Introduction
There is an increasing interest in the application of the Sterile Insect Technique (SIT) to Dipteran pests (Kuba et al., Reference Kuba, Kohama, Kahinohana, Yamagishi, Kinjo, Sokei, Nakasone, Nakamoto, McPheron and Steck1996; Jessup et al., Reference Jessup, Dominiak, Woods, de Lima, Tomkins, Smallridge, Vreysen, Robinson and Hendrichs2007; Resilva et al., Reference Resilva, Obra, Zamora and Gaitan2007; Estes et al., Reference Estes, Nestel, Belcari, Jessup, Rempoulakis and Economopoulos2012). Due to the invasive nature of many fruit flies (Ekesi et al., Reference Ekesi, Nderitu and Rwomushana2006; Cini et al., Reference Cini, Ioriatti and Anfora2012; Papadopoulos, Reference Papadopoulos and Shelly2014), SIT is considered a viable method to eradicate them from areas where they have been newly introduced or established (Suckling et al., Reference Suckling, Kean., Stringer, Cáceres-Barrios, Hendrichs, Reyes-Flores and Dominiak2014). However, before attempting a costly and logistically demanding SIT application against a new target species, it is crucial to develop basic information regarding their biology and the effects of radiation and artificial rearing on the general fitness (Hendrichs et al., Reference Hendrichs, Robinson, Cayol and Enkerlin2002).
Towards this aim, we report the effects of irradiation on the longevity and competitiveness of a laboratory strain of the Ethiopian fruit fly Dacus ciliatus (Loew) (Diptera: Tephritidae), a significant pest of plants in the family Cucurbitaceae (i.e., cucumbers, zucchinis, melons, etc.) that is listed by the European and Mediterranean Plant Protection Organization as an A1 quarantine pest (EPPO, 2011). An early radiation sterility study (Huque & Ahmad, Reference Huque and Ahmad1969) indicated partial male sterility at a dose of 50 Gy, and full sterility at doses of 85 and 100 Gy. More recently, detailed dose–response experiments (Rempoulakis et al., Reference Rempoulakis, Castro, Nemny-Lavy and Nestel2015a) showed that it is possible to fully sterilize D. ciliatus males with 140 Gy of gamma rays, while 120 Gy have been proposed as a preferable sub-sterilizing dose. Additionally, a study describing the mating behavior of D. ciliatus and investigating the compatibility and competitiveness of laboratory and wild populations in field cages (Rempoulakis et al., Reference Rempoulakis, Nemny-Lavy, Castro and Nestel2015b), provided encouraging results for a future SIT implementation. The present study investigated the effects of a sub-sterilizing dose of radiation (120 Gy) to: (a) the longevity of irradiated laboratory D. ciliatus flies as compared with untreated laboratory flies and (b) the ability of laboratory irradiated males to reduce the fertility rates of wild females when introduced into a wild population under laboratory conditions. We chose the 120 Gy radiation dose in order to follow a similar methodology with the behavioral studies described in Rempoulakis et al., Reference Rempoulakis, Nemny-Lavy, Castro and Nestel2015b (i.e., using the dose that is closest to the one inducing full sterility). Based on the resulting competitiveness index (C), and in order to achieve a significant reduction in the egg hatch rates of wild female flies, we further calculated the effective laboratory irradiated: wild male ratios in a theoretical field release scenario.
Materials and methods
Origin of insects
Laboratory colony
D. ciliatus pupae were collected from a laboratory colony established at the Agricultural Research Organization of Israel (ARO) for more than 4 years (>45 generations). The rearing conditions and propagation method of this colony have been described in details elsewhere (Rempoulakis et al., Reference Rempoulakis, Afshar, Osorio, Barajas Aceves, Szular, Ahmad, Dammalage, Sto Tomas, Nemny-Lavy, Salomon, Vreysen, Nestel and Missirlis2014, Reference Rempoulakis, Castro, Nemny-Lavy and Nestel2015a).
Wild flies
For the competitiveness experiment, wild flies were collected in the Arava area (Ein Yahav), South Israel, from infested melons (Cucumis melo) of the variety Galia and kept under the laboratory conditions described by Rempoulakis et al. (Reference Rempoulakis, Nemny-Lavy, Castro and Nestel2015b). Due to low initial number of wild insects, we kept the emerged wild flies at low density in large entomological cages, offered to them fresh zucchinis for oviposition, and collected pupae from the first filial generation to conduct the experiments.
