Introduction
The common housefly, Musca domestica L. (Diptera: Muscidae), is a synantropic and cosmopolitan species. The geographical origin of the taxon is unclear, but was probably in the Palaeartic region or the Middle East (Skidmore, Reference Skidmore and Junk Publishers1985). Houseflies colonize habitats wherever mankind and domestic animals occur. The morphological variability of houseflies is remarkable; there is much diversity in the mechanism of sex determination, and environmental differences among geographically distant housefly populations may also cause genetic differentiation (Marquez & Krafsur, Reference Marquez and Krafsur2002). Studies in genetic diversity and the consequences of rearing different strains provide evidence for natural selection and demonstrate differences in the adaptive values of different genotypes (Sokal & Sullivan, Reference Sokal and Sullivan1963). This raises the question of whether the genetic variability of the housefly is reflected in some aspects of its biology and phenotypic variation of adaptive traits. This variability can help shed light on some aspects of applied biology, for example, it is important to understand the development and the special characteristics of a strain when establishing colonies with a specific target, such as the larval degradation of animal waste. Beard & Sands (Reference Beard and Sands1973) suggested that certain housefly strains might degrade manure better than others. The degradation process could be influenced by factors other than biodegradation potential, for instance, the volume of eggs produced by the female flies from different strains. This aspect is crucial for improving the degradation process, as the final weight of degraded manure depends directly on the egg production of the housefly strain (Cicková et al., Reference Cicková, Pastor, Kozánek, Martínez-Sánchez, Rojo and Takac2012).
Martínez-Sánchez et al. (Reference Martínez-Sánchez, Smith, Rojo, Marcos-García and Wall2006) showed that the geographic origin of fly populations affected their biological parameters. They reported differences in survival, size and development time in two European populations of Lucilia sericata (Meigen) (Diptera: Calliphoridae). Moreover, these differences could be modified and attenuated after several generations had been reared in a laboratory. The captive environment may reduce the proportion of individuals breeding, and may affect their survival rates; however, some of them could persist (Frankham, Reference Frankham2005).
Strain differences affecting life histories are evident in every species that has been studied in the laboratory or in sample plots (Sokal & Sullivan, Reference Sokal and Sullivan1963). This study aimed to demonstrate that three strains of M. domestica that were genetically different due to the fact that they had different origins, and that had been adapted to laboratory rearing for some generations, still presented differences in their biological parameters and morphology. It also endeavours to show how these differences could be reduced once more generations were reared in the laboratory. In addition, we aimed to evaluate whether these differences would still be present after hybridizing the strains: would the colonies maintain their mating ability? Or, on the other hand, would the hybridization affect their development? and would the hybrids obtained from our strains improve some aspects of their artificial rearing? In order to answer these questions, the present study investigates the following aspects separately: (1) biological, morphological and genetic differences of three strains with different origins: Central Europe (stain A), South America (strain B), and South-Western Europe (strain C) and (2) the possibility of hybridization between the three of them and the differences in their biological parameters.
Material and methods
Flies used for the experiments came from three different strains. Slovak strain (A) was established as laboratory colony in 1998, Venezuelan strain (B) and Spanish strain (C) were established in 2006 as previously described (Ludoski et al., Reference Ludoski, Djurakic, Pastor, Martínez-Sánchez, Rojo and Milankov2014).
Flies used for the experiments were obtained by rearing the larvae in pig manure at low density (0.5 ml eggs per 1 kg of manure) in order to obtain high-quality pupae (18–20 mg). Once the larvae pupated, the pupae were separated from the manure by flotation. Adults were maintained under a constant temperature, humidity and photoperiod (22±0.2 °C, 57±1.5% RH, 14:12 L:D) in experimental cages (40×40×40 cm) and provided with water and food ad libitum.
In order to establish differences between housefly strains and their hybrids, the following parameters were measured: egg production and viability, and development and variation of morphometric traits. To measure the egg production of flies, the following methodology was used: an oviposition substrate was offered and replaced everyday, from the third day after emergence. After 14 h, the oviposition substrate was removed from the cages and eggs were separated from the medium and weighed. This protocol was repeated everyday for 15 days after the flies started to lay eggs. Hatchability of the eggs was also measured: a sample of 100–150 eggs was kept in Petri dishes and placed on a filter paper with a sponge soaked in water to prevent dehydration. Twenty-four hours later, the number of hatched and non-hatched eggs was counted and the percentage of hatchability was calculated by the difference of number of hatched eggs and non-hatched eggs divided by the total number of eggs. This process took place three times during the experiment (days 3, 9 and 15 of egg collection).
