Introduction
Alien insect species have become an increasing concern worldwide due to their significant ecological and economic impact (Mack et al., Reference Mack, Simberloff, Lonsdale, Evans, Clout and Bazzaz2000; Mooney & Hobbs, Reference Mooney, Hobbs, Mooney and Hobbs2000; Pimentel et al., Reference Pimentel, Lach, Zuniga and Morrison2000; Clarke et al., Reference Clarke, Armstrong, Carmichael, Milne, Raghu, Roderick and Yeates2005; Roll et al., Reference Roll, Dayan and Simberloff2007; Kenis et al., Reference Kenis, Auger-Rozenberg, Roques, Timms, Péré, Cock, Settele, Augustin and Lopez-Vaamonde2009). For example, in North America alone, invasive species (insects and other organisms) have been estimated to cause damage and losses of ca. US $137 billion per year (Pimentel et al., Reference Pimentel, Lach, Zuniga and Morrison2000). According to Kenis et al. (Reference Kenis, Auger-Rozenberg, Roques, Timms, Péré, Cock, Settele, Augustin and Lopez-Vaamonde2009), the majority of studies on the ecological effect of invasive insect species have been carried out in the past eight years, with two thirds of them occurring in North America and only about 5% in Europe. Invasions of herbivorous arthropods in North America reflect historical patterns of movement, with the vast majority of introduced species in Canada being of Paleartic origin, particularly from Europe (Langor et al., Reference Langor, DeHaas, Foottit, Langor and Sweeney2009). Nevertheless, recent increases in trade with China have been accompanied by matching increases of invasive species of such origin (Langor et al., Reference Langor, DeHaas, Foottit, Langor and Sweeney2009). Based on the latter, it is expected that augmented trade from North America to Europe will also be accompanied by new biological invasions. Data by Kenis (Reference Kenis and Wittenberg2006) shows that in Switzerland there are over 300 established alien insect species, with numbers likely to increase as a result of recent liberalization of human movement within the Schengen Territory and expanding trade with commercial partners worldwide.
The speed at which invasions occur has been significantly increased in recent times as a result of mainly two factors: (i) expanding international trade and tourism (Mumford, Reference Mumford2002; Work et al., Reference Work, McCullough, Cavey and Komsa2005; Westphal et al., Reference Westphal, Browne, MacKinnon and Noble2008), and (ii) global warming (Dukes & Mooney, Reference Dukes and Mooney1999; Ricciardi, Reference Ricciardi2007). Global warming is also opening new pathways for alien organisms, as, for example, formerly impenetrable mountain passes gradually become milder, allowing the survival of adults that are wind carried (Chapman et al., Reference Chapman, Reynolds, Smith, Smith and Woiwod2004; Torrez-Diaz et al., Reference Torrez-Diaz, Cavieres, Munoz-Ramirez and Arroyo2007). Also, many invasive species, establishing transient populations that would normally vanish during cold winter months, now survive as a result of winter or, more importantly, spring months that are becoming milder (Cannon, Reference Cannon1998; Rosati & Van Laerhoven, Reference Rosati and Van Laerhoven2007).
Among the most worrisome groups of invasive insects, fruit flies (Diptera: Tephritidae) stand out, as their invasive capacity impacts society along several axes. (i) On an ecological level, invasive species, particularly highly polyphagous ones, can displace native species or compete for essential resources with them (Duyck et al., Reference Duyck, David and Quilici2004). (ii) On an economic level, severe economic damage is inflicted as infested fruit and vegetables become unmarketable and inedible. In addition, the presence of invasive species severely restricts trade (Aluja & Mangan, Reference Aluja and Mangan2008). (iii) On a social level, invasive species such as the Medfly, Ceratitis capitata (Wiedemann) or Bactrocera invadens Drew, Tsuruta & White can reap havoc, as wide-ranging eradication efforts can cause the appearance of secondary pests as natural enemy populations dwindle or entire local economies are sent into disarray because formerly stable trade commodities suddenly become scarce or cannot be exported anymore (Mumford, Reference Mumford2002; Drew et al., Reference Drew, Tsuruta and White2005).
Here, we dwell on the relatively recent invasion into Switzerland of a fruit fly belonging to the genus Rhagoletis Loew native to North America (R. completa Cresson), detailing its current distribution within the country, analyzing some biotic and abiotic factors that explain its establishment and rapid expansion and offering hypotheses as to possible invasion routes. Flies within the genus Rhagoletis are distributed in North and South America, all of Europe (Austria, Bulgaria, France, Germany, Greece, Italy, Netherlands, Norway, Poland, Portugal, Spain, Sweden, Switzerland, Turkey (EPPO/CABI, Reference Smith, McNamara, Scott and Harris1996)) and parts of Eurasia
In Switzerland, only Rhagoletis cerasi is endemic; but, in the past two to three decades, Rhagoletis completa and Rhagoletis cingulata (Loew) have invaded the country, most likely from Italy where the two species were first reported for Europe (Duso, Reference Duso1991; Norrbom, Reference Norrbom2004). R. completa was initially found near Venice and in the Friuli region (Duso, Reference Duso1991), and its presence in Italy was shortly thereafter confirmed in Milano, Pavia, Novara, Varese and Sondrio (Ciampolini & Trematerra, Reference Ciampolini and Trematerra1992). The first formal reports of the presence of R. completa and R. cingulata (originally reported as R. indifferens) in Switzerland originate from trap captures in the Ticino region (Merz, Reference Merz1991; Mani et al., Reference Mani, Merz, Brugnetti, Schaub, Jermini and Schwaller1994; Lampe et al., Reference Lampe, Burghause, Krauthausen, Alford and Backhaus2005), indicating that these flies possibly invaded the country in the mid- to late 1980s. As is the case with other non-European species of Tephritidae, R. completa was regulated as a quarantine pest in Switzerland and presently still possesses this status (Swiss Federal Council, 2001). As noted above, R. indifferens Curran was also reported in Switzerland (Merz, Reference Merz1991; Mani et al., Reference Mani, Merz, Brugnetti, Schaub, Jermini and Schwaller1994), but B. Merz later acknowledged that the specimens had been misidentified and were in fact R. cingulata (Merz & Niehuis, Reference Merz and Niehuis2001). So, there is no formal evidence that R. indifferens is or was ever present in Switzerland or anywhere else in Europe.