Irradiation
A batch of 1000 laboratory pupae was irradiated with a dose of 120 Gy in air atmosphere, as described by Rempoulakis et al. (Reference Rempoulakis, Castro, Nemny-Lavy and Nestel2015a). Irradiated and non-irradiated laboratory pupae and wild pupae were allowed to emerge in separate large (50 × 40 × 40 cm3) entomological cages. Within the first day of adult life, flies were sorted by sex with the use of an aspirator and placed in small single sex cages (30 × 30 × 30 cm3) in groups of ~200 individuals. Adult diet (hydrolyzed yeast and sugar 1:3 wt/wt) plus water was provided to them ad libitum. The sexed flies were kept under laboratory conditions for ca. 10 days to allow for sexual maturation. After sexual maturation, flies from both sexes were combined.
Assessment of mortality
The mortality of sexually mature irradiated and non-irradiated laboratory insects from all possible mating combinations in groups of 20 pairs per cage (see fig 1 and Rempoulakis et al., Reference Rempoulakis, Castro, Nemny-Lavy and Nestel2015a for details) was recorded every two days, when dead insects were removed from the cages. The mortality of all cohorts during the first 9 days after adult emergence was negligible (data not shown). Mortality was assessed for a period of 50 days, which is sufficient to establish the effects of irradiation upon longevity of tephritids (Carey et al., Reference Carey, Liedo, Muller, Wang, Love, Harshman and Partridge2001). Mortality was assessed from three different cohorts of irradiated and non-irradiated insects, belonging to different generations.

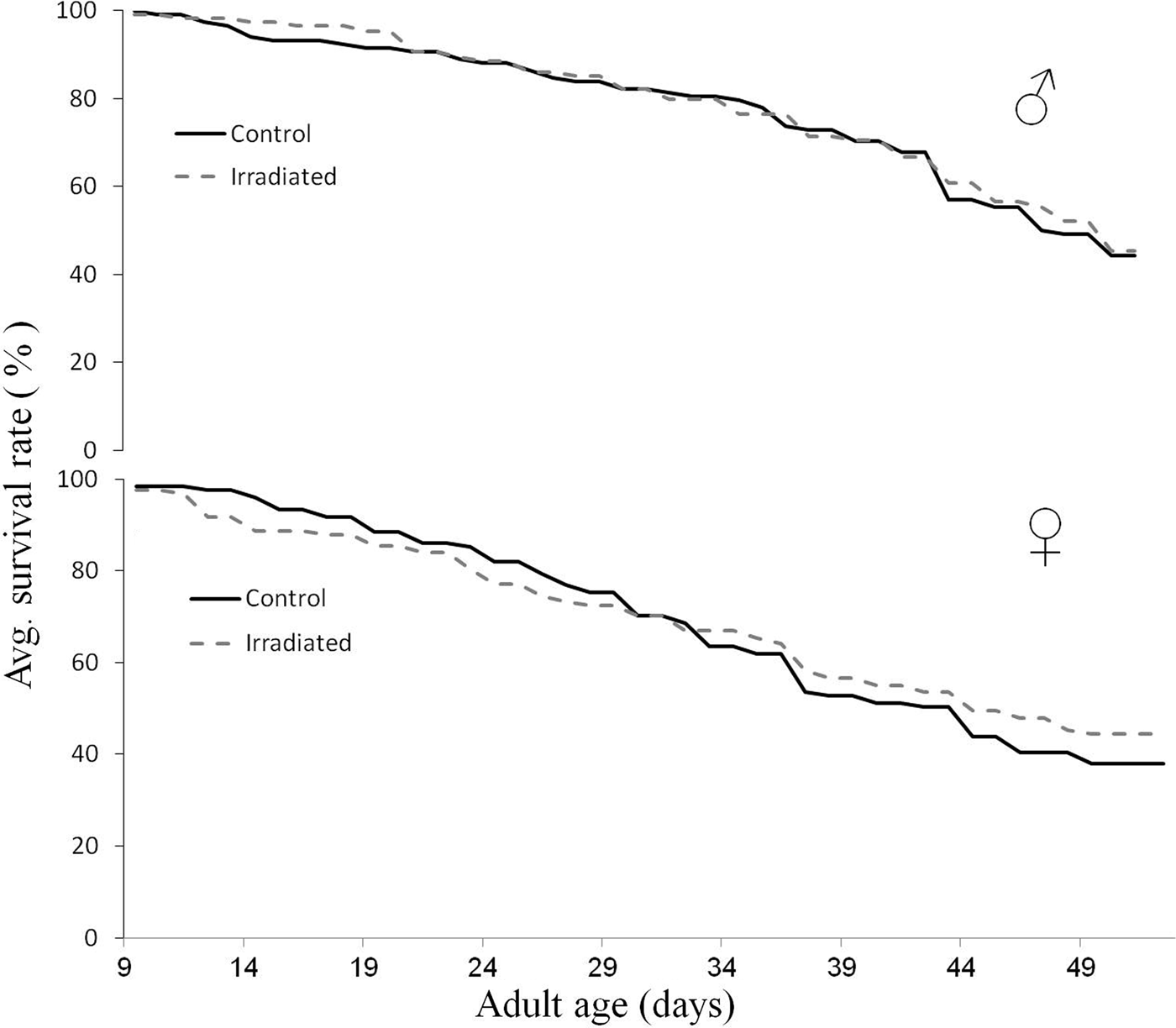
Fig. 1. Survival trends of irradiated (120 Gy) and non-irradiated male (♂) and female (♀) laboratory D. ciliatus maintained with food and water for 50 days. A Kaplan–Meier survival analysis (Log-rank pair wise comparisons) was employed on pooled longevity data from three replications (a total of ca. 120 insects per sex and treatment). Data used for the analysis and figure derive from the 4 possible strain combinations (i.e., treatments) in a single experiment: (1) non-irradiated ♂ X non-irradiated ♀, (2) non-irradiated ♂ X irradiated ♀, (3) irradiated ♂ X non-irradiated ♀, and (4) irradiated ♂ X irradiated ♀. No differences were observed among irradiated and non-irradiated males (χ 2 = 0.42; df = 1; P = 0.51), and also among irradiated and non-irradiated females (χ 2 = 0.80; df = 1; P = 0.36).
