Introduction
The tomato leaf miner Tuta absoluta (Povolny 1994) (Lepidoptera: Gelechiidae) is one of the most devastating pests for tomato crops (Solanum lycopersicum). Native in South America, it was first detected in Europe (Spain) in 2006 (Urbaneja et al., Reference Urbaneja, Vercher, Navarro, Garcıia and Porcuna2007), before spreading throughout the Mediterranean basin countries where it reached high damaging levels to tomato cultivations (Potting, Reference Potting2009). This pest causes damage by feeding on the leaves, fruits, flowers, and stems, producing mines and galleries that reduces the photosynthetic capacity of the plant and severely degrades the crop commercial values (Desneux et al., Reference Desneux, Wajnberg, Wyckhuys, Burgio, Arpaia and Narv́aez-Vasquez2010, Reference Desneux, Luna, Guillemaud and Urbaneja2011). In addition to the tomato, T. absoluta has also been found to attack other cultivated and wild Solanaceae, such as pepper, eggplant, tobacco, potato, black nightshade, and the garden bean (Ferracini et al., Reference Ferracini, Ingegno, Navone, Ferrari, Mosti, Tavella and Alma2012). The most common control strategy against T. absoluta in South America countries has been the application of chemical insecticides. Yet, their intensive usage have led to severe and widespread resistance cases that now affects many insect populations (Siqueira et al., Reference Siqueira, Guedes and Picanco2000; Lietti et al., Reference Lietti, Botto and Alzogaray2005; Haddi et al., Reference Haddi, Berger, Bielza, Cifuentes, Field, Gorman, Rapisarda, Williamson and Bass2012). For this reason, various biological control agents have been investigated to be used in combination with chemical control, such as the predators (Nesidiocoris tenuis, Podisus nigrispinus), the egg parasitoids (Trichogramma pretosium), and the entomopathogens (Beauveria bassiana, Bacillus thuringiensis) (Giustolin et al., Reference Giustolin, Vendramim, Alves and Vieira2001; Rodriguez et al., Reference Rodriguez, Gerding and France2006; Mollá et al., Reference Mollá, González-Cabrera and Urbaneja2011; Sellami et al., Reference Sellami, Zghal, Cherif, Zalila-Kolsi, Jaoua and Jamoussi2013).
The lepidopteran larval gut contains various enzymes involved in food digestion such as proteases and β-glucosidases. Particularly, β-glucosidases act as exoenzymes which hydrolyse the terminal non-reducing residues in β-D-glucosides from a variety of β-D-glycoside substrates (di and/or oligosaccharides) with the release of glucose (Terra &Ferreira, Reference Terra and Ferreira1994). These enzymes are a powerful tool for degradation of plant cell walls and play an important role in the hydrolysis of cellulose and hemicelluloses, largely composed of polymers of β-bond linked glucose molecules, and in the cleavage of the carbohydrate moieties of glycoproteins present in insect food. Plants produce secondary metabolites like glucosides that are converted into toxic aglycones in insects’ midgut by the activity of digestive enzymes such as glucosidases and are thereby defended against herbivore attacks (Wei et al., Reference Wei, Semel, Bravdo, Czosnek and Shoseyov2007). In order to prevent this phenomenon, different strategies were adopted by insects like the reduction of β-glucosidases activity and thus glucosides hydrolysis at the highly alkaline pH in the larval midgut lumen, sequestration of glycosides, and instability of the released aglycones (Morant et al., Reference Morant, Jorgensen, Jorgensen, Paquette, Sánchez-Pérez, Moller and Bak2008; Dobler et al., Reference Dobler, Petschenka and Pankoke2011). Furthermore, larval gut environment could contain various families of proteases, among them are serine proteases (trypsins, chymotrypsins, and elastases), Cysteine proteases, Aspartic proteases, aminopeptidases, and carboxypeptidases (Srinivasan et al., Reference Srinivasan, Giri and Gupta2006). Trypsins and chymotrypsins are known to dominate the lepidopteran larval gut environment and contribute up to 95% of the total digestive activity (Patankar et al., Reference Patankar, Giri, Harsulkar, Sainani, Deshpande, Ranjekar and Gupta2001). In addition, T. absoluta larval gut proteases activated B. thuringiensis Cry protoxins, so the active Cry toxins (65 kDa) bound to specific receptors on the Bruch Border Membrane Vesicules (BBMV) of T. absoluta midgut, which results in cell lyses (Bravo et al., Reference Bravo, Gill, Soberón, Gilbert, Iatrou and Gill2005; Jamoussi et al., Reference Jamoussi, Sellami, Nasfi, Krichen-Makni and Tounsi2013). Dammak et al. (Reference Dammak, Ben Khedher, Boukedi, Chaib, Laarif and Tounsi2016) demonstrated that Cry1Aa toxin binds to six proteins in the BBMV of T. absoluta having molecular sizes of 150, 80, 65, 44, 30, and 20 kDa. It was also demonstrated that a lipopeptide biosurfactant extracted from Bacillus amyloliquefaciens was toxic against T. absoluta larvae via its recognition of five putative receptors located in the BBMV of T. absoluta with sizes of 68, 63, 44, 30, and 19 kDa (Ben khedher et al., Reference Ben Khedher, Boukedi, Kilani-Feki, Chaib, Laarif, Abdelkefi-Mesrati and Tounsi2015).
The aim of this study was the investigation of T. absoluta larvae digestive physiology by the characterization of gut β-glucosidase and proteases enzymes. Effects of D-glucono-δ-lactone and soybean trypsin inhibitors (SBTI) on T. absoluta were studied as they could prevent digestion and assimilation of nutrients in the larvae gut causing death by starvation.
Materials and methods
Preparation of the gut extracts proteins from T. absoluta larvae
The development of the T. absoluta larva needs four instars to reach the chrysalides. Early-fourth-instar larvae growing at 26 ± 2°C were collected from tomato leaves, chilled on ice for 30 min, and dissected to isolate the guts. Each ten guts were homogenized in water and samples were centrifuged at 15,000 × g for 15 min at 4°C. The recovered supernatant contained the gut extract enzymes. On the other hand, gut tissues obtained in the pellet were used in β-glucosidase analyses as described by Marana et al., Reference Marana, Terra and Ferreira2000. The protein content was determined by using Bio-Rad Protein Assay (München, Germany) and bovine serum albumin as standard protein according to the method of Bradford (Bradford, Reference Bradford1976).