To study the development of each house fly strain, eggs collected from colonies were incubated until the first larvae hatched. One hundred first instar larvae (younger than 24 h) were transferred to containers (8×8 cm and 3 cm deep) with 50 g of swine manure. Containers with manure and larvae were placed in boxes (18 cm diameter, 10 cm deep) with sand in the bottom; boxes were covered with lids in order to maintain the moisture of the medium. Each lid had an opening covered with gauze to aerate the environment and keep larvae from escaping. Maintaining moisture in the manure is important so that surface of the manure does not dry and larvae can move freely around the medium. Each colony type was replicated four times. When larvae pupated, pupae were collected, weighed and individualized in Petri dishes. Time of development, larval and pupal survival were also measured.
Differences in wing morphometry of each kind of population were analysed following the methodology of Gobbi et al. (Reference Gobbi, Martínez-Sánchez and Rojo2013). First, wings were removed and stuck on a paper sheet in preparation for pictures of left wings to be taken. As a measure of the overall size of wings, we computed centroid size, which is the square root of the sum of the squared deviation of landmarks around their centroid (Klingenberg et al., Reference Klingenberg, Mc Intyre and Zaklan1998). Landmarks can be visualized in fig. 1. Wing size was calculated as centroid size, using TpsDig 2.16 software (Rohlf, Reference Rohlf2010). The differences in the parameters above were measured to establish: (a) differences among strains and (b) differences between hybrids and their parental strains.

Fig. 1. Wing of Musca domestica indicating landmarks taken for measuring of centroid size.
Differences among strains
These experiments took place during the spring of 2008. In order to measure the egg production of flies, 1000 pupae were placed in cages of 40×40×40 cm; water and a mixture of sugar and milk powder in a proportion of 2:1 was provided ad libitum. Two different oviposition substrates were used: paper soaked in milk and pig manure. Substrates were covered with black paper as previously described (Pastor et al., Reference Pastor, Cicková, Kozánek, Martínez-Sánchez, Takác and Rojo2011). Six cages with 1000 pupae were set for each kind of strain. Three cages were offered with paper and milk and three with pig manure as the oviposition substrate. On the 15th day of egg collection, dead adults were removed and counted. Once the experiment had finished, emergence of the 1000 pupae introduced in each cage was measured, as well as sex ratio. The rest of the parameters (egg hatchability, development, weight of pupae and wing morphometry) were measured following the methodology described above.
For genetic analysis, a 348 base pair fragment of the 5′-part of the mitochondrial gene cytochrome c oxidase subunit I (COI) was sequenced (Cummings & Krafsur, Reference Cummings and Krafsur2005). A total of 12 samples were compared, four from the strain B (fourth generation reared in the laboratory), three from the strain C (sixth generation) and five from the strain A (third generation). A haplotype minimum spanning network of DNA sequences obtained was reconstructed using statistical parsimony (Templeton et al., Reference Templeton, Crandall and Sing1992), as implemented in the software TCS1.21 (Clement et al., Reference Clement, Posada and Crandall2000) using a 95% connection limit. This network uses information on inferred mutational steps between the haplotypes and groups closely related haplotypes together. The specimens used for analysis were captured in the strains, from different generations.
Hybridization of strains
This experiment took place during the winter of 2009. Pupae of each strain were individualized in Petri dishes and, once adults emerged, were placed in experimental cages (19×19×19 cm) to obtain the following combinations: controls of pure strains (50 males and 50 females in each replicate), and all possible combination of males/females from different strains (50 females–50 males in each combination and replicate). Each combination was replicated three times. In order to produce enough adults (900 adults of each strain), 1000 pupae of each strain were individualized. Following the methodology described above, the parameters measured were: egg production, hatchability of eggs and larval development.
Additionally, two more parameters were measured in this experiment: fertility of the first (F1) and second (F2) generations. To measure the fertility of F1 in each combination of strains, pupae obtained were individualized and, once emerged, placed in experimental cages with water and food (sugar and milk powder). The number of males and females were recorded, as well as the date of emergence. On the third day after emergence, oviposition substrates were offered; the first three egg-layings were collected and the hatchability of the eggs measured. Additionally, the sex ratio of the second generation of the combination of strains A and B was studied. Morphometry and weight of pupae of first generation hybrids were not measured.