Laboratory competitiveness test
An overall competitiveness test (Fried, Reference Fried1971) was conducted according to FAO/IAEA/USDA (2014) guidelines. We assessed the induced fertility from wild males (expressed as egg hatch rate) using 40 couples of wild males × wild females, and from irradiated laboratory males using 40 couples of irradiated males × laboratory non-irradiated females. We housed those insects in large Perspex cages (50 × 40 × 40 cm3; 0.08 m3). In parallel, we conducted a competitiveness experiment using a 3:1:1 ratio of irradiated laboratory males to wild males and females (120:40:40 flies) in a much larger Perspex cage (200 × 60 × 60 cm3; 0.72 m3), to allow for sufficient space for females to escape, should they reject any pursuing males. In all mating arenas, we provided small lemon tree branches as mating sites (Rempoulakis et al., Reference Rempoulakis, Nemny-Lavy, Castro and Nestel2015b) and adult diet/water, and allowed the insects to mate for a day. After 24 h we removed the males, and assessed female fecundity and fertility for 1 week. We exposed egg collecting devices (zucchini slices) and counted the produced eggs and their hatch rates as described in Rempoulakis et al. (Reference Rempoulakis, Castro, Nemny-Lavy and Nestel2015a). We conducted three egg collections, 2 days apart from each other, and we duplicated the competitiveness experiment using fresh insects similarly reared and irradiated, for a total of six replicates.
Statistical analysis
The longevity of irradiated and non-irradiated laboratory flies was analyzed with the use of a Kaplan–Meier survival analysis (Carey, Reference Carey1993), and pair wise comparisons were conducted using a log-rank model in the statistical program JMP for Windows (SAS inc.). One way analysis of variance followed by Tukey's HSD post-hoc multiple comparisons was used to infer differences in fecundity (log n + 1-transformed data) and fertility (arcsine transformed data) between wild control, laboratory control and competitiveness treatments. Data were transformed as mentioned above in order to reduce heteroscedasticity (Zar, Reference Zar1999). The overall competitiveness (C) of the laboratory males was calculated by adopting the methods described in Haisch (Reference Haisch1970); Fried (Reference Fried1971). For calculating the variance of C from the experimental replications, we adopted the method described in Hooper & Horton (Reference Hooper and Horton1981). The calculation of hypothetical field release scenarios relied on the observed wild and laboratory irradiated fertility values, and the derived C index (see details in Table 2).
Results
The survival rates of irradiated and non-irradiated male (A) and female (B) flies are presented in fig. 1. No significant differences were found in the survival patterns of irradiated (for both sexes) and non-irradiated flies (χ 2 = 1.13; df = 1; P = 0.28). More than 40% of irradiated and non-irradiated male and female flies, maintained on food and water, survived for longer than 50 days. Furthermore, no survival differences were found between male and female insects, regardless of their irradiation status (χ 2 = 2.29; df = 1; P = 0.12).
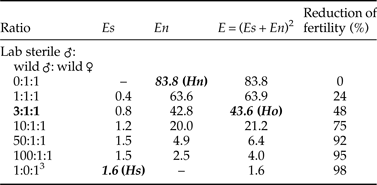
Results on the egg production and hatch rates of the competing and non-competing strains are presented in Table 1. The inclusion of laboratory males in the competition arena reduced significantly the egg hatch rates, but not the amount of produced eggs when compared with the wild control (Table 1). Using the fertility results in Fried's equation, the overall competitiveness (±SE) was found to be 0.32 ± 0.07, which indicates that irradiated laboratory and wild males were not equally competitive (i.e., three laboratory males were required in order to affect fertility to the same extent as one wild male). Table 2 presents the expected fertility values resulting from various laboratory: wild release ratios, assuming that the two male types are acting independently. Since the competitiveness index is not affected by the laboratory: wild male ratio, we calculated that a release ratio of 1:1 (laboratory: wild males) is expected to reduce the egg hatch rate of a wild population by ~25%, while a 10:1 ratio will reduce it by ~75% and a 100:1 ratio by ~95%.