Effect of pH and temperature on β-glucosidase activity
β-glucosidase activity was monitored by the release of p-nitrophenol (p-NP) from the substrate para-nitrophenyl-β-D-glucopyranoside (p-NPG; ACROS/ORGANICS). A reaction mixture (500 µl) consisting of a volume of 300 µl comprising the gut extract enzymes (5 µl) diluted in 100 mM sodium acetate, and 200 µl of 1 mM p-NPG solution, was incubated for 15 min under the optimal temperature. The control contained all the reactants except the enzymatic preparation. The reaction was stopped by the addition of 600 µl of 400 mM Glycine-NaOH buffer, pH 10.8, and the absorbance was measured at 400 nm. One unit of the enzymatic activity was determined as the amount of enzyme required to release 1 µmol of pNP per minute under the assay conditions (Saibi et al., Reference Saibi, Amouri and Gargouri2007). Firstly, the effect of pH on β-glucosidase activity was examined at 37°C in pH range of about 3–5 and 6–8 by using sodium acetate and Tris-HCl buffers, respectively. Secondly, the effect of the temperature on β-glucosidase activity was determined in 100 mM acetate buffer (pH 5) over a temperature range of 10–80 °C (Saibi et al., Reference Saibi, Amouri and Gargouri2007).
β-glucosidase zymogram analysis
β-glucosidase activity was revealed by zymogram analysis via native-polyacrylamide gel electrophoresis (PAGE) using BioRad Mini-PROTEAN Tetra Cell Apparatus (Laemmli & Favre, Reference Laemmli and Favre1973). After electrophoresis of gut extract proteins (100 and 200 µg), the gel was incubated firstly for 15 min in 100 mM sodium acetate buffer pH 5 and then in presence of 3 mM MUGlc (methylumbellypheryl-β-D-glucoside, BioBasic Canada Inc) for 10 min at 30°C. The enzymatic reaction was revealed by exposing the gel to ultraviolet light (366 nm). The fluorescence (445 nm) emitted by the released methylumbelliferol indicates the presence of β-glucosidase activity on the gel (Davis, Reference Davis1964; Saibi et al., Reference Saibi, Amouri and Gargouri2007).
Proteolytic activity and effect of temperature and pH
A volume of 7 µl of gut extract proteins were incubated with 500 µl of 1% casein (Accumix) in 50 mM glycine-NaOH buffer (pH 11.0), for 30 min at 40°C. The proteolytic reaction was stopped by adding 500 µl of 20% trichloroacetic acid (TCA, PROLABO) and the samples were incubated for 15 min at room temperature. After centrifugation for 15 min at 15,000 × g, absorbance of supernatants was measured at 280 nm by comparing with a control sample incubated without proteases (Kembhavi et al., Reference Kembhavi, Kulharni and Pant1993). Total proteolytic activity was firstly tested at six different temperatures (from 20 to 70 °C) using the Na2CO3 buffer (pH 9.6). After that, different pH values (6.0–12.0) were tested at 40°C using phosphate (50 mM; pH: 6.0, 7.0), Tris-HCl (50 mM; pH: 8.0), and glycine-NaOH (50 mM; pH: 9.0, 10.0, 11.0, 12.0) buffers.
Trypsin activity was assayed by the method of Benjakul et al. (Reference Benjakul, Visessanguan and Thummaratwasik2000) with a slight modification using 200 µl of diluted gut extract proteins added to 1000 µl of 0.5 mM BApNA (N-α-benzoyl-L-arginine p-nitroanilide, Sigma Chemical Co. (USA)) in 50 mM glycine-NaOH buffer (pH 11.0). Chymotrypsin activity was determined by adding 10 µl of the gut extract proteins to 990 µl of 100 mM SApNA substrate solution (N-Succinyl-L-Ala-L-Ala-L-Pro-L-Phe-p-nitroanilide, Sigma Chemical Co. (USA)) (Del Mar et al., Reference Del Mar, Brodrick, Geokas and Largman1979). The mixtures were incubated for 15 min at 40°C. The mean values and the standard deviations of the enzymatic activity corresponded to three independent experiments.
Zymogram analysis of the protease activity
Gut extract proteins of T. absoluta larvae (100 µg) were mixed with Laemmli sample buffer but without boiling denaturation, separated by 13% Tris-glycine sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and processed for zymogram analysis. The gel was washed three times with 2.5% (v/v) Triton X-100 and one time with distilled water. Then, it was incubated in substrate solution (casein 20 mg ml−1 or Cry protoxins 10 mg ml−1) in 50 mM glycine-NaOH buffer (pH 11) with gentle agitation, for 2 h at 37°C (Oppert et al., Reference Oppert, Kramer, Johnson, Upton and McGaughey1996, Reference Oppert, Kramer, Beeman, Johnson and McGaughey1997; Budatha et al., Reference Budatha, Meur and Dutta-Gupta2008). The gels were stained in (50% (v/v) ethanol, 10% (v/v) acetic acid, and 0.25% Coomassie Brilliant Blue R-250) solution and then destained with (5% (v/v) ethanol in 7% (v/v) acetic acid) solution. Clear bands of protease activities were visible against a dark background.
Protease inhibition assays
Inhibition of the T. absoluta gut extract proteases was assayed by using the protease inhibitors phenylmethylsulfonyl fluoride (PMSF, 5 mM, Biochemica), ethylene diamine tetra acetic acid (EDTA, 5 mM, Fluka), ethylene glycol tetra acetic acid (EGTA, 5 mM, Boehringer Mannheim GmbH), SBTI (1 mg ml−1, Thermofisher Scientific), Leupeptin (50 µg ml−1, Biochemica (AppliChem)), Pepstatin (1 µg ml−1, Biochemica (AppliChem)), iodoacetamid (5 mM, Amersham), N-α-tosyl-L-phenylalanine chloromethyl ketone (TPCK, 5 mM, Sigma-Aldrich), and N-Ethylmaleimide (NEM, 5 mM, Amersham).
