of navel orangeworm eggs consumed, number of mite eggs laid and percent hatch of remaining eggs was recorded. The experiment was repeated four times, each time with 5–12 replicates as determined by mite availability. Hatch rate of a corresponding number of A. transitella eggs was also recorded in both experiments, as a control for egg viability.
Development and fecundity
Development time of an F2 generation colony of B. keegani was evaluated at 25°C for mites fed on fresh A. transitella eggs. There were 14 replicates. Each replicate consisted of a single mite egg. Mite development was also evaluated under high heat conditions at 32.2°C and 30% r.h. There were 12 high heat replicates, each consisting of five mite eggs. To quantify any reduction in survival under high heat conditions, there were additionally five control replicates of five mites each, reared at room temperature. For all treatments, F2 generation females from the appropriate laboratory colony were isolated for 24 h to obtain eggs. Eggs were transferred onto paper towels within petri dish arenas using a moist paint brush and provided with a minimum of ten A. transitella eggs per mite. Amyelois transitella eggs were replaced daily and the petri dishes were searched for mite exuviae at this time. For the high heat treatment consisting of a pool of five mites, total development time from egg to adult was recorded as time until the last remaining mite molted to the adult life stage.
Fecundity tests were conducted over 72 h using young mated female mites from a F2 or later generation of each colony. The age of female mites was standardized by removing all adults from a colony 11–14 days prior to conducting an experiment. For each test, individual pairs of male and female mites were transferred to petri dishes. Males were included to ensure that females were not limited in mating opportunities, as we have observed that females may need to mate more than one time for continuous egg laying. The total number of eggs laid was recorded after 72 h.
The first set of fecundity tests were conducted 16–19 June 2009 to determine whether there was a loss in fecundity for mites reared on frozen eggs and to quantify any fitness benefits of adult moth bodies. Provision treatments included: (i) fresh A. transitella eggs, (ii) A. transitella eggs frozen at 0°C, (iii) A. transitella eggs frozen at −20°C, and (iv) recently dead adult A. transitella moth bodies in addition to fresh A. transitella eggs. The second set of fecundity experiments were conducted 11–14 August 2009 for two high-heat colonies fed (i) frozen A. transitella eggs and (ii) frozen A. transitella eggs and recently dead adult A. transitella moth bodies. A third set of experiments was conducted from 14–17 September 2009 and compared the fecundity of mite colonies reared at room temperature (25°C) and fed (i) frozen A. transitella eggs, (ii) frozen A. transitella eggs and recently dead adult A. transitella, and (iii) Ephestia eggs. Fecundity tests at this time were also conducted under high heat conditions (32.2°C) for mites fed frozen A. transitella eggs, and mites fed frozen A. transitella eggs in addition to adult moth bodies (dead). There were eight replicates.
Phoresy
This experiment was conducted in the laboratory under 16:8 (L:D) lighting in Bioquip 24-in3 collapsible aluminum cages. Prior to the experiment, recently emerged A. transitella moths were placed in a mite colony for 24 h to inoculate. A destructive sample of five adult moths were taken from the mite colony and frozen in individual petri dishes sealed with parafilm. The number of mites on each of these moths was later counted to determine the level of infestation at the onset of the experiment. Twenty-one pairs of female and male moths were checked for the presence of mites, released into each experimental cage-replicate and allowed to mate and oviposit for five days. Within the cage, there was a petri dish suspended on a sponge over a water moat barrier. There was also a water barrier between the cage and the countertop to prevent mite escape. Within the petri dish were four almonds and four paper towel squares surrounding a one ounce pile of navel orangeworm bait (mashed almonds and almond oil) to lure A. transitella to lay eggs. The number of mites and navel orangeworm eggs in the petri dish was recorded after five days. All A. transitella in the cage were collected, and the remaining number of mites on a subsample of eight adult moths per cage was counted, as well as all A. transitella eggs laid on the cage walls.
Statistical analyses
Count data were square root transformed prior to analysis, and proportion data were arcsine square root transformed. Fecundity data were log transformed. Analysis of variance (ANOVA) or standard regression was employed for analyses as appropriate. Non-parametic Wilcoxan/Kruskall-Wallace tests are reported where data were non-normal and conformed to other assumptions of the test. Welch's ANOVA is reported where sample variances are unequal.
Results
Egg predation
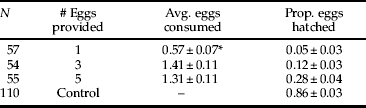
Mites that were provided with one egg over 24 h either consumed the entire egg or did not feed, with a median of one egg consumed. Mites provided with three or five eggs consumed a median of 1.25 to 1.5 eggs, respectively, and there was no significant difference between the two treatments. A maximum of three eggs was consumed by any one mite in the 24-h experiment. Non-parametric Wilcoxon rank sum tests showed proportion A. transitella hatch was significantly higher in control eggs that were not exposed to mites (table 1; P<0.0001).
Table 1. Egg predation by B. keegani when adult female mites were provided with one, three, or five A. transitella eggs.