Statistical analyses
After testing the data for adherence to normality using the Kolmogorov–Smirnov test, parametric data were analysed using a one-way analysis of variance (ANOVA, F) followed by a post hoc test (Tukey). In the first experiment (differences among strains), this test enabled us to investigate the differences in the daily amount of eggs deposited per cage, the percentage of emerged adults, adult mortality after 15 days and the centroid size of wings among strains. A Student's t-test was performed to investigate the differences in the number of eggs deposited between oviposition substrates as well as differences in mortality after 15 days between males and females. In the second experiment (hybridization of strains), an ANOVA followed by a Tukey test was used to characterize differences in the accumulated weight of deposited eggs, survival, sex ratio and length of total and pupal development.
For the non-parametric-independent samples, a Kruskal–Wallis (H) followed by a Dunn's test was used. In the first experiment, these tests characterized the differences in the hatchability of deposited eggs, survival for each stage, pupal weight and time of development among different populations of M. domestica. A Mann–Whitney test was used to analyse differences in daily egg weight observed between the oviposition substrates used among the three strains. In the second experiment, a Kruskal–Wallis test was used to analyse differences in daily egg weight, hatchability of eggs and length of larval development.
Results
Differences among strains
No differences among strains were observed in the percentage adult mortality (see table 1) after 15 days (H=0.08, df=2, P>0.05). However, there were differences between dead males and females (t=−2.09, P<0.05). Mortality after 15 days of adult emergence was higher in female flies. There were also differences in the daily production of eggs per female among strains when the substrate used was paper soaked in milk, where the highest production was obtained in the strain A (F=9.55, df=2, 132, P<0.001) (table 1). When the substrate used was swine manure, no differences among strains were found (F=2.21; df=2, 132; P>0.05). Upon comparing the cumulative weight after 15 days of egg collection, we only found differences between the strain A (using paper soaked in milk as the oviposition substrate) and the strain C, but no differences were found between the strain B and the other two (F=9.09; df=5, 12; P<0.05) (see fig. 2). Regarding the differences between the two types of substrates used, oviposition was higher in paper soaked in milk than swine manure, but these differences were only significant in results obtained in the strain A, where quantities of eggs deposited in milk were higher than in manure (t=2.82, P<0.01). Egg production appeared to be more or less constant during 15 days of egg collection; it did not seem to decrease in the last days of the experiment. However, what could be observed is that the tendency across time is more or less the same in the three strains. Flies required less time to start to lay bigger quantities when paper soaked in milk was offered, while it appeared that more time was needed to adapt to manure. No differences were found in the percentage of hatchability of eggs among strains of housefly (80.55%±2.37).

Fig. 2. Accumulated number of eggs per female (mean±SE) of strains from Slovakia (strain A), Venezuela (strain B) and Spain (strain C) in two different oviposition substrates. Number of eggs was obtained by the correlation y=9118.40x±17.089. Columns with the same letters are not significantly different (P<0.05, n=270).
Table 1. Daily number of eggs produced per female and percentage of mortality of adults after 15 days (males/females) (mean±SE) in three different strains: A (Slovakia), B (Venezuela) and C (Spain). Means followed by the same letters are not significantly different (P<0.05, n=18).

Although final survival was around 50% (table 2) and no significant differences were observed in the final survival among strains (H=4.15, df=2, P>0.05), there were differences in larval (H=9.27, df=2, P<0.01), and pupal survival rates (H=6.27, df=2, P<0.05).
Table 2. Percentage of survival (mean±SE, n=12) from larva to pupa, from pupa to adult and final survival and time of development (mean±SE, n=626) from larva to pupa, from pupa to adult and total time of development of three different strains of housefly: A (Slovakia), B (Venezuela) and C (Spain). Means in rows followed by the same letters are not significantly different (P<0.05).
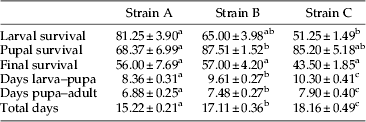
As can be observed in table 2, total development from larva to adult was different in the three strains (H=469.77, df=2, P<0.001), being faster in the strain A than in the other two, followed by strain B; total development was 3 days slower in the strain C than in the strain A. Differences among the three populations were also observed in larval development time (H=510.33, df=2, P<0.001) and in time from pupation to emergence were also observed among the three different strains (H=209.84, df=2, P<0.001). Finally, differences in the pupation process were also observed. For the strain A, on the first day of pupation (eighth of larval development) more than 70% of the larvae pupated, followed by 25% on the second day, while on the final 2 days only a few pupae were collected. For the strain B, on the second and third day (ninth and tenth day of larval development) 97% of pupae were collected. The strain C needed one more day to start pupation, and the majority of pupae (53%) were collected on day 3 (tenth day of larval development). Although pupation occurred at different times in each strain, all three different strains completed pupation within 4 days, which means that it took 4 days from the moment when the first until the last larva pupated.