Table 1. Dacus ciliatus fecundity (eggs/collection) and fertility (% egg hatch) from the laboratory competitiveness study. Average values ± SD are presented. Sample size within parenthesis. Within rows, values with different letters differed significantly at P < 0.05 (ANOVA).

1 Wild control (40 wild ♂:40 wild ♀), laboratory control (40 irradiated laboratory ♂: 40 non-irradiated laboratory ♀), competition arena (120 irradiated laboratory ♂: 40 wild ♂:40 wild ♀); data derived from two experiments, and three egg collection dates per experiment.
2 Statistics: One way ANOVA, F = 5.18; df = 2, 15; P = 0.019.
3 Statistics: One way ANOVA, F = 58.1; df = 2, 15; P < 0.001.
Table 2. Expected fertility (E) expressed as % of egg hatch, and fertility reduction (% of the wild control egg hatch) in a wild population of Dacus ciliatus following the theoretical release of different ratios of irradiated laboratory males. A competitiveness index (mean ± SD) value of 0.32 ± 0.161 was used for calculation of laboratory (Es) and wild (En) males contribution to the expected fertility (E); C was calculated from the egg hatch rates (Hn, Hs, Ho) from the control and competitive arenas and S/N ratio 3:1. Values derived from the experiment are presented with bold characters.

1 Competitiveness, C = N/S*Hn − Ho/Ho − Hs, where N = wild males and S = lab sterile males (adapted from Fried, Reference Fried1971).
2 Expected fertility,E = N*Hn + S*Hs/N + S (adapted from Fried, Reference Fried1971).
3 Laboratory females were used for this treatment (FAO/IAEA/USDA, 2014).
Discussion
The survival of flies maintained with food and water is a good indicator of long-term somatic effects of irradiation (Nestel et al., Reference Nestel, Galun and Friedman1986). In this study, the survival of irradiated and non-irradiated flies was very similar, suggesting a strong tolerance of D. ciliatus to irradiation. This conclusion is strengthened with previously investigated fitness parameters, such as emergence and flight ability of irradiated insects (Rempoulakis et al., Reference Rempoulakis, Castro, Nemny-Lavy and Nestel2015a). Huque & Ahmad (Reference Huque and Ahmad1969) reached similar conclusions in their study in which adult longevity of irradiated D. ciliatus (100 Gy) was not significantly affected when compared with non-irradiated insects. Similar resistance to radiation, as expressed by longevity, has been reported for other tephritids (Carey et al., Reference Carey, Liedo, Muller, Wang, Love, Harshman and Partridge2001; Calkins & Parker, Reference Calkins, Parker, Dyck, Hendrichs and Robinson2005; San Andres et al., Reference San Andres, Ortego and Castañera2007). Notably, the life expectancy at birth (e o) for D. ciliatus has been calculated to be ca. 123 days (Ryckewaert et al., Reference Ryckewaert, Deguine, Brévault and Vayssières2010), strengthening our results (i.e., < 60% mortality found at 50 days of adult age) (fig. 1).
As expected, the Fried test results (i.e., that measure-induced sterility) suggest that irradiated laboratory males are less competitive than their wild counterparts. The calculated competitiveness index (0.32) is in accordance with results previously observed from field cage compatibility and competitiveness tests (Rempoulakis et al., Reference Rempoulakis, Nemny-Lavy, Castro and Nestel2015b). In the field cage study, the Relative Sterility Index value observed from similarly irradiated males was 0.33. In conclusion, the findings from this study add to the existing knowledge on radiation biology of D. ciliatus, and are encouraging for a future SIT application against this insect pest.
Acknowledgements
We thank Rami Sadeh for helping in the collection of wild material and Bio-fly Company (Sde Eliahu, Israel) for allowing the use of the 60Co source. We also thank AP Economopoulos and F Missirlis for comments to earlier versions of the manuscript. We particularly thank two anonymous reviewers for their valuable comments that improved this paper. This study was partially financed by a grant from the IAEA Technical Cooperation (ISR 5018) and by the Chief Scientist Fund of the Ministry of Agriculture and Rural Development, Israel (Grant no. 131-1676).
Disclosure
The authors have no conflicts of interest.