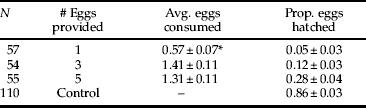
* Significantly different from other treatments at P<0.0001.
Regression analyses indicated egg-laying by B. keegani was significantly correlated with the number of A. transitella eggs consumed during the 72-h predation experiment (y=−0.75+1.26x, r 2=0.65, P<0.0001; fig. 1). Female mites laid an average of 5.82±0.44 eggs and proportion hatch of offspring was 0.73±0.07. Mean proportion hatch of A. transitella eggs was 0.37±0.03 in treatments and 0.77±0.04 for control eggs.

Fig. 1. Regression analysis of eggs laid by B. keegani in relation to the number of A. transitella eggs consumed over 72 h. Markers are weighted by the number of samples.
Development and fecundity
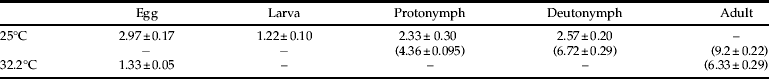
Mites were able to complete development on A. transitella at room temperature as well as under high heat conditions (table 2). However, mite survival was significantly reduced under high heat conditions (χ2=8.49, df=1, P<0.004). Mean proportion survival was 0.27±0.07 under high heat conditions and 0.88±0.12 for room temperature control mites. All eggs hatched by day three in both high heat and control treatments.
Table 2. Duration1 of B. keegani (Acari: Ascidae) life stages when reared on A. transitella at 25°C and 32.2°C.
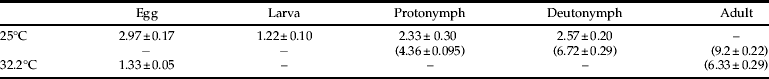
1 In days, with days from egg-lay to each life stage in parentheses below.
Mites provisioned with fresh A. transitella in addition to moth bodies laid significantly more eggs than mites provided frozen eggs (F 3,26=6.16, P=0.0026; fig. 2a; additional post hoc comparison at P=0.05). There was no significant difference in the fecundity of mites fed eggs stored at 0 or −20°C. Mite fitness was lower when reared under high heat conditions in August 2009. Females laid an average of 2.25±0.37 eggs over 72 h and 2.85±0.40 eggs when provided with moth bodies in addition to eggs. There was no significant difference between the two treatments. During the September experiment, mites provided moth bodies in addition to frozen eggs were significantly more fit than those provided frozen eggs alone, E. kuehniella eggs or mites reared under high heat conditions (fig. 2b; F 4,35=6.19, P=0.0009). There was no significant difference in fitness between mites reared on frozen A. transitella or E. kuehniella eggs.

Fig. 2. Mite fecundity measured over 72 h. Treatments were combinations of resource provisions and rearing temperature: (a) June 2009. Fresh A. transitella eggs, fresh eggs and moth bodies, and eggs frozen at 0 and −20°C; (b) September 2009. Frozen A. transitella eggs, A. transitella eggs and adult moth bodies, Ephestia eggs, frozen A. transitella eggs and adult moth bodies in addition to frozen A. transitella eggs.
Phoresy
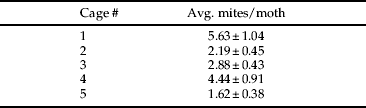
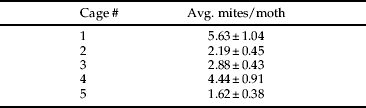
Moths sampled at the onset of the experiment were infested with an average of 18.1±2.31 mites per moth with no significant difference in the presence of mites on male and female moths. At the conclusion of the experiment, the number of mites remaining per moth was highly variable by cage (table 3), with an average of 3.35±1.29 mites per moth. There was no significant bias in the presence of mites on male and female moths. Average A. transitella eggs laid on each substrate within the cage was as follows: almonds, 3.2±0.80; paper toweling, 2.4±1.29; petri dish, 65.8±11.58; cage, 95.2±19.33. There were no eggs laid directly on the A. transitella bait, and this substrate was excluded from statistical analysis. Egg laying by A. transitella was significantly higher on the petri dish and the cage than it was on other surfaces (F 3,16=47.83, P<0.0001). Mites were successfully transferred to the petri dish by adult moths, although in low numbers (0, 0, 1, 8, 3 mites per replicate, respectively).
Table 3. Average number of B. keegani mites (±standard error) remaining on a subsample of eight female and eight male adult A. transitella moths per cage at the conclusion of a caged phoresy experiment conducted from 23–28 March 2010.
Discussion
The results indicate that B. keegani is able to feed and develop on eggs of A. transitella and E. kueniella. Temperature affected development time, and development times at 25° and 32.2°C fell within the expected linear range of comparable estimates from a study where B. keegani was reared on beetle eggs at 22.2° and 26.7°C, and mite offspring hatched in 1.9±0.34 to 4.0±0.44 days (Barker, Reference Barker1967). In the same study, there was approximately 3% mortality of B. keegani at 26.7°C, suggesting that lower survival at 32.2°C in our experiments could indicate that this temperature is near the upper tolerance threshold.