The weight of the pupae was different between strains (H=48.74, df=2, P<0.001), being lowest in the strain B (11.67±0.28 mg). No significant differences were observed between the other two strains, although the strain C appeared to produce heavier pupae (12.82±0.22 mg) than the strain A (12.62±0.22 mg). Differences in the centroid size of different strains were observed among flies from strain A and the other two strains (F=83.00; df=5, 450, P<0.001), where flies from strain A (males and females) were bigger than the other ones (fig. 3).

Fig. 3. Centroid size of adults from each strain (pixels; mean±SE, mean±SD) separated by gender (m: male, f: female). Columns with the same letters are not significantly different (P<0.001, n=558). Slovakia (SLK, strain A), Venezuela (VNZ, strain B) and Spain (SPN, strain C).
DNA analysis results revealed four haplotypes present in the three strains studied, two in the strain B (HTIII and HTIV) and one in each of the other strains: HTII (strain C) and HTI (strain A). The haplotype network is shown in fig. 4. The rectangle represents the haplotype most likely to be ancestral for this set of sequences (HTIII), as determined using the TCS software. HTIV differs from HTIII by one nucleotide change, whereas HTII and HT1 differ by three nucleotide changes each from the ancestral haplotype.

Fig. 4. Haplotype network for Musca domestica with different origins. The size of the circles is related to the number of specimens. Each line between the circles indicates one nucleotide change (n=12). Slovakia (strain A), Venezuela (strain B) and Spain (strain C).
Hybridization of strains
No large differences were observed in daily egg weights recorded in each pure strain and their mixtures (H=31.31; df=8; P<0.01). Significant differences were only observed between the pure strain B and the hybrid strain resulting from mixing C-males with B-females, where the biggest quantities of eggs were collected from the pure strain B (table 3).
Table 3. Daily weight of eggs, sex ratio and percentage of survival (mean±SE) from larva to pupa, from pupa to adult and final survival of control pure strains of housefly and the first generation of hybrids resulting from different combinations between males and females of each strain (A: Slovakia, B: Venezuela and C: Spanish). Means in columns followed by the same letters are not significantly different (P<0.05, n=45).

When comparing cumulative egg weights after 15 days of egg collection (fig. 5), more differences were observed (F=4.63; df=8, 18; P<0.05). The hybrids of A-males and C-females produced more eggs than the original strain A and the hybrids of A-males and B-females. However, hybrids of C-males and B-females laid lower quantities of eggs than the hybrids of the C-strain with A-strain. No differences were observed in percentages of hatchability among original strains or their hybrids in either the first or second generation, being 75.92±1.31% for the first generation and 91.55±2.42% for the second generation.

Fig. 5. Accumulative weight of eggs (AWE) (g; mean±SE) during 15 days in three different strains of Musca domestica: Slovakia (SLK, strain A), Venezuela (VNZ, strain B) and Spain (SPN, strain C) and the combinations of males (m) and females (f) of each strain. Columns with the same letters are not significantly different (P<0.05, n=399).
No differences were observed in total survival among pure strains and hybrids. There were also no differences in larval or pupal survival (table 3). Differences in sex ratios were observed between hybrids of A-females and B-males and the rest of original strains and hybrids due to the fact that only males emerged from the eggs obtained from this mixture of strains. There were also significant differences between hybrids obtained from mixtures of strains A and C; the ratio of males was higher when A-females were mixed with C-males (F=8.98; df=8, 36; P<0.001) and more females were produced when the combination was the opposite (table 3).
Differences were observed for the length of development (table 4). When comparing total development between pure strains and their hybrids, differences are observed (F=9.63; df=8, 83; P<0.001). Differences were also observed in days taken to transition from larva to pupa (F=3.87; df=8, 83; P<0.01), but only between the pure strain C and the hybrid strain of C-females and B-males, where the pure strain was faster. Differences were also observed between the pure strain B and the mixture of A-males with B-females for the days taken to transition from pupa to adult; development of the hybrid strains was faster than the pure strain (H=53.65; df=8, P<0.001).
Table 4. Time of development (mean±SE) from larva to pupa and from pupa to adult of three different strains of housefly and the first generation of hybrids resulting from different combinations between males and females of each strain (A: Slovakia, B: Venezuela and C: Spain). Means in columns followed by the same letters are not significantly different (P<0.05, n=2160).

