Introduction
The host range of insect species is an important parameter in modern agriculture because significant decisions are based on this knowledge, such as the development or the release of a novel biological control agent in an area (Sheppard et al., Reference Sheppard, van Klinken and Heard2005; van Lenteren et al., Reference van Lenteren, Bale, Bigler, Hokkanen and Loomans2006). However, studies have shown extreme plasticity in the host range of insect species that can occur very slowly or in a relatively very short time frame, with changes potentially observable in human life time in the latter. The factors facilitating the host shift and subsequently the pest status have been thoroughly reviewed by Price et al. (Reference Price, Denno, Eubanks, Finke and Kaplan2011). Thus, native herbivores have been reported to shift to pest status, becoming threat to agro-ecological environments. Notable cases of pest outbreaks have been linked with climate change (Patterson et al., Reference Patterson, Westbrook, Joyce, Lingren and Rogasik1999; Giorgi & Lionello, Reference Giorgi and Lionello2008; Jepsen et al., Reference Jepsen, Hagen, Ims and Yoccoz2008; Traill et al., Reference Traill, Lim, Sodhi and Bradshaw2010; Kocsis & Hufnagel, Reference Kocsis and Hufnagel2011; Parkash et al., Reference Parkash, Ramniwas and Kajla2013) or with changes in land use patterns. For example, the pollen beetle Meligethes aeneus (Fabricius) shifted to oilseed rape after the expansion of the crop cultivation in Northern Europe. The insect adapted successfully to the new environment and rapidly developed resistance to insecticides used for control, a combination that made pest control extremely difficult in many cases (Hokkanen, Reference Hokkanen2000; Slater et al., Reference Slater, Ellis, Genay, Heimbach, Huart, Sarazin, Longhurst, Müller, Nauen, Rison and Robin2011).
Here, we present a striking case of host shift for the moth Bactra bactrana (Kennel, 1901) (Tortricidae), a typical stem borer of sedges with a widespread distribution, that is reported for the first time to infest pepper as a fruit borer. B. bactrana infestation was associated with economic loses in greenhouse crops of Crete, Greece. A comparative analysis for identification of the pest is provided and the main biological attributes are summarized.
First pest report
Location and crop
On 9 May 2014, reports of extensive damages on a greenhouse pepper crop in the area of Tympaki (Makrimaliana) were communicated to our laboratory (fig. 1). Initial observations indicated feeding activity of an unknown insect pest. An onsite inspection was immediately arranged with the local agronomists of the Tympaki Agricultural Cooperative. The damage levels were estimated based on fruit production of the particular day. Approximately 30% of the fruits were infected. A month after the initial report (10 June 2014), a second sweet pepper greenhouse was reported infested, with fruits bearing the same symptom pattern. The location was again in the area of Tympaki, however, at a different site (Anadasmos) approximately 7 km east of the initial report. The percent of infested fruit was estimated at 15% in this case.

Fig. 1. Map of Crete showing the areas that the infestations by B. bactrana were detected: No 1, first pest report at Makrimaliana, No 2, second pest report at Anadasmos. Dark square is Heraklion, where the Institute is located. Side map: Crete in relation to mainland Greece.
Damage description
Infested fruits were bearing multiple holes on the surface, sometimes surrounded by intense green discoloration (fig. 2a, b). The opening of the hole was occasionally covered by silk fibres. Within the fruit, Lepidoptera larvae were found causing extensive damage on the internal surface followed by dark discoloration (fig. 2c). In some cases, secondary infestations were observed and macroscopic symptomatology denoted bacterial infestation. No symptoms or damage was observed on any other parts of the plant vegetation. Dense numbers of moths were found hiding under the debris on the soil surface. Moths were easily detected as they were flying in response to human activity during the thorough inspection process.