Although there was some loss in fecundity for B. keegani when fed on frozen eggs of A. transitella and E. kuehniella, we observed four females that survived between 45–48 days while still laying eggs. However, concerns may arise for quality control over generational time if mass rearing is later considered. Mites provisioned with adult moth bodies were the more fecund in most tests, suggesting that B. keegani may also feed on adult moths and this could be a useful resource to boost mite fitness in the laboratory and the field. High retention of mites on adult moth bodies at the conclusion of the phoresy experiment also suggests that adult moths may be a food resource for B. keegani. Behavioral and physiological changes resulting in higher egg laying in response to the mere detection of adult moths serve as an alternative explanation that was not effectively investigated in this study. There was additional evidence for phoresy on adult moths, as some mites were also successfully transferred by A. transitella to locations where moth eggs were present. The ambiguous relationship of B. keegani to adult moths warrants further investigation, as it will greatly affect their potential population dynamics relative to prey. Reduced fitness effects and mortality of adult A. transitella by B. keegani may be better likened to the population dynamics of a gregarious parasitoid or parasites, whereas predation on A. transitella eggs reflects classical predator-prey dynamics.
Future prospects
The use of biological control as a pest management tactic relies on the confluence of many factors in predator and prey biology, as well as their population dynamics. Predatory mites must exhibit a high level of adaptability and microhabitat association in common with the target pest. They must also be able to successfully navigate the ecological landscape to locate prey through adaptation to plant structures and defenses or by closely following the host arthropod life cycle. B. keegani may well be able to overcome environmental variables by their phoretic use of adult moths.
For implementation, successful pest management programs using augmentative, inoculative releases or conservation must be cost effective and have a low risk of intraguild predation and non-target effects on native arthropods. Predatory mites have been among the few case studies proven to be cost effective for mass rearing (Collier & Van Steenwyk, Reference Collier and Van Steenwyk2004). Eggs of Ephestia are produced in insectaries for rearing of generalist Trichogramma parasitoids, and this may reduce the costs of rearing B. keegani. Our findings suggest B. keegani should also be considered as a possible alternative to B. tarsalis for biological control of E. kueniella in stored products, as B. tarsalis has been found to be effective only under specific environmental conditions (Cobanoglu, Reference Cobanoglu2008).
In agricultural systems, predatory mites must also be able to perform well in the presence of anthropogenic influences, to persist at high population densities relative to their prey in the presence of pesticides and where landscape disturbance may be high. Orchards can be relatively stable agricultural habitats. For navel orangeworm, the use of pesticides is difficult because of the population dynamics of the pest (Meals & Caltagirone, Reference Meals, Caltagirone, Nechols, Andres, Beardsley, Goeden and Jackson1995), and the current program relies on highly effective cultural practices (Zalom et al., Reference Zalom, Barnett and Weakley1984). Some biological control may occur from natural (Ellers & Klein, Reference Ellers and Klein2009) and augmentative parasitism (Zalom et al., Reference Zalom, Pickel, Bentley, Haviland and Van Steenwyk2009), but the natural role of predators has traditionally been thought to occur in low numbers for California orchard crops (Wade, Reference Wade1961). This integrated pest management system may fair well for well-timed inoculative or augmentative releases of a predator.
Future studies should determine field fitness and adaptability of this species, and a gross evaluation of whether this predator can regulate A. transitella populations. Blattisocius keegani has promising attributes for biological control, as well as potential for environmental adaptability, as they can tolerate high heat and low humidity conditions characteristic of California's Central Valley agricultural production. B. keegani may be of reduced risk for intraguild predation on some mite species because we were not able to successfully rear them on two spotted mite, and prior studies have shown they will not feed on mold mites. Food product contamination by B. keegani should be minimal for hulled almonds. Prey specificity to Lepidoptera, as well as fitness effects on adult A. transitella, warrant further investigation. Interspecific preferences between A. transitella and other insects, and intraspecific prey preference between adult and egg life stages of A. transitella may greatly affect the degree and rate at which B. keegani can regulate and track this prey population. Further questions involve the field inoculation procedure and optimal release strategy for establishment of this predator onto reproductive populations of A. transitella.
Predatory mites have been previously studied for biological control of pest arthropods in orchard and field crop agro-ecosystems, with specific focus on control of spider mites. There is no well-documented study where a mite has been augmented specifically to target Lepidoptera rather than pest mites, thrips, scales or whiteflies. Future field implementation of B. keegani for biological control would be novel, if effective, and further the study of non-phytoseiid mites as biological control agents of insect pests.
Acknowledgments
We would like to thank Zainulabeuddin Syed and the Leal Laboratory at UC Davis, Steve Tebbets, Pat Noble, and Gail Sergent of USDA-ARS, and Franz Niederholzer of UC ANR Cooperative Extension for their help in rearing navel orangeworm and supplying materials.