Rhagoletis completa belongs to the suavis species group where it has been placed together with R. boycei Cresson, R. suavis (Loew), R. juglandis Cresson, R. zoqui Bush and R. ramosae Hernández-Ortiz (Bush, Reference Bush1966; Hernández-Ortíz, Reference Hernández-Ortíz1985; Smith & Bush, Reference Smith, Bush, Aluja and Norrbom2000). It is native to Midwestern USA and north-eastern Mexico (Bush, Reference Bush1966; Smith & Bush, Reference Smith, Bush, Aluja and Norrbom2000; Chen et al., Reference Chen, Opp, Berlocher and Roderick2006) and was described from specimens collected in the USA in the late 1920s (Cresson, Reference Cresson1929). In the USA, it was originally restricted to the Midwestern part of the country, but between 1922 and 1925 it was reported in California (Boyce, Reference Boyce1934). On the west coast, the fly now ranges from southern California as far north as Washington State (Chen et al., Reference Chen, Opp, Berlocher and Roderick2006). In Mexico, it is restricted to the north east (Smith & Bush, Reference Smith, Bush, Aluja and Norrbom2000) particularly the states of Coahuila, Nuevo León, and Tamaulipas (J. Rull, unpublished data). The known hosts of R. completa are Juglans nigra L., J. microcarpa Ber., J. californica S. Whatson, J. hirsuta Manning, J. hindsii, Jepson ex R.E. Smith, J. major (Torr.) A. Heller and J. regia L. (Bush, Reference Bush1966; Smith & Bush, Reference Smith, Bush, Aluja and Norrbom2000). In addition to infesting walnuts, R. completa has been reported infesting peaches, Prunus persica L. (Boyce, Reference Boyce1934), although this host affiliation is rare (Smith & Bush, Reference Smith, Bush, Aluja and Norrbom2000). The few additional details on its biology can be summarized as follows: it goes through obligate diapause, is univoltine, is considered oligophagous, and is attacked by very few parasitoids (Boyce, Reference Boyce1934; Legner & Goeden, Reference Legner and Goeden1987; Kasana & AliNiazee, Reference Kasana and AliNiazee1995, Reference Kasana and AliNiazee1996; Ovruski et al., Reference Ovruski, Wharton, Rull and Guillen2007). European populations in Italy exhibit one generation a year, with adult emergence spanning from early July to the second half of August. Oviposition occurs from late July to early September, with peaks between the 5th and 18th of August. First instar larvae have been recorded since early August, and mature larvae leave husks from late August onwards to pupate in the soil (Duso & Dal Lago, Reference Duso and Dal Lago2006).
Economic damage is caused by R. completa larvae, especially when the infestation occurs in an early stage of walnut development from July until mid-August. Damage has been reported to occur on 74–91% of fruit in untreated orchards, with fruit not developing fully and producing small nuts or on occasion shrivelled, mouldy kernels (Olhendorf, Reference Olhendorf2000; Duso & Dal Lago, Reference Duso and Dal Lago2006). Furthermore, there is the suspicion that heavily infested fruit facilitates the penetration of pathogens into the edible nut, such as the fungus Marssonina juglandis (Lib.) Magnus and particularly the bacteria Xanthomonas campestris pv. juglandis (Pierce (Dye)), causing the actual nut (kernel) to shrink and lose weight and also to rot, producing considerable (sometimes total) yield loss as a consequence of mould growth and malformation of the kernel (Hislop & Allen, Reference Hislop and Allen1983; Coates, Reference Coates2005). Despite the fact that feeding activity of R. completa larvae in late infestations usually does not damage the commercially valuable kernel, it does interfere with the natural separation of the pulp from the nut shell, and this can render commercialization cumbersome or impractical. Black stains have to be removed from the nut skin with high water pressure or nuts must be bleached as consumers refuse or hesitate to buy stained nuts (Hislop & Allen, Reference Hislop and Allen1983; Olhendorf, Reference Olhendorf2000).
Considering the fact that the history of R. completa in Europe and particularly Switzerland is fairly recent and that, since it was first reported in Italy by Duso (Reference Duso1991) and Ciampolini & Trematerra (Reference Ciampolini and Trematerra1992), its presence has been confirmed in other European countries such as Slovenia (Seljak, Reference Seljak1999), Austria (C. Lethmayer, personal communication), Germany (EPPO, 2004) and France (EPPO, 2008; Bouvet, Reference Bouvet2009), we decided to investigate the current distribution of R. completa in Switzerland, as possibly global warming was generating suitable conditions for its expansion through the Alpine divide into formerly colder areas (Studer et al., Reference Studer, Appenzeller and Defila2005; IPCC, 2007).
A second goal of our survey was to try to gain insight into possible invasion/expansion routes into/within the country (and into neighbouring countries) and to identify potential sources of environmental resilience to the invasive alien. In the spirit of Primack et al. (Reference Primack, Ibanez, Higuchi, Lee, Miller-Rushing, Wilson and Silander2009), we were also interested in gaining insight into the ability of R. completa to persist in a new environment with changing climate.