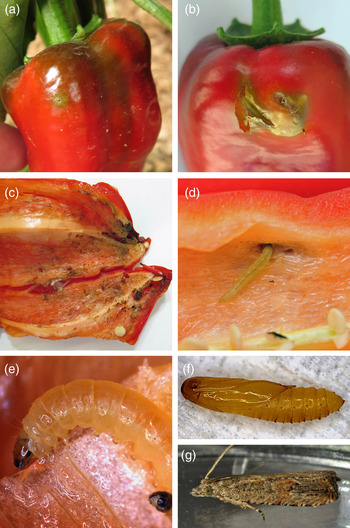
Fig. 2. Damages and general aspect of immatures and adult. (a) and (b) external surface of sweet peppers infested by B. bactrana Cracks on the dry epidermis of the fruit can also be occasionally detected as the supporting tissue underneath is damaged by the galleries created by the mining activity of the larvae; (c) and (d) internal surface of the fruit showing galleries and tissue deterioration; (e) details of larva; (f) pupa; (g) adult.
Specimen collection
Infected peppers as well as unidentified adult moths were collected for examination in the laboratory. Collected fruits were stored in an insect proof cage, under controlled conditions (16 h L: 8 h D, 25 ± 1°C) to allow larval development. Once development was completed, most larvae exited the fruit, moved under the tissue paper that was placed at the bottom of the container, and pupated within a silk cocoon. Pupae were collected, placed separately in a Petri dish and were monitored daily. Emerged adults matched the morphology and the wing colour pattern of the field collected adults.
Species identification
General dissection procedures followed standard methods for genitalia preparation for the optical microscope (Robinson, Reference Robinson1976). Tools for dissection and cleaning included spring micro-scissors, Dumont forceps (number 5) and fine model brushes (5/0) under a MZ9.5 Leica stereomicroscope in glass embryo dishes of 30 mm. Optical microscopic images were taken through a Leica Macroscope Z16 APO equipped with a digital camera DFC 500. Images were edited by Photoshop CS3 (Adobe).
Nomenclature of the taxa follows Gilligan et al. (Reference Gilligan, Baixeras, Brown and Tuck2012). General and genitalia terminology follows Horak (Reference Horak and Kristensen1999) and Klots (Reference Klots and Tuxen1970), respectively. Setal map interpretation follows Hinton (Reference Hinton1946). Five male adults, and two last instar larvae were examined. Based on the comparison with museum material and publications later discussed, the specimens were assigned to B. bactrana.
The species B. bactrana
Introduction to the genus Bactra and distributional notes
Bactra Stephens 1829 is a worldwide distributed genus of Olethreutinae including about a hundred species (Gilligan et al., Reference Gilligan, Baixeras, Brown and Tuck2012). It is a rather homogeneous monophyletic group of moths adapted to grass habitats. Their larvae are internal feeders mostly on Cyperaceae (sedges), but have been recorded also on other representatives of the order Poales as Juncaceae and Poaceae. Horak (Reference Horak2006) updated the diagnosis of the group and discussed the main relations with other genera of Olethreutini. The elongate wings with a cryptic grass habitat adapted wing pattern is a key characteristic and allows superficial identification of the adults, but, the species level taxonomy is far from obvious. The best taxonomical references on the genus are still the works by Diakonoff (Reference Diakonoff1956, Reference Diakonoff1962, Reference Diakonoff1963, Reference Diakonoff1964). Aarvik (Reference Aarvik, Karsholt, van Nieukerken and de Jong2013) included nine species of Bactra in Europe including Canary Islands and Azores. B. bactrana is distributed across Western and Southern Europe. Other Bactra species generally have broad distributions, although few are considered geographically restricted taxa. Bactra lancealana (Hübner, 1799), Bactra furfurana (Haworth, 1811) and Bactra robustana (Christoph, 1872) are the most widely distributed representatives. Complementary to Bactra lacteana Caradja, 1916, which is mostly distributed in central and northern countries, Bactra venosana (Zeller, 1847) shows similar distributions to that of B. bactrana. Bactra suedana Bengtsson (Reference Bengtsson1990) has a Nordic distribution (Bengtsson, Reference Bengtsson1990). Finally, Bactra legitima Meyrick, 1911 is an African species present in the Canary Islands and Bactra minima Meyrick, 1909 a South Asiatic representative that penetrates in the Canary Islands and Azores (Karsholt & Vieira, Reference Karsholt, Vieira, Borges, Cunha, Gabriel, Martins, Silva and Vieira2005; Aarvik, Reference Aarvik2008). Most of the records of B. bactrana are referred to its junior synonym Bactra graminivora Meyrick. Diakonoff (Reference Diakonoff1962) complained about the lack of reliable records for this species, which is systematically confused with B. lancealana. Out of continental Europe, B. bactrana records include Central Asia (India, Pakistan) and Africa (north and tropical, including Madagascar and Canary Islands). This suggests a broad distribution through the temperate regions of the Old World.