The in vitro inhibition assays were carried out in 50 mM glycine-NaOH buffer, pH 11.0, where the gut extract proteases (10 µL) and each inhibitor were pre-incubated in the buffer for 30 min at 30°C prior to the addition of the casein substrate. The experiment was conducted in triplicate.
The in gel inhibition assays were carried out by SDS-PAGE (13%) separation of the gut extract proteins (100 µg), followed by the incubation of the gel in 50 mM glycine-NaOH buffer (pH 11.0) supplemented with a protease inhibitor (PMSF 5 mM, SBTI 1 mg ml−1, or TPCK 5 mM) for 30 min at 30°C. After that, the gel was incubated in 50 mM glycine-NaOH buffer (pH 11.0) containing casein substrate (20 mg ml−1) for 2 h at 37°C with gentle agitation (Wagner et al., Reference Wagner, Mohrlen and Schnetter2002).
Effect of protease inhibitors on the δ-endotoxins activation by gut extract proteases
The δ-endotoxins (Cry1 (130 kDa) and Cry2 (65–70 kDa) protoxins) were isolated from a sporulating culture of Bacilus thuringiensis kurstaki reference strain HD1 in the liquid medium described by Zouari et al., (Reference Zouari, Dhouib, Ellouz and Jaoua1997) where the CaCO3 was added for pH stabilization in order to produce the parasporal crystals during the growth. Flasks containing 50 ml of culture medium were incubated for 72 h at 30°C and 200 rpm in a rotary shaker. Then, digestion of HD1 protoxins (20 µg) were conducted with different quantities of gut extract proteins (20, 2, 0.4, 0.1, 0.01 µg) in presence of PMSF (5 mM), Benzamidine (5 mM), or SBTI (1 mg ml−1) protease inhibitors for 30 min at 30°C using the Na2CO3 buffer (pH 9.6). Protoxins without proteases and inhibitors as well as protoxins with each inhibitor were used as control.
Insect bioassay