Materials and methods
Collection sites and climate parameters
Samples were collected in a total of 71 sites scattered throughout Switzerland. A complete list of the localities is provided in Appendix 1, we first targeted valleys located along known paths of warm transalpine winds from the south (Föhn valleys). In particular we targeted two regions: (i) valleys in the cantons Grisons and Uri; and (ii) the flatlands of the entire canton Valais all the way from Brigg to Lake Geneva. Climatic regions, as a possible discriminating factor for incidence of R. completa and walnut infestation levels, were determined for individual sites on the basis of a simplified scheme following Müller (Reference Müller1980). We further discriminated among regions with distinct climatic conditions, such as the Jura Mountains, the midlands between Lake Geneva and Lake Constance, the north face of the Alps, the south face of the Alps beyond the Alpine divide and finally the Valais due to special conditions regarding adiabatic wind formation. Meteorological 30-year spring temperature means (March–May 1961–1990) were used as an integrating parameter reflecting growing season length and potential, respectively, and winter temperatures (December–February 1961–1990) were used as an integrating parameter reflecting winter length and potential for survival during hibernation. Such parameters were retrieved for all walnut collection sites from the software ‘Atlas of Switzerland’ Version 2.0 (Project KLIMA90: Aschwanden et al., Reference Aschwanden, Beck, Häberli, Haller, Kiene, Roesch, Sie and Stutz1996). Climatic parameters for each collection site are listed in Appendix 1.
For two possible south–north invasion pathways through the Gotthard region of the central Swiss Alps, yearly widths of the cold barrier unsuitable for establishment of R. completa around the Alpine divide were calculated from 1961 to 2010 on the basis of the homogeneous temperature series of Switzerland (Begert et al., Reference Begert, Schlegel and Kirchhofer2005) applied to the digital climate map of spring temperatures implemented with the software ‘Atlas of Switzerland’ Version 2.0 (Aschwanden et al., Reference Aschwanden, Beck, Häberli, Haller, Kiene, Roesch, Sie and Stutz1996). Resolution of distance measurements was 1 km.
Fruit sampling and processing
Most (>95%) samples were gathered from tree canopies by direct harvest or with the aid of a 4 m telescopic scissor (expandable PVC tubing with a sharp curved scissor at end; Wallace, Enfield, CT, USA). Between 20 and 35 nuts were collected in every site (Appendix 2); but, if fruit were plentiful and heavily infested and we received explicit permission by the tree owners, up to 250 nuts were sampled. Fruit samples were placed in 10-l transparent plastic bags that were labelled (date, name of collection site) and placed in plastic crates so as to allow us to maintain the upper end of the bag open to permit for aeration.
After collection, we weighed each bag the same day and then gently transferred fruit samples inside 33×22×9-cm transparent Polystyrol containers, with a screened cover to allow for aeration (Lagerdosen PS glasklar, Semadeni AG, Ostermündingen, Switzerland). On the bottom of the container, we placed a layer of pure 0.1–0.6 mm quartz sand (minimum 93.3% SiO2; Carlo Bernasconi AG, Bern, Switzerland) as a pupating medium. Since R. completa larvae wander before pupating and also creep along container walls/roof, we used three heavy duty rubber bands to guarantee that the container cover was tight, as otherwise many larvae would have escaped. In those cases where there was evidence of infestation by R. completa larvae, we took five such fruit from the sample for a single-fruit experiment, weighed them and placed them individually in 180-ml transparent Polystyrol cups (Joghurtbecher PS glasklar, Semadeni AG, Ostermündingen, Switzerland), into which we had also placed a layer of quartz sand in the bottom. The 180-ml cups were tightly covered with a plastic lid into which we had poked at least 100 holes with a needle to allow for aeration. Cups for the single-fruit experiment and containers with the remaining samples were then shifted to an exterior covered warehouse (i.e. ambient temperature) built with wire mesh to allow for ample aeration and protect samples from wild scavengers. Containers were randomly distributed on the warehouse subdivisions (bottom and top), avoiding stacking to allow for maximum aeration. Cups for the single-fruit experiment were placed in a plastic tray grouped by collection site.
Processing of larvae, pupae and adults
Approximately 45–60 days after fruit had been placed in the warehouse, we started to separate the pupae from the sand and debris. Fruit had totally disintegrated inside, but the hardened skin was mostly intact and had to be cracked open to search for dead larvae or pupae. To facilitate pupal recovery, we removed all fruit and then added water to the container so as to allow pupae/dead larvae to float. Prior to this, we had carefully inspected every fruit to remove any pupae or dead larvae that remained inside skin or nut crevices. Dead larvae were surprisingly well preserved and easily recognizable as their white body contrasted with the dark liquid of the decayed pulp. At this stage, we counted all nuts in the sample as fruit had not been individually counted when originally placed in the Polystyrol containers. This information was needed to calculate the number of larvae per fruit in the overall sample.
Both larvae and pupae were transferred into a colander, rinsed with tap water and placed in 90-mm filter paper circles to let them dry. The next day at the latest, dead larvae and pupae were counted and viable pupae transferred into a 180-ml transparent Polystyrol cup with a layer of moistened quartz sand in the bottom. Some pupae were rotten or hollow and were discarded after they had been tallied so as to avoid fungal growth that could damage the healthy pupae. In the case of the single-fruit experiment, each individual pupa was weighed. After weighing, all pupae from a particular collection site that had been kept individually were placed together with those stemming from the larger sample (same location). From this pool, we separated 20 pupae (if so many were available), transferred them into 0.5-ml Eppendorf tubes and placed them at –80°C in an ultra freezer for future genetic analysis.