Biology – host range
Larvae of Bactra are stem borers. According to the food plant database compiled by Brown et al. (Reference Brown, Robinson and Powell2008), there are records of food plants for 19 species of Bactra. Food plants include mostly Cyperaceae of the genera Cyperus (18 records), Schoenoplectus (two), Carex (four), Bolboschoenus (two), Eriophorum (three), Scirpus (five), Cladium (one) and Kyllinga (five). Few records make reference to Poaceae (Cynodon, two records) and Juncaceae (Juncus, nine records). Trematerra & Ciampolini (Reference Trematerra and Ciampolini1989) updated all current biological knowledge, including food plants and life cycle, with special reference to the European species. According to these authors, B. bactrana is a bivoltine species overwintering as larvae in the stem of the plant.
Diagnosis of B. bactrana infestation
Identification of B. bactrana adults and its allies
Figures 2g and 3a show the general appearance of the adult of B. bactrana. Wing pattern of Bactra are cryptically variable and of difficult interpretation. No specific identification should be taken based on wing pattern. The shape and design of the forewing is characteristic of the genus. The forewing is elongate, with pointed apex. General colour is light to dark brown with greyish suffusion. As in most Olethreutinae, forewing upperside transversal elements appear degenerated. Forewing pattern includes typical strigulae variably developed on the costa. Scales on discal cell and from discal cell to apex tend to be variably darker giving a longitudinally striated general aspect to the forewing. Male genitalia examination (fig. 3b) is important for identification and the only reliable morphological way for identification of Bactra species. In all Bactra, the tegumen is much more developed than the vinculum. The tegumen may be rather globose, incurved, with the uncus-socii strongly connected. Preparing the tegumen separately (fig. 3c) increases the diagnostic power of this character. The vinculum is strongly connected to the juxta and phallus. The uncus usually is bent and edged by rod like spines. The sacculus is globular with an internal sac opened obliquely. There are typically 7–8 spines in the spc1 of B. bactrana. Additional short spines (or bristles) may appear at the posterior edge of the sacculus (Ms series; medio-saccular series of Diakonoff, Reference Diakonoff1962); their diagnostic value is more reduced. The cucullus is elongate in B. bactrana with the costa slightly sinuous, moderately pointed in the apex; the ventral edge is curved, somewhat quadrangular at the distal third, covered by hairs and bristles to the edge of the valva.

Fig. 3. Adult identification. (a) B. bactrana (from Greece); (b) male genitalia; (c) tegumen. Scale bars a = 2 mm; b and c = 200 µm.
Larval description of B. bactrana and notes
In spite of a relatively well-known biology in some species of Bactra, little detailed information on the larval identification is available. The first data on the larvae of B. bactrana may have been collected by Fletcher (Reference Fletcher1932) but the author is unclear about the true identity of the larvae examined. Swatschek (Reference Swatschek1958), based on B. lancealana, and McKay (Reference McKay1959), based presumably on B. verutana, discussed the characteristics of the genus. Figure 4 includes a setal map of the B. bactrana larvae examined. Typical olethreutine character is the presence of a L2 closer to L1 than to L3 on a trisetose group in T1. However, SD1 is closer to SD2 than to XD2 where McKay (Reference McKay1959) finds them equidistant. On A7 the group SV is trisetose in B. bactrana and B. lancealana, but bisetose in B. verutana. A8 and A9 coincident in the three species with a common pinacula for D1 (anterior) and SD1 (posterior).

Fig. 4. Larval chaetotaxy. (a) Thorax and abdomen; (b) head frontal view; (c) head left side. Scale bars = 0.5 mm.