A solution of either the β-glucosidase inhibitor D-glucono-δ-lactone (De Melo et al., Reference De Melo, da Silveira and Carvalho2006) or the trypsin inhibitor SBTI (Zhou et al., Reference Zhou, Liu and Tsou1989) was displayed until dry on tomato leaf surfaces at concentrations of 7.1 or 10 mg cm−2, respectively. One tomato leaf and ten T. absoluta third instar larvae were placed in Petri plate and then incubated in the insect culture room at 26 ± 2°C and under a photoperiod of about 14 h light/10 h dark during 72 h. The experiment condition was repeated three times and the mortality percentage was calculated in R. (R Core Team, 2015).
Results
β-glucosidase activity from T. absoluta larvae
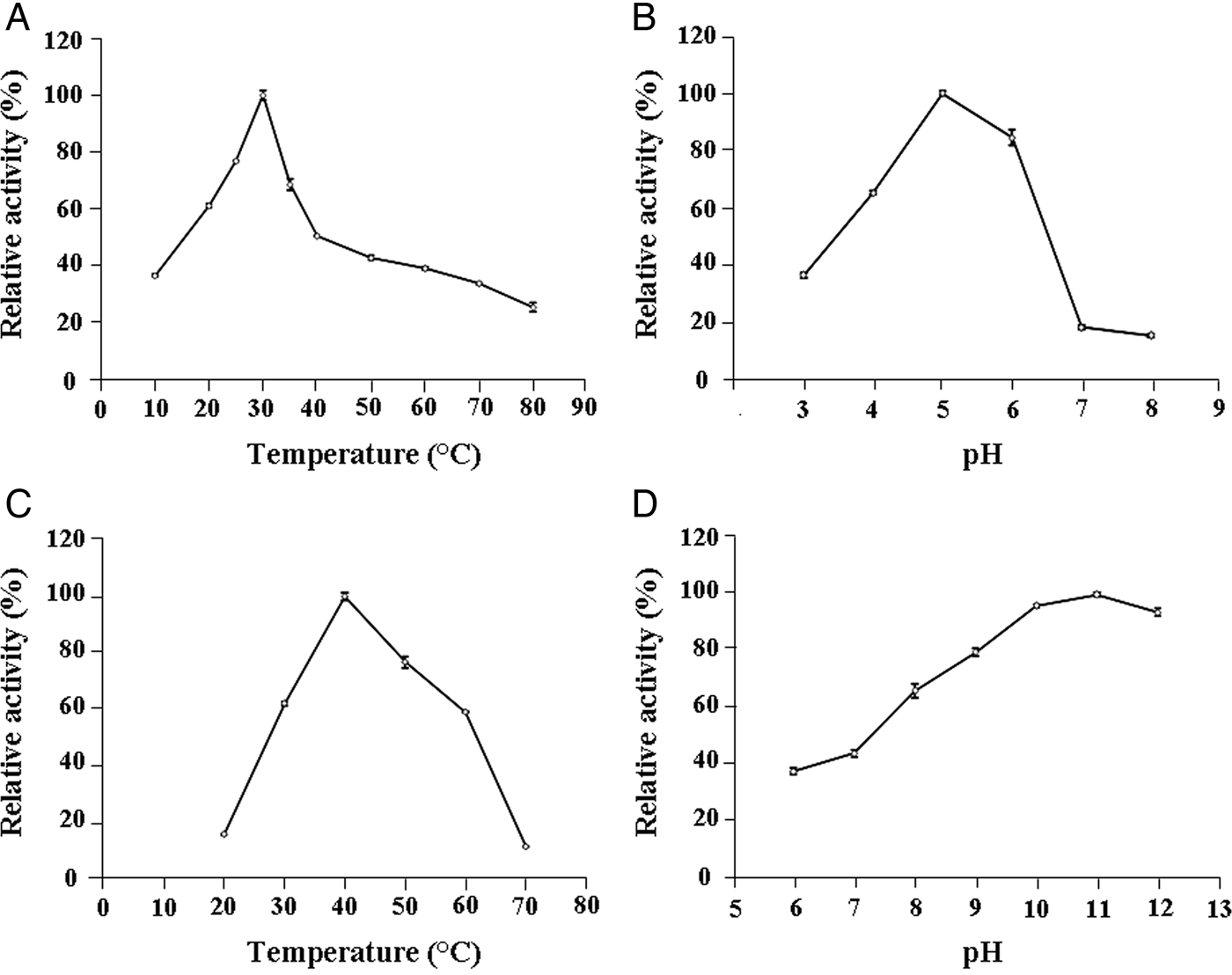
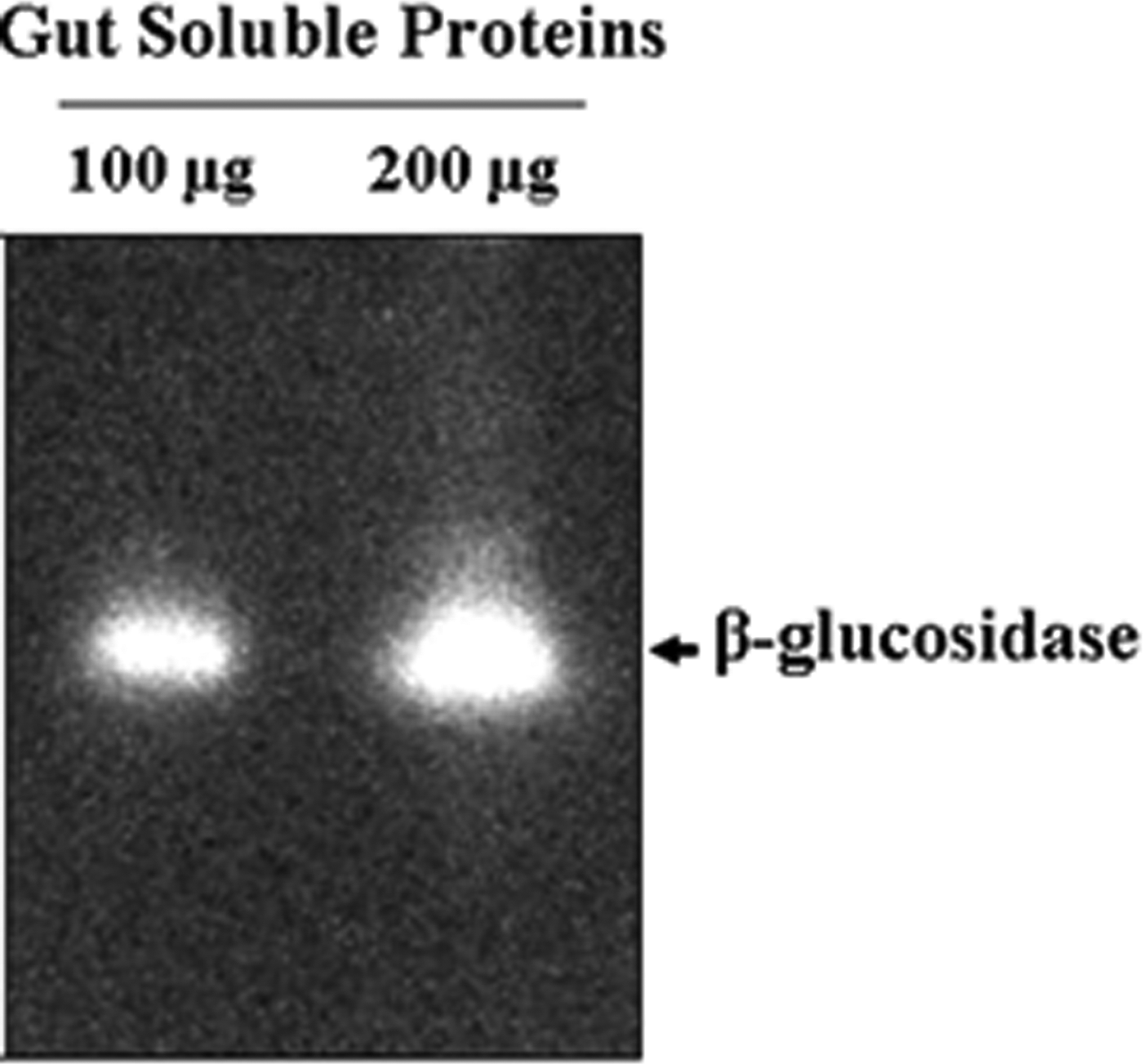
The optimal β-glucosidase activity was accomplished at around 30°C and a progressive decline reaching 50% of residual activity was detected at 40°C (fig. 1A). Additionally, pH variation of the enzymatic reaction showed an optimal activity at pH 5 and a drastically decrease to 17% of residual activity at pH 7 (fig. 1B). β-glucosidase activity recorded at optimal conditions of pH 5 and 30°C was 3.67 ± 0.06 U ml−1 which correspond to a specific activity of about 183 ± 15 U mg−1 related to gut extract proteins. Moreover, zymogram analysis showed a clear band corresponding to an important β-glucosidase enzyme activity (fig. 2).

Fig. 1. Effects of temperature and pH on T. absoluta β-glucosidase activity (A, B) and proteolytic activity using casein as substrate (C, D). Each data point is a mean of three experiments; error bars depict a standard deviation of the mean values.

Fig. 2. Zymogram analysis of T. absoluta larvae β-glucosidase. The gut extract proteins (100 and 200 µg) were separated in native PAGE and the β-glucosidase activity was revealed using 3 mM of MUGlc substrate.
Effects of temperature and pH on the gut extract proteases
The total proteolytic activity performed in the Na2CO3 buffer (pH 9.6) revealed an optimal temperature of about 40°C (fig. 1C). Beyond this temperature, the residual activity declined reaching around 11% at 70°C. Additionally, examination of the pH effect using buffers with pH ranging from 6.0 to 12.0 and a temperature of 40°C showed that the highest casein hydrolysis occurred at a range of pH 10.0–11.0 (fig. 1D).