After the separation, rinsing and counting procedure, all pupae were transferred into a climatic chamber kept at 13°C until early January, and then the temperature was raised to 27°C to artificially break the diapause. During the test, the pupae/sand were moistened regularly. All cups containing the pupae were then transferred to a room kept at 20°C, 70% RH, 16∶8 L∶D cycle (long day) to monitor adult emergence. As flies emerged, they were transferred into 0.5-ml Eppendorf tubes and quickly frozen. Samples were placed together with the pupae from the same collecting site in the same ultra freezer (see previous paragraph) at −80°C for future genetic analysis.
Statistical analyses
All statistical analyses were run with the aid of XLSTAT version 2008.2.03 (Addinsoft, Andernach, Germany). The presence of R. completa in relation to the sampling site was analysed by multiple logistic regression to determine the influence of the climatic region within Switzerland, mean spring temperatures and mean winter temperatures with a single simultaneous analysis of incidence. The infestation level at the sites was measured as infestation per fruit and infestation per fruit mass (i.e. per kilogram of fruit). Both variables were analysed across all the sampled sites by simple factorial analyses of covariance (ANCOVA) to determine the influence of the factor climatic region and the covariates mean spring temperature and mean winter temperature. The same ANCOVA approach was applied to analyse the effect of the climate parameters on mean pupal weight measured in the overall samples (Appendix 1).
Data from the single fruit experiment were averaged for each site in order to avoid the inclusion of dependent data points in the analysis. They were first analysed to determine the possible influence of the climatic region, mean spring temperatures and mean winter temperatures on fruit size by simple factorial ANCOVAs. The sites where no flies were found in the experiment were excluded from the analysis. Fruit weight was log10 transformed in order to achieve a data structure not significantly different from the normal distribution as confirmed by Shapiro-Wilk test (W=0.96, P=0.27). Subsequently, the number of pupae per fruit was also analysed by ANCOVA to determine the possible influence of the factor climatic region, the covariates mean spring temperature, mean winter temperature and fruit weight, as well as the factor mean pupal weight in a particular fruit. Finally, mean pupal weight was analysed by ANCOVA to determine the possible influence of the factor climatic region, and the covariates mean spring temperature, mean winter temperature, fruit weight and number of pupae per fruit.
The shrinking trend of the cold barrier around the Alpine divide was tested by means of a robust non-parametric correlation based on Kendall's Tau, representing the difference between the probability that in the observed data distance is reduced over time vs. the probability that time and distance behave independently.
Results
Distribution of R. completa in Switzerland
As detailed in fig. 1, R. completa presently occurs in most lower-altitude regions of Switzerland. Of the 71 sites in which we collected walnuts, we found infested fruit in 42 sites (52.5% of total) distributed in 14 cantons. In the Ticino region, south of the Alpine divide, R. completa is fully established, as well as in the other smaller areas across the main mountain ridge towards Italy in the south-eastern parts of Switzerland (e.g. Bondo, Brusio). In the Valais, R. completa was found in the entire valley, even in the most eastern upper parts less than ten kilometres away from the Alpine divide (fig. 1). Also, in the canton Uri, R. completa occurs just about ten kilometres north of the Gotthard Massif forming the Alpine divide in this central region. North of that region, however, the species could not be found in the Lake Lucerne area. Along the upper Rhine Valley and its watershed, R. completa occurs only in the warm areas around Chur about 40 km north of the Alpine divide (fig. 1).

Fig. 1. Current distribution of R. completa in Switzerland with possible invasion and expansion routes. Green circles represent sites where infested fruits were collected, and white circles represent sites where fruits were collected but no infestation was determined. The grey circle represents the locality of Martina (Grisons) where no walnut trees were found despite suitable altitude and climatic conditions for tree survival. Alpine divide drawn as main physical and climatic barrier (light blue line). Four possible invasion/expansion routes are depicted: green arrows (most likely), blue (likely), pink (possible) and red (unlikely). DE, dead end (i.e. at present not yet passed physical/climatic barrier). Graphics by J. Samietz.
The incidence of R. completa in the sampling sites is significantly influenced by climate (multiple logistic regression, overall score Χ 26=34.84, P<0.0001). Among the parameters tested, however, only mean spring temperature significantly explains the incidence of R. completa (Χ 21=8.30, P=0.004), whereas climatic region (Χ 24=5.40, P=0.25) and mean winter temperature (Χ 21=0.013, P=0.91) had no effect. The regions where the fly is not present but the host (J. regia) is are located in the central part of the country in areas where the 30-year mean spring temperature fluctuates between 5°C and about 7°C, or falls bellow these mean values (cantons Glarus, Luzern, Nidwalden, Obwalden, Schwyz, and parts of Bern, Grisons and Jura) (Appendix 1).
Infestation patterns
Fruit infestation levels varied greatly between the sites sampled and spanned from 0 to 259.32 larvae per kilogram of fruit (Appendix 1). Although the maximum infestation levels were detected in the outskirts of the city of Bern within the central midlands of the northern pre-alpine region, heavily infested fruit was also collected in various other sites distributed all over Switzerland, such as Brusio (Grisons), Courrendin (Jura), Chur (Grisons) and Etzgen (Aargau) (details in fig. 2 and Appendix 1). The number of larvae per fruit varied between 0.08 and 9.88, averaging 1.55 for all infested samples (Appendix 1).

Fig. 2. Degree of infestation (larvae per kg of fruit) in infested localities across Swiss cantons, illustrating the fact that sites with the highest levels of infestation by R. completa show no discernible geographical pattern. Graphics by J. Samietz.
Infestation level per fruit was significantly influenced by mean spring temperature (ANCOVA, F 1,68=4.97, P=0.029). Climatic region and mean winter temperature, however, had no significant effect (ANCOVA, factor: F 4,68=1.32, P=0.274; covariate: F 1,68=2.70, P=0.106). Thus, the discriminating influence of spring temperature exhibits the same pattern in all climatic regions (no interaction).