The economic importance of B. bactrana globally
Zhang (Reference Zhang1994) compiled records of eight economically important species of Bactra in Asia (B. bactrana, Bactra commensalis, B. minima, Bactra phaeopis and B. venosana), Europe (B. furfurana and B. lancealana) and North America (B. verutana). However, the consideration of economic importance requires consideration in relation to a geographical perspective. Some species of sedges (e.g., Cyperus rotundus) are among the most invasive species of plants, and information available on their negative effects is considerable (Riemens et al., Reference Riemens, van der Weide and Runia2008). Their distribution has been facilitated by human activity and there is no doubt that some species of Bactra have followed their distribution. Their narrow range of food plant hosts has made them adequate candidates for weed control. A good example is provided by the introduction into the Hawaiian Islands (from the Philippines) of B. venosana. The presence of this nutgrass natural enemy in Hawaii was confirmed by Swezey (Reference Swezey1926) and was the subject of interesting research (Poinar, Reference Poinar1964a , Reference Poinar b ). The series of papers by Frick and collaborators (Frick & Garcia, Reference Frick and Garcia1975; Frick, Reference Frick1978; Frick & Wilson, Reference Frick and Wilson1980; Frick, Reference Frick1982) are also informative. However, Cyperus species (especially Cyperus esculentus) are commercially beneficial plants in some areas of the world, either as food (Sánchez-Zapata et al., Reference Sánchez-Zapata, Fernández-López and Pérez-Alvarez2012) or as biofuel (Zhang et al., Reference Zhang, Hama, Ali and Nan1996). Consequently, Bactra species may be considered as pests wherever nut-grass is considered as a crop (Tsung-Kai, Reference Tsung-Kai1978).
Current status and pest management
In this study, we found that B. bactrana can cause significant damages to commercial scale greenhouse crops and can therefore be considered as a potential future pest of sweet peppers. There are very few reports on B. bactrana as a pest and therefore the potency of the species and its damage capacity are basically unknown. To date, there are no additional reports of infestations by B. bactrana in the Tympaki region of Crete. However it is not until the end of the next cropping season that we will have a clear perspective on the level of expansion of the infestations. Unfortunately, valid predictions on infestation expansion at this stage are currently impossible due to absence of necessary information. Basic studies on the biology of B. bactrana may be required in order to estimate the risks from this novel pest of sweet peppers.
Based on the records of the Tympaki Agricultural Cooperative, once the infestation was detected it was immediately controlled with insecticide. The first greenhouse received three applications of the insecticides indoxacarb and chlorantraniliprole. The second greenhouse received one application of spinosad, lufenuron and beta-cyfluthrin. All these insecticides are registered for Lepidoptera control in greenhouse sweet peppers. Pest control treatments were ongoing until acceptable damage levels were obtained. Efficacy of application has not been evaluated. However, based on preliminarily laboratory observation, the vitality of the larvae within the fruit was not affected after insecticide application. In all cases, larvae that were extracted from sprayed fruits responded normally to stimulus (disturbance with a fine paint brush) and completed development under laboratory conditions. This suggests that pest chemical control should focus mainly on the first instar larvae before entering the fruit and on the eggs laid on the plant surface. Beneficials preying on Lepidoptera eggs or egg parasitic hymenoptera may also play a key role in a revised integrated pest management (IPM) scheme. It has been demonstrated that under field conditions, natural parasitism by the egg paraitoid Trichogrammatoidea bactrae Nagaraja can successfully restrict the moth populations (Visalakshy & Jayanth, Reference Visalakshy and Jayanth1995). Control of the pest could also be achieved by targeting the adults as they come in contact with the treated plant foliage during oviposition. However, insecticides with adulticide activity should be identified and this may not be easy, since registered novel chemistries are in most cases efficient but exclusive larvicides (Roditakis et al., Reference Roditakis, Stavrakaki, Bassi and Rison2014). Monitoring and eventually mating disruption by pheromone traps is another possible future step (Booij & Voerman, Reference Booij and Voerman1984). Based on laboratory observations, pupation is expected to occur in the soil or under protected niches. Potentially, soil inhabiting beneficials (i.e., entomopathogenic nematodes) may be an important parameter in a successful pest control scheme.