In vitro and in gel protease inhibition assays and trypsin and chymotrypsin protease activities
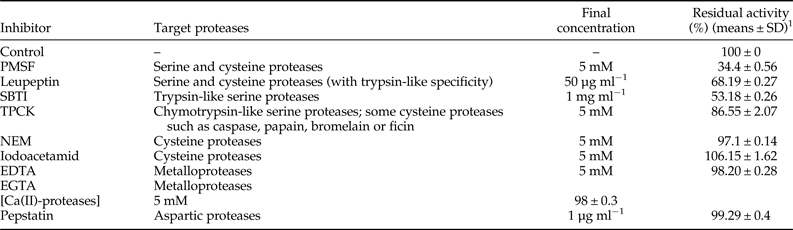
By comparing with the control without any protease inhibitor, we showed that the highest inhibition effect was obtained using the PMSF, a common serine and cysteine proteases inhibitor, since we detected 34.4% of residual activity. A residual activity of 68.19% was detected using the leupeptin as serine and cysteine proteases inhibitor with trypsin-like specificity. In addition, residual protease activity was assessed 53.18% using the SBTI, a trypsin-like serine proteases inhibitor. A residual activity of 86.55% was observed using the TPCK, a chymotrypsin-like inhibitor. Furthermore, Cysteine proteases were not detected since the proteolytic activity was similar to the control using the NEM or the iodoacetamid inhibitors. EDTA or EGTA chelating agents and the Pepstatin did not cause significant proteases inhibition, suggesting that hardly any of the metalloproteases and the aspartic proteases classes were present. These results are highly suggestive that serine proteases are the primary proteases in T. absoluta larval gut (Table 1).
Table 1. Inhibitors effects on gut extract proteases from T. absoluta fourth instar larvae tested with casein.

1 Total proteases activity measured in the absence of any inhibitor was taken as 100%. The remaining protease activity was measured after pre-incubation of the midgut proteases with each inhibitor at 30°C for 30 min. Values are the means ± standard deviation from three independent experiments.
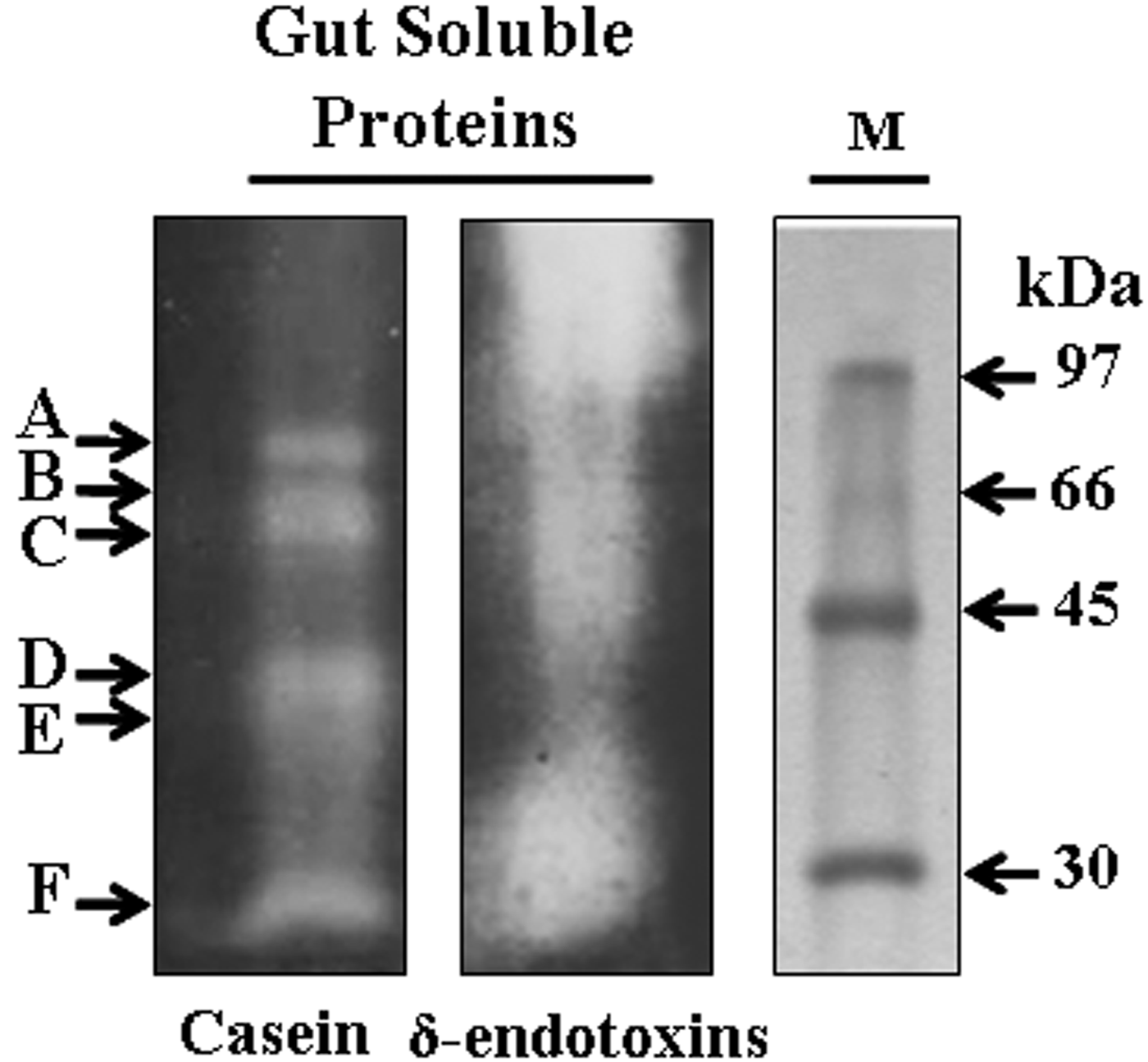
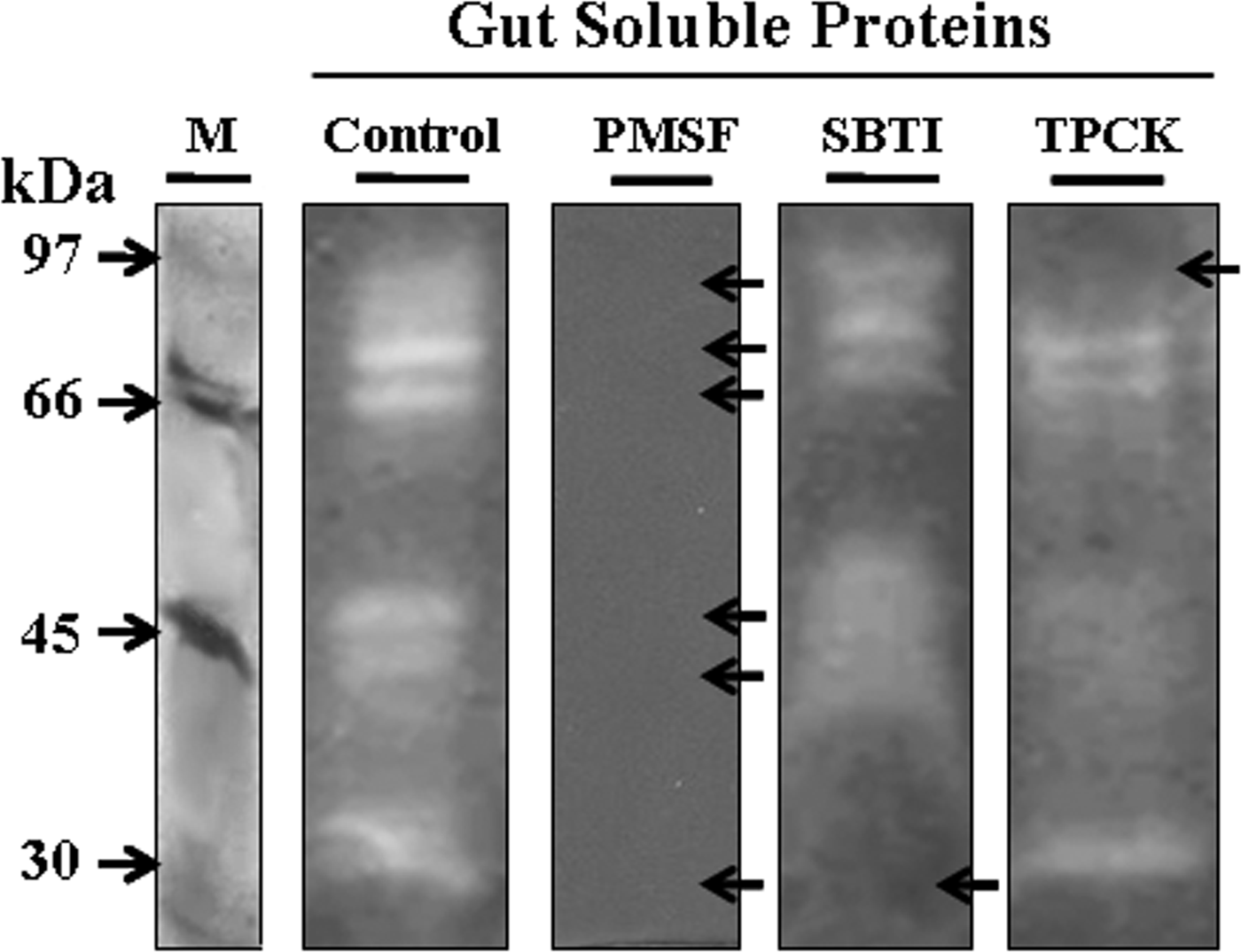
Specificities of the proteases were further determined by zymogram analysis using gut extract proteases previously incubated with specific inhibitors. It revealed at least six proteases by casein hydrolysis corresponding to A, B, C, D, E, and F bands with molecular weights of approximately 73, 66, 55, 40, 35, and 28 kDa, respectively. Furthermore, when using B. thuringiensis (strain HD1) δ-endotoxins as susceptible protoxins, the zymogram analysis revealed the major role of the A, B, C, and F proteases in δ-endotoxins activation but the D and E proteases do not clearly contribute in Cry proteolysis (fig. 3).