Infestation rate per fruit mass was likewise significantly influenced by mean spring temperature (ANCOVA, F 1,68= 5.49, P=0.022). Climatic region and mean winter temperature had no significant effect (ANCOVA, factor: F 4,68=2.41, P=0.059; covariate: F 1,68=3.65; P=0.061). As was the case for infestation level per fruit, the discriminating influence of spring temperature exhibits the same pattern in all climatic regions (no interaction).
The effects of seasonal temperatures on infestation level per fruit and infestation rate per fruit mass were confirmed when analysed separately. Whereas infestation by R. completa was not related to winter temperatures (per fruit: Spearman's r=0.307, P=0.111; per fruit mass: Spearman's r=0.315, P=0.092), infestation rates were significantly correlated with spring temperatures (fig. 3a, per fruit: Spearman's r=0.575, P<0.0001; fig. 3b, per fruit mass: Spearman's r=0.577, P<0.0001).

Fig. 3. Level of infestation of R. completa (a) per walnut fruit sampled and (b) per kilogram fruit as linear functions of meteorological 30-year averages of spring temperatures (March–May) in Switzerland on 69 locations. Refer to the text for statistics.
Pupae weighed, on average, 7.66±0.21 mg (Appendix 1) and weight was not influenced by any of the climatic parameters analysed (ANCOVA, factor climate region: F 4,39= 0.198, P=0.938; covariates: spring temperature: F 1,39=0.007, P=0.934; winter temperature: F 1,39=1.34, P=0.255).
Single-fruit experiment
In the case of the subsample of walnuts that were kept individually after harvest, individual fruit weight ranged from 19.54 g, for a nut sampled in Aigle (Vaud), to 119.2 g, for a nut sampled in Le Guercet (Valais); but this parameter was not influenced by the climatic parameters analysed (log10-transformed; ANCOVA, factor climatic region: F 4,159=0.694, P=0.603; covariates: spring temperature: F 1,159=1.44, P= 0.241; winter temperature: F 1,159=0.164, P=0.689). For mean fruit mass in the samples, refer to Appendix 2.
Infestation level measured as the number of pupae per fruit was significantly influenced by individual fruit weight (log10-transformed; ANCOVA, F 1,31=8.67, P=0.008) but not by mean pupal weight nor any of the climatic parameters analysed (ANCOVA, factor climatic region: F 4,31=0.379, P= 0.821; covariates: spring temperature: F 1,31=2.28, P=0.146; winter temperature: F 1,31=0.059, P=0.810; pupal weight: F 1,31=0.016, P=0.901). The discriminating influence of fruit weight on infestation per fruit exhibits the same pattern in all climatic regions (no interaction). The corresponding relationship between infestation per fruit and log10-transformed fruit weight is plotted in fig. 4 (for regression statistics, see graph).

Fig. 4. Level of infestation of R. completa per walnut fruit as linear function of individual fruit weight in a single-fruit experiment (n=160) with samples from 32 locations in Switzerland. Dashed lines: 95% confidence of the regression slope. Dotted lines: 95% confidence of the prediction range.
Weight of single pupae in individually kept fruit varied from 4.30 mg in Contone (Ticino) to 10.32±0.26 mg in Bondo (Grisons), both located south of the Alpine divide (Appendix 2). Overall (considering all fruit kept individually independent of site of collection), pupae weighed a mean 7.66±0.21 mg. Importantly, pupal weight was neither influenced by individual fruit weight and pupal number per fruit nor by any of the climatic parameters analysed (ANCOVA, factor climatic region: F 4,31=0.624, P=0.657; covariates: spring temperature: F 1,31=0.024, P=0.879; winter temperature: F 1,31= 1.00, P=0.329; infestation pupae per fruit: F 1,31=0.0038, P= 0.956; log10-transformed fruit weight: F 1,31=3.52, P=0.559).
Based on the above, it becomes clear that there is no trade-off between fruit size, fruit infestation level and pupal weight. To illustrate the link between the variables, pupal weight was plotted as a function of log10-transformed fruit size and degree of infestation in fig. 5. The nearly horizontal three-dimensional least-square mesh visualizes and confirms the ANCOVA results. That is, the smallest pupae did not stem from the most infested or smallest fruit (fig. 5).

Fig. 5. Mean pupal weight of R. completa as multiple linear function of individual fruit weight and infestation level per walnut fruit in a single-fruit experiment (n=160) with samples from 32 locations in Switzerland. Mesh shows three-dimensional least-square relationship which reveals no effect of the two parameters (for statistics see text).
Parasitism
A total of 6476 pupae were processed in the entire study. Of these pupae, 3675 were followed until break of diapause and adult fly emergence. In the case of the rest, we only checked for parasitoid emergence. Not a single parasitoid emerged in any case.
Alpine divide as a climatic barrier
From 1961 to 1990, in the Gotthard region of the central Swiss Alps, the width of the cold barrier around the Alpine divide, based on ≤7°C spring temperatures unsuitable for establishment of R. completa, was on average 43.0±26.0 km between the Ticino Valley (south) and the Reuss Valley (north) and 38.4±16.0 km between the Brenno Valley (south) and the Rhine Valley (north). In the past ten years (2001–2010), the width for the possible south-north invasion pathways shrunk to only 21.8±5.6 km between the Ticino and Reuss Valleys and 19.6±5.7 km between the Brenno and Rhine Valleys (fig. 6). During 2007, a record year with respect of spring temperatures, the shortest width was reached with only 11 km between sites with favourable temperatures across the Brenno and Rhine Valleys (fig. 6). The observed trend of shrinking climatic barrier is highly significant for both analysed pathways across the Gotthard region (Ticino-Reuss Valley: Kendall's Tau=−0.469, P<0.0001; Brenno-Rhine Valley: Kendall's Tau=−0.462, P<0.0001).