Conclusion
This is the first report of B. bactrana developing as fruit borer on a solanaceous host, such as Capsicum annuum. Based on the observed damage levels in a commercial scale crop, it is suggested that B. bactrana can be considered as a potential pest for sweet pepper. However, the most striking finding in this study is the rare and notable shift in host range. This finding is even more important if one considers that other species of this genus, such as Bactra verutana Zeller, have been used in augmentative releases to facilitate biological weed control (Frick & Chandler, Reference Frick and Chandler1978; Prick et al., Reference Prick, Hartley and King1983). The value of biological control has been demonstrated in numerous studies (van Lenteren & Woets, Reference van Lenteren and Woets1988; Cruttwell McFadyen, Reference Cruttwell McFadyen1998; Thomas & Reid, Reference Thomas and Reid2007; Bale et al., Reference Bale, Van Lenteren and Bigler2008; Naranjo et al., Reference Naranjo, Ellsworth and Frisvold2015). However, there are some limitations and risks involved when releasing natural enemies (Collier & Van Steenwyk, Reference Collier and Van Steenwyk2004; Andersen et al., Reference Andersen, Ewald and Northcott2005; Delfosse, Reference Delfosse2005; Barratt et al., Reference Barratt, Howarth, Withers, Kean and Ridley2010; Simberloff, Reference Simberloff2012). Delfosse (Reference Delfosse2005) analyzed the risks of biological control and listed factors that are linked to uncertainties with respect to hazards that can result from the release of natural enemies. One of the least investigated parameters is the adaptation of the biocontrol agent population to the new environment (Hufbauer & Roderick, Reference Hufbauer and Roderick2005; Phillips et al., Reference Phillips, Baird, Iline, McNeill, Proffitt, Goldson and Kean2008). Several cases of rapid adaptive evolution for biocontrol agents have been reported (Bean et al., Reference Bean, Dalin and Dudley2012; Szűcs et al., Reference Szűcs, Schaffner, Price and Schwarzländer2012) and such changes in key life parameters have resulted in significant adverse effects on non-target organisms, that could not be predicted prior to the release of the organism (Louda et al., Reference Louda, Pemberton, Johnson and Follett2003). For example, the weevil Rhinocyllus conicus Froeh exhibited both geographical and host range expansion significantly effecting native thistles and subsequently the density of native tephritid flies (Louda et al., Reference Louda, Kendall, Connor and Simberloff1997). Recently, Lu et al. (Reference Lu, Siemann, He, Wei, Shao and Ding2015) demonstrated that additional factors, such as the climate change, are forcing biocontrol agents to evolve. Global warming is driving shifts in phenology and geographical range of species, influencing the effect of biocontrol agents on non-targets (Simberloff, Reference Simberloff2012).
Our study is a striking example of the unexpected risks involved in the release of insect biocontrol agents. It is not clear why and how this transition from a stem feeder to a fruit borer occurred. Several factors facilitating host plant shifts have been summarized by Price et al. (Reference Price, Denno, Eubanks, Finke and Kaplan2011). Unfortunately, with the current level of knowledge, it is not possible to identify the causes of this phenomenon. Most of all, we are unaware of a report on the presence of B. bactrana in Crete prior to this report. A recent invasion to the island could be an explanation. However, based on the published data of the species distribution, B. bactrana has been previously reported in areas where the cultivation of sweet pepper under glass is extensive for at least a decade (i.e., Southern Europe such as Spain and Italy). This suggests that a number of factors coexisted in the area of Tympaki that eventually enabled this rare host shift. It will be important to identify the factors that drive the host shift, thus potentially enabling prediction of potential damage prior to its occurrence or efficient screening of biocontrol agents for potential non-target effects.
Acknowledgements
The authors would like to thank the agronomists Mr K. Nikoloudis (Tympaki Agricultural Cooperative) and Mr J. Sklivakis (Biotopio) for their assistance in the infestation detection and monitoring as well as Dr N. Roditakis (NAGREF) and Dr O. Karsholt (NHM, Denmark) for their guidance during the identification process.