Fig. 3. Zymogram analysis of T. absoluta larvae gut proteases. Gut extract proteins (100 µg) were separated in SDS-PAGE and revealed using casein (20 mg ml−1) or Cry protoxins (10 mg ml−1) as substrates. M: low molecular weight (Amersham).
Additionally, fig. 4 demonstrated that PMSF inhibited the activity of almost all proteases at 5 mM concentration, indicating that the proteases revealed by the zymogram belonged to serine and/or cycteine protease classes. Interestingly, the SBTI and TPCK inhibited the 28 and 73 kDa proteases at 1 mg ml−1 and 5 mM which could indicate the presence of trypsin-like and chymotrypsin-like serine proteases, respectively. The proteases corresponding to the bands of 66, 55, 40, and 35 kDa were unaffected by SBTI or by TPCK, but their inhibition by PMSF is highly suggestive that they are serine proteases.

Fig. 4. Zymogram analysis of inhibition of T. absoluta larvae gut proteases by protease inhibitors. Gut extract proteins (100 µg) were incubated with an inhibitor (PMSF 5 mM, SBTI 1 mg ml−1, or TPCK 5 mM) or without inhibitor (Control) and then separated in SDS-PAGE. Protease activities were revealed using the casein. M: low molecular weight (Amersham).
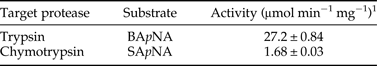
Moreover, trypsin and chymotrypsin activities present in the gut extract proteins were estimated at 27.2 ± 0.84 and 1.68 ± 0.03 µmol min−1 mg−1 using the specific synthetic substrates BApNA and SApNA, respectively (Table 2).
Table 2. Trypsin and chymotrypsin activities in gut soluble proteins of T. absoluta fourth instar larvae.
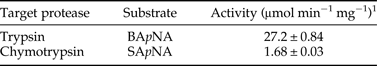
1 Gut soluble proteins were incubated with synthetic serine protease substrates at 40°C for 15 min. Activity values were the mean ± standard deviation from three independent experiments.