Fig. 6. Distance of cold barrier around the Alpine divide based on 7°C spring temperature from 1961 to 2010 for two possible south–north invasion pathways through the Gotthard region (Ticino – Reuss; Brenno – Rhine). Lines show ten-years running mean. Map insert depicts areas with mean spring temperatures for 1961–90 (yellow), 1989–2010 (orange), 2001–2010 (Red), and the record year 2007 (dark red). Graphics by J. Samietz(![]() , Ticino – Reuss valley;
, Ticino – Reuss valley; ![]() , Brenno – Rhine valley).
, Brenno – Rhine valley).
Discussion
Several findings from this study merit discussion. (i) R. completa is now firmly established in most of Switzerland. The only areas still free of the pest are those where mean spring temperatures fall below 7°C or in all mountainous areas where walnut trees cannot grow. We propose factors possibly explaining its current distribution in this country and also hypothesize on possible invasion/expansion routes within Switzerland and to neighbouring countries. (ii) The sites exhibiting the highest rates of infestation are distributed all over Switzerland, with no discernible geographical pattern. (iii) We did not find a significant correlation between the degree of infestation (i.e. pupae per fruit) and pupal weight and between fruit weight and pupal weight (i.e. the smallest pupae did not stem from the most infested or smallest fruit). We discuss all the above to raise awareness on the risk of invasion of other pestiferous fruit fly species that pose potential threats, such as the apple maggot, R. pomonella, and other species in the same genus or, in the longer term, tropical species, such as the Medfly or the Oriental fruit fly (Meixner et al., Reference Meixner, McPheron, Silva, Gasparich and Sheppard2002; Clarke et al., Reference Clarke, Armstrong, Carmichael, Milne, Raghu, Roderick and Yeates2005).
Possible invasion and expansion routes
A recent literature review revealed that most biological invasions in Canada follow a defined pattern of introduction and spread, with some species being directly introduced but many others expanding their range from original entry points in the US (Langor et al., Reference Langor, DeHaas, Foottit, Langor and Sweeney2009). Identifying invasion routes becomes, therefore, a highly relevant endeavour. Incursion of invasive species from the Mediterranean across the Alps is most likely limited by the climatic limits of the species and by the orographic structure of the alpine region – especially by the Alpine divide and the main valleys. Mountain ranges create a natural climatic and physical barrier that somehow had to be crossed by R. completa since it was first reported in Europe in northern Italy (Duso, Reference Duso1991; Ciampolini & Trematerra, Reference Ciampolini and Trematerra1992). In fig. 1, we propose four possible routes of entry of R. completa into Switzerland and for its expansion within the country. At this stage of the analysis, we ignore if another pathway for entry into the country could have potentially been the incidental or accidental importation of infested fruit by car. Such founder events will be explored by us in subsequent studies on the population genetics of R. completa since they have been found to leave genetic signatures (Berlocher, Reference Berlocher1984; Chen et al., Reference Chen, Opp, Berlocher and Roderick2006).
Given the widespread distribution in the Valais, even in the upper valley less than ten kilometres away from the Alpine divide, a very plausible transalpine crossing point is represented by Route 1 (fig. 1; green arrows) through which adult flies would have crossed the Simplon area into the upper Valais from northern Italy. This would also be supported by Föhn winds occurring in that region that can form quite strong and warm adiabatic currents. Additionally, the Valais is a narrow valley in itself, where strong adiabatic winds that change direction during the day are generated, possibly aiding adults to quickly expand from the upper Valais towards Lake Geneva. In both cases, flies could also have been introduced via infested fruit by nursery owners driving into the country by car.
Another very likely incursion route into the southern Ticino and plausible alpine crossing is represented by Route 2 (fig. 1; blue arrows). Adult flies likely moved into Switzerland from northern Italy along the prealpine lakes in that area (Lugano and Maggiore). Once established in the Ticino, a region where walnuts are plentiful and widespread, adult flies may have crossed the Alpine divide through the narrow Gotthard Pass into the Canton of Uri, where they could have established founder populations. Again, this may have been supported by the strong Föhn winds occurring there in the case of southern currents. From Uri, a canton where walnuts are also common in farms, backyard gardens and parks, flies could have expanded into the rest of the country via the cantons of Schwyz, Zürich, Grisons and St Gallen. Initial expansion from the Canton of Uri into northern Switzerland could have occurred via the Lake Lucerne and the Reuss Valley. We note, however, that we did not find R. completa directly north of Uri and in sites around Lake Lucerne. So, it is also possible that founder populations in Uri stemming from the Ticino may have disappeared after a harsh winter; and, if such would have been the case, Route 2 may well represent a dead end.
A third potential alpine crossing, albeit a non-parsimonious one, is represented by Route 3 (fig. 1; red arrows), where flies would have entered the Rhine Valley and its watershed from the Lake Como region in Italy via the Splügen Pass, aided by Föhn winds and then moved into St Gallen, Aargau, Schaffhausen, Basel and the Jura, Neuchâtel and Valais (opposite direction as Route 1). However, the locations in which we discovered R. completa populations (Chur, Zizers) are quite distant from the Alpine divide (40 km), and we therefore consider this hypothesis as weak. It is also possible that the flies found in western Grisons arrived via invasion routes one or two (or both).