Proteases involved in B. thuringiensis δ-endotoxins proteolysis
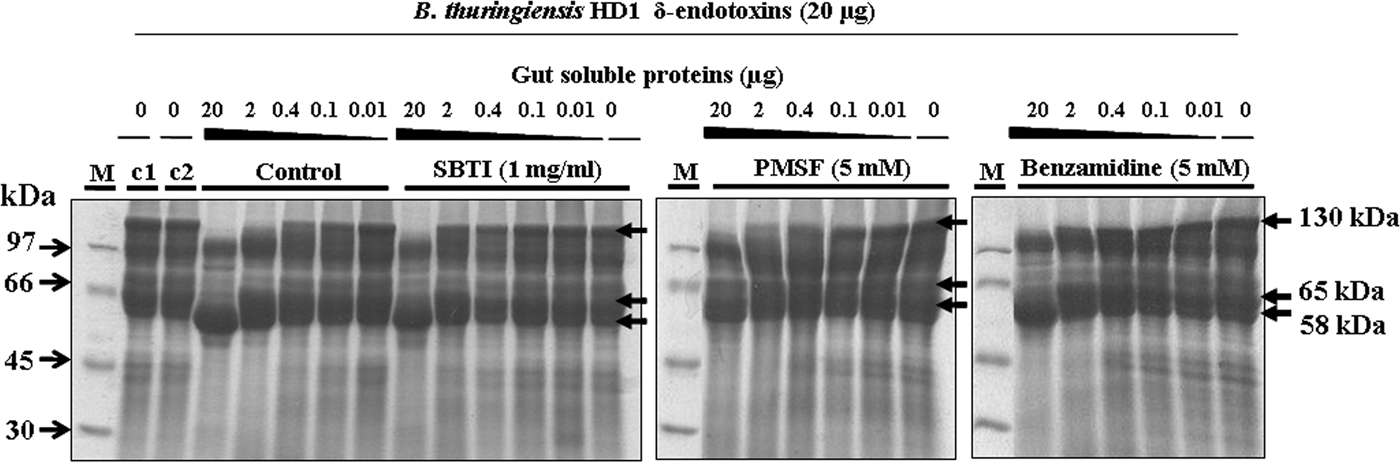
Activation of the Cry protoxins from B. thuringiensis kurstaki HD1 was tested by incubating the Cry (20 µg) with the gut extract proteins (20, 2, 0.4, 0.1, or 0.01 µg) and the PMSF (5 mM), the SBTI (1 mg ml−1), or the Benzamidine (5 mM) inhibitors. The SDS-PAGE analysis showed that, without protease inhibitors, the 130 kDa band intensity corresponding to the Cry1 protoxins decreased progressively by applying raised gut extract proteins of 0.01, 0.1, and 0.4 µg, and disappeared when using 2 and 20 µg, while the activated 65 kDa toxin appeared progressively. Besides, we noted the progressive proteolysis of the 65 kDa toxin into a 58 kDa resistant core, particularly with 2 and 20 µg gut extract proteins. Addition of PMSF, SBTI, or Benzamidine protease inhibitors partially inhibited the activation of Cry1 protoxins as the residual 130 kDa protoxins was higher than that without the inhibitors, notably with 2, 0.4, and 0.1 µg of gut extract proteins (fig. 5).

Fig. 5. SDS-PAGE analysis of inhibition of B. thuringiensis HD1 δ-endotoxins activation by protease inhibitors. The δ-endotoxins (20 µg) were incubated with T.absoluta midgut proteins (20, 2, 0.4, 0.1, 0.01, or 0 µg) without and with SBTI (1 mg ml−1), PMSF (5 mM), or Benzamidine (5 mM). Controls: C1: δ-endotoxins without protease, C2: δ-endotoxins without protease incubated 30 min at 30°C. M: low molecular weight (Amersham).
In vivo effect of D-glucono-δ-lactone and SBTI on T. absoluta larvae
Bioassays experiment using inhibitors showed interesting results. In fact, the recorded mortality percentages of T. absoluta third-instar larvae were about 76 and 46% after 72 h, when using D-glucono-δ-lactone per leaf (7.1 mg cm−2) and SBTI per leaf (10 mg cm−2), respectively.
Discussion
The knowledge of gut enzymes from target insects followed by bioassays was necessary to establish novel insect control strategies. Here, the β-glucosidase required for the glucose release from nutrient polysaccharide and proteases responsible for protein proteolysis in the larva gut of tomato leaf miner T. absoluta were examined. Hence, a significant β-glucosidase activity was revealed during this report. In fact, β-glucosidase was localized as a membrane bound enzyme (Ferreira & Terra, Reference Ferreira and Terra1983). It was rarely described in insects like the lepidoptera Erinnysis ello (Santos &Terra, Reference Santos and Terra1985), Spodoptera frugiperda (Marana et al., Reference Marana, Terra and Ferreira2000), and Bombyx mori (Byeon et al., Reference Byeon, Lee, Gui, Kim, Kang, Lee, Sohn and Jin2005), contrarily to the well-studied fungal and bacterial β-glucosidases (Li et al., Reference Li, Chir, Tanaka and Chen2002; Wallecha & Mishra, Reference Wallecha and Mishra2003).
Among the six detected T. absoluta gut proteases, trypsine-like proteases activity (BApNA substrate) was about 16 times the chymotrypsin-like proteolytic activity (SApNA substrate). Such proportion was similar to some lepidopteran larvae like Helicoverpa armigera and Heliothis virescens (trypsin 90%, chymotrypsin 5%) but different from Spodoptera exigua and S. frugiperda (trypsin 7%, chymotrypsin 85%), and Manduca sexta (trypsin 10%, chymotrypsin 80%) (Broadway & Duffey, Reference Broadway and Duffey1986; Johnson et al., Reference Johnson, Narvaez, An and Ryan1989; Johnston et al., Reference Johnston, Le, Brough, Hilder, Gatehouse and Gatehouse1995; Srinivasan et al., Reference Srinivasan, Giri and Gupta2006). Interestingly, zymogram analysis and inhibition assays of the T. absoluta proteolytic activity demonstrated that (1) the 28 kDa (F) and the 73 kDa (A) should be trypsin-like and chymotrypsin-like serine proteases, respectively, (2) the B, C, D, and E proteases may represent serine proteases since they were inhibited by PMSF but could not be trypsin-like nor chymotrypsin-like serine proteases as no clear inhibition was obtained with specific SBTI and TPCK inhibitors, (3) cysteine proteases, aspartic proteases, and metalloproteases were not detected in the T. absoluta larvae gut. We noted that serine proteases, particularly trypsins and chymotrypsins, dominated the lepidopteran larval gut environment and contributed to about 95% of the total digestive activity. In addition, elastases (1%), carboxypeptidases (1%), and aminopeptidases (5%) activities were very low in almost all lepidopteran species, while cathepsin B-like proteases (1%) and metalloproteases (1%) were present in few lepidopteran larvae with little proportions (Johnston et al., Reference Johnston, Gatehouse and Anstee1993, Reference Johnston, Le, Brough, Hilder, Gatehouse and Gatehouse1995; Broadway, Reference Broadway1995; Gatehouse et al., Reference Gatehouse, Norton, Davison, Babbe, Newell and Gatehouse1999; Hegedus et al., Reference Hegedus, Baldwin, O'grady, Braun, Gleddie, Sharpe, Lydiate and Erlandson2003; Srinivasan et al., Reference Srinivasan, Giri and Gupta2006).
The larva gut was principally composed of the foregut, the midgut, and the hindgut, where the pH varies according to insect orders and affects the pH optima of digestive enzymes (Applebaum, Reference Applebaum, Kerkut and Gilbert1985). The β-glucosidases in larvae guts of lepidopteran insects are generally most active in slightly acid pH conditions (Ghadamyari et al., Reference Ghadamyari, Hosseininaveh and Sharifi2010). Hence, we found that the optimal pH value was about 5 units for T. absoluta comparing with 6 units in Bombyx mori (Lepidoptera: Bombycidae) (Byeon et al., Reference Byeon, Lee, Gui, Kim, Kang, Lee, Sohn and Jin2005) and 5.5 units for Glyphodes pyloalis (Lepidoptera: Pyralidae) (Ghadamyari et al., Reference Ghadamyari, Hosseininaveh and Sharifi2010). Additionally, the optimal pH for the T. absoluta proteolytic activity (about 10–11 units of pH) was similar to that of the lepidopteran larvae midgut (about 8–12) (Terra & Ferreira, Reference Terra and Ferreira1994). The optimal temperature for T. absoluta β-glucosidase activity was about 30°C, which belong to the optima temperature range (20–50°C) of larvae gut β-glucosidase activities from most lepidopteran insect (Riseh et al., Reference Riseh, Ghadamyari and Motamediniya2012). Interestingly, the optimal temperature for the total proteolytic activity of the T. absoluta was about 40°C, despite the insect growth was maximal at about 28–30°C and could be stopped at 40°C.
The variability of δ-endotoxins toxicity was frequently attributed to its gut proteolytic activation, generally performed by trypsin-like and chymotrypsin-like serine proteases in various insect groups, as well as to their interactions with the midgut receptors (Miranda et al., Reference Miranda, Zamudio and Bravo2001; Díaz-Mendoza et al., Reference Díaz-Mendoza, Farinós, Castañera, Hernández-Crespo and Ortego2007). The zymogram indicated the role of 73, 66, 55, and 28 kDa serine proteases in Cry protoxin activation among them are chymotrypsin-like (73 kDa) and trypsin-like (28 kDa) proteases. Although, the 40 and 35 kDa proteases were classified as serine proteases but they did not clearly involve in δ-endotoxins proteolysis indicating that only specific proteases are responsible of Cry activation. Moreover, the presence of PMSF, SBTI, or Benzamidine inhibited the activation of the Cry protoxins, confirming the implication of T. absoluta serine protease classes in Cry activation.
Many novel insect control strategies were based on biological compounds followed or not by transgenic plant approaches (Silva et al., Reference Silva, Alcazar, Macedo, Oliveira, Macedo, Abreu, Santos and Sales2006). β-glucosidase and serine proteases of the digestive tract as well as enzyme inhibitors represent important roles in the interaction between insects and plants (Broadway & Duffey, Reference Broadway and Duffey1986; Broadway, Reference Broadway and Michaud2000; Marana et al., Reference Marana, Terra and Ferreira2000). Interestingly, bioassays showed that addition of natural β-glucosidase inhibitor (D-glucono-δ-lactone) or serine protease inhibitor (SBTI) to tomato leaves resulted in 76 or 46% of T. absoluta larvae death, respectively, demonstrating the particular effectiveness of the inhibitors. We note that T. absoluta larvae rearing were done on their natural diet (tomato leaves) which could influence the expression of some genes in the insect. Bioassays using β-glucosidase inhibitors against lepidopteran pests are scarce in the literature although that β-glucosidase is the most common carbohydrolase enzyme in lepidopteran. Furthermore, feeding diet supplemented with SBTI inhibitor reduced the activity of Mamestra Configurata (Lepidoptera: Noctuidae) larval midgut protease by 80% (Hegedus et al., Reference Hegedus, Baldwin, O'grady, Braun, Gleddie, Sharpe, Lydiate and Erlandson2003) and Spodoptera frugiperda larvae trypsin in the lumen, the cells, and the cell secretions (Lwalaba et al., Reference Lwalaba, Weidlich, Hoffmann and Woodring2010).
Our findings lead towards the design of highly specific pest control strategies. In fact, we propose the employment of β-glucosidases inhibitor, proteases inhibitor, and B. thuringiensis δ-endotoxins individually or in combination. As well, we consider that the inhibitor natural function could be expanded upon in the development of insect resistant transgenic plants.
Acknowledgments
This work was supported by grants from the Ministry of Higher Education and Scientific Research, Tunisia. The authors wish to thank Mr Zouhair Essafi, an English professor for having proofread this paper.