Finally, Route 4 (fig. 1; pink arrows) would suppose that R. completa crossed the Alps in southern France or at least in the southernmost and, thereby, warmer parts of the Alpine divide and then would have invaded Switzerland through the Rhone valley. Although the presence of R. completa in France has only been recently formally acknowledged (EPPO, 2008; Bouvet, Reference Bouvet2009), it is likely that invasion into this country occurred a long time ago and that the species is now firmly established as being the case in Switzerland. Recent invasion of the species into Germany (EPPO, 2004) likely happened by crossing the Alpine divide, either via France or more likely by one of the hypothesized routes through Switzerland. At least, the first unofficial reports of the presence of R. completa in Germany stem from localities in southern Baden – the tri-border region of France, Germany and Switzerland (Kirsten Köppler, personal communication). Of course, it is also possible that infested walnuts could have been carried into the country by car. In our planned genetic analysis of European populations, the genetic structure of R. completa populations will be analysed along the lines of Meixner et al. (Reference Meixner, McPheron, Silva, Gasparich and Sheppard2002) and Michel et al. (Reference Michel, Rull, Aluja and Feder2007), including reference sampling in the areas of origin, to further elucidate the dynamics of the Swiss invasion.
Considering all the above, global warming over the past 50 years, and especially after 1990, appears to have facilitated the crossing of R. completa over the highest regions of the Swiss Alps. As analysed here, from 1985 on, there is a progressive reduction of the dividing effect around the Alpine divide (fig. 6). The ten-year running means of both pathways analysed here progressively fell from 1987 on, about the time when R. completa was introduced into Europe. When considering recent climate change scenarios, during the next two to three decades this climatic barrier will further shrink to mean width values of about 15 km (Samietz, unpublished data; IPCC, 2007). But even considering current crossing distances – on average only about 20 km and as little as 11 km in record years (e.g. 2007) – Alpine crossing aided by Föhn winds or even by active flight has become much more likely than it was only some decades ago. This should be taken into account when monitoring species establishment in the Mediterranean Basin and, thereafter, in the Alpine valleys.
The lack of a clear geographical pattern with respect to levels of infestation could be indicative of the fact that local microclimate influences larval development. However, results from the single-fruit experiment clearly indicate that fruit weight is positively correlated to infestation level by R. completa (fig. 4). That is, large fruit provide more resources for feeding larvae and, therefore, produce more pupae and eventually adults. Nevertheless, pupal weight, a key fitness parameter, shows no trade-off with fruit size and degree of infestation (fig. 5). That is, the smallest pupae did not necessarily stem from fruit that harboured the largest number of larvae. Therefore, we believe that the observed infestation patterns can be better explained by varying levels of secondary metabolites (allelochemicals) in fruit, in turn determined by cultivar, age of tree or by growing conditions, rather than microclimate. In support of the above, differences in walnut cultivar susceptibility to R. completa have been reported in California (Boyce, Reference Boyce1934; Hass, Reference Hass1937; Opp & Zermeño, Reference Opp and Zermeño2001; Coates, Reference Coates2005). We believe that until more accurate information on the effect of cultivar (plant chemistry) can be gathered, presence absence data, rather than infestation levels, is better suited to make inferences on potential invasion routes.
Monitoring and management of invasion
Considering the fact that two Nearctic pest species of Rhagoletis have invaded Switzerland and neighbouring countries in the past 20–30 years, preventive detection trapping for Rhagoletis pomonella a key pest of apples, R. indifferens a key pest of cherries, R. fausta a potential pest of cherries, and other Rhagoletis species in the suavis group as potential pests of walnuts should be established along probable invasion routes. An advantage, in this respect, is that all of these species are visually attracted to yellow panels, and immature adults of all species respond to ammonium carbonate (Aluja & Rull, Reference Aluja, Rull, Aluja, Leskey and Vincent2009). Therefore, a single trapping device could be employed to monitor all of these species. We also believe that if global warming trends persist, potentially allowing tropical species to survive in formerly impenetrable areas because of the presence of permanent snow or ice (and concomitant cold air), preventive trapping routes, placed along strategic valleys such as the Valais to detect the potential presence of species such as B. dorsalis, C. capitata or A. fraterculus, may be warranted.
In the USA, yellow traps baited with ammonium acetate are recommended to monitor R. completa populations (Riedl & Hoying, Reference Riedl and Hoying1981; Riedl et al., Reference Riedl, Barnett, Coates, Coviello, Joos and Olson1989). Once the first adult flies emerge, insecticides such as spinosad and malathion have been applied in California to entire tree canopies (Riedl et al., Reference Riedl, Barnett, Coates, Coviello, Joos and Olson1989; Van Steenwyk et al., Reference Van Steenwyk, Zolbrod and Nomoto2003). An organic alternative to insecticides applied in California is represented by clay-based kaolin products (e.g. Surround®) (Coates & Van Steenwyk, Reference Coates and Van Steenwyk2002). Among the European countries recently invaded by R. completa, France, a country with large commercial walnut-producing areas, has approved the restricted use (limit of 120 days) of thiacloprid and phosmet for conventional agriculture and spinosad for organic nut production in 2009 (guidelines of EU Directive 2000/29, Annex A) (Bouvet, Reference Bouvet2009).
The potential danger R. completa represents to peach growers in Central Europe is a topic that merits attention. This fly has been shown to attack peaches in California, where, fortunately, its diapause schedule causes it to emerge at the end of the peach harvest season, allowing for the implementation of a ‘pest-free harvest period’ (Yokoyama & Miller, Reference Yokohama and Miller1993, Reference Yokoyama and Miller1994). Nevertheless, it has to be kept in mind that plasticity in diapause length facilitated expansion of R. completa from its native range in the midwestern US (5°C) to California, where warmer winter temperatures prevail (10°C) (Chen et al., Reference Chen, Opp, Berlocher and Roderick2006). R. completa may possess the genetic variability necessary to evolve diapause lengths to match the fruiting phenology of potential European host plants. We, therefore, suggest the establishment of a preventive monitoring network to help reduce the potential danger posed by R. completa to peach growers in Hungary and Romania, as this species has already been detected in Austria and Slovenia (Seljak, Reference Seljak1999; Lethmayer, Reference Lethmayer2008).
Insights into the biology and ecology of Rhagoletis completa
Our study yielded many insights into the biology and ecology of this invasive pest and complements nicely the work of pioneers such as Cresson (Reference Cresson1929) and Boyce (Reference Boyce1934) (for a more recent review see Prokopy & Papaj, Reference Prokopy, Papaj, Aluja and Norrbom2000). For example, quantitative data on rates of infestation were retrieved (e.g. larvae per fruit), which in turn shed light into the oviposition behaviour of females, which, as far as is known, lay one egg per oviposition bout but commonly reuse previously used oviposition punctures. Our findings coincide with those of Papaj (Reference Papaj1994) and Nufio et al. (Reference Nufio, Papaj and Alonso-Pimentel2000) who, working with other walnut infesting species (R. boycei and R. juglandis), also report the presence of multiple larvae per fruit, the result of multiple ovipositions by the same female or conspecifics. Our discoveries on the close temperature relationship between R. completa and sites where it is present expand our knowledge on the ecology of this tephritid fly, as previously pointed out by Chen et al. (Reference Chen, Opp, Berlocher and Roderick2006) in California for introduced populations of R. completa and by Filchak et al. (Reference Filchak, Roethele and Feder2000) for host races of R. pomonella. This is important, as gene–environment interactions related to the effect of local environment and host phenology can produce genetically differentiated populations adjusted to different hosts (Berlocher, Reference Berlocher1984).
As is common with invasive species (Torchin et al., Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003), we found that parasitism is currently nil in Switzerland. That is, the pest escaped from an important mortality factor, at least for a while. Previously, Feder (Reference Feder1995), working with the apple maggot, R. pomonella, documented the fact that, by shifting hosts (from the native hawthorn (Crataegus mollis (Torr. & A. Gray) Scheele) to the introduced apple (Malus domestica Borkh.)), the degree of parasitism fell from a mean of 46% to a mean of 13%. Similarly, R completa is attacked by Aganaspis alujai in its native range, while no parasitoids have ever been recorded in association with this species in California (Boyce, Reference Boyce1934; Legner & Goeden, Reference Legner and Goeden1987; Ovruski et al., Reference Ovruski, Wharton, Rull and Guillen2007).
In conclusion, we were not only able to firmly establish the current distribution of R. completa in Switzerland but gained significant insights into its biology and ecology, which will become handy when management schemes in the invaded European countries have to be designed. Additionally, our findings here highlight the rapid pace with which Rhagoletis pest species of Neartic origin can establish and expand within Europe with perhaps global warming allowing crossing of the Alps much more rapidly than it was the case some decades ago. Rhagoletis cingulata is supposed to have a similar invasive history as R. completa and currently is becoming a nuisance in sour-cherry growing regions of Germany and neighbouring countries (K. Köppler, personal communication). Considering current global warming trends and particularly the ever increasing volume of international trade, additional invasions represent a realistic potential threat that should be taken seriously (Work et al., Reference Work, McCullough, Cavey and Komsa2005). Therefore, identifying potential entry routes, climate parameters promoting or hindering expansion, potential host species, better understanding of environmental resilience to invasive species and prospecting remedial measures should become a high priority to minimize potential damage to fruit production in European countries by other potentially invasive fruit fly species.
Acknowledgements
We thank all walnut tree owners that granted us permission to collect samples throughout Switzerland and gratefully acknowledge Patrizia and Tobias Eichelberg, Christina Marazzi, Rahel and Peter Überschlag, Barbara and Steve Leib and Betty Benrey for facilitating the identification of collection sites and arranging permissions by walnut-tree owners to collect samples. We also thank Luigi Colombi (LC), Mauro Genini (MG) and Patrick Kehrli (PK) for helping us collect samples in the entire Ticino (LC), the middle and lower Valais (MG) and Nyon and Geneva (PK). We thank Neil Villard for guiding us through the Jura region and Kathrin Annaheim, Hans Ulrich Höpli and Elizabeth Razavi for expert technical support and Alberto Anzures Dadda and Suzzette Tamez-Cruz for helping us prepare the final version of this manuscript. Financial support was furnished by the Mexican Council for Science and Technology (CONACyT) through a competitive Sabbatical Year Fellowship to MA (Reference No. 79449), and a competitive Research Grant to JR (Reference No. CB 2005-25889-50008Q), by Aline Schünemann who provided a rental car to travel throughout most of Switzerland, by Agroscope Changins-Wädenswil ACW (internal funds managed by JS) and by the Instituto de Ecología, A.C. (salaries of MA and LG). MA would like to thank the Agroscope Changins-Wädenswil ACW for allowing him and his family to live in the guest house and for providing laboratory facilities and administrative support during his Sabbatical. We also thank two anonymous reviewers and the editor for constructive comments.
Appendix 1. Survey results for Rhagoletis completa in Switzerland organized according to climatic region and decreasing mean spring temperatures within region. Spring temperatures: March–May 1960–1990; winter temperatures: December–February 1961–1990.
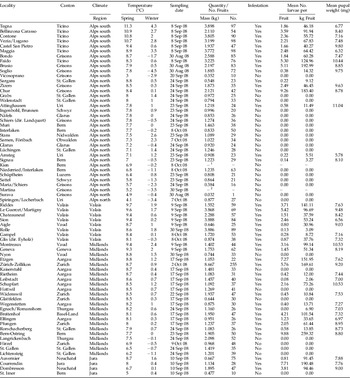
1 Sampling impossible but no obvious infestation.
2 no nut trees albeit sufficient growing conditions.
Appendix 2. Infestation patterns of Rhagoletis completa in the single-fruit experiment (five fruits per locality) listed in the same order as in table 1.