Finally, no differences were observed in the sex ratios of the second generation of hybrids of strain A and B (F=0.84; df=2, 7; P>0.05).
Discussion
It is important to understand the biology of the insects when working with them in mass-rearing conditions, and this knowledge has to be used to introduce continuous improvements in the technology. An effective operation of a mass-rearing facility requires maintenance of high fecundity of the adults and high survival of the immature stages (eggs, larvae and pupae), as well as regular quality control tests to ensure high quality of produced insects (Cicková et al., Reference Cicková, Kozánek and Takác2013). This study shows that the selection of the strain is a key point for mass-rearing programmes as the differences found affected egg production, time of development and size of individuals and the genetical results obtained from this study lend support to these observed biological differences between the three strains: four haplotypes were found and each strain had a different haplotype.
The strains studied had differences in their life history: the strain A (Central Europe, Slovakia) had already been adapted to laboratory conditions previously to this experiment; the strains B (South America, Venezuela) and C (South-West Europe, Spain) were collected and adapted in our laboratories. In experiments with Drosophila melanogaster Meigen, 1830 (Diptera: Drosophilidae), adaptation appeared to be complete after approximately 60 generations (Gilligan & Frankham, Reference Gilligan and Frankham2003); consequently, our strains (with the exception of the strain A) were not completely adapted in the present experiments. These differences are reflected in the current study. The egg production of the strain A was higher, whilst collecting large quantities of eggs from the other strains was difficult and its developmental time was longer. It is well known that it can take several generations until the strain adapts to laboratory conditions and can be reared normally (Carpenter & Bloem, Reference Carpenter and Bloem2002). Thus, differences in fertility could be due to the adaptation to captivity conditions as the results obtained in this parameter changed from the first experiment to the second one, performed one year later. Sometimes, fluctuant environmental conditions can lead to the selection of particular genotypes that are easily mass reared (van Lenteren, Reference Van Lenteren and van Lenteren2003): this change in the strain C was observed after the summer of 2008, when a high mortality in the adults occurred due to a devastating infection with Entomophtora muscae. The colonies recovered but the population levels decreased for one or two generations. Differences in developmental time and size are also remarkable: strain C had a slower development; adults of the strain A were bigger and pupae from strain B lighter.
It is important to consider all these parameters, as differences at experimental scale could represent great advantages in the mass rearing of this insect in a manure degradation facility. The egg production, for example, is particularly significant, as it can be a bottleneck for the potential quantity of manure to be processed by the housefly larvae (Pastor et al., Reference Pastor, Cicková, Kozánek, Martínez-Sánchez, Takác and Rojo2011). The reduction of the time of development is also remarkable, as it implies that the process of degradation is also reduced and larger quantities of the residue could be processed in the same period of time. The size of the individuals is also important as it provide us information about adult fitness and female fertility. Moreover, it is important to obtain larger individuals if the sub-products of the process (larvae or pupae) will be commercialized.
Sokal & Taylor (Reference Sokal and Taylor1976) reported that ecological properties of hybrid populations of housefly were not the same as those of their parent strains. Martínez-Sánchez et al. (Reference Martínez-Sánchez, Smith, Rojo, Marcos-García and Wall2006) found an intermediate tendency in the larval behaviour of hybrids of L. sericata in comparison with their parent strains. However, in our study, when comparing pure strains and the hybrids resulting from them, we cannot arrive at any definitive conclusion. Regarding egg production, some hybrids produced larger quantities of eggs than their pure strains (mixtures of strains A and C) and some of them laid smaller quantities than their pure strains (mixtures of strains B and C). Another trait that appeared to be different was the developmental time. Differences were observed between pure strains and some of its hybrids and it seemed that the male parent determined the length of the developmental time of the hybrids.
When mixing strains and obtaining hybrids, the most remarkable aspect found was the abnormal sex ratio of hybrids resulting from the mixture of A-females and B-males, the offspring of this mixture gave only males, which were fertile and had a normal sex ratio in their descendants; normal sex ratio was also observed when mixing the same strains but the contrary sexes. Same sex ratio was observed in five replicates, being the eggs of two of them collected in a different day than the other three. Multiple sex determining factors co-exist in many populations of the housefly (Dübendorfer et al., Reference Dübendorfer, Hediger, Burghardt and Bopp2002). In standard strains, females are XX and males are XY, whereby the Y chromosome carries a male determining factor (M Y); other strains exist in which the factor M is located on other autosomes; thus, males are heterogametic and females are homogametic. (Hilfiker-Kleiner et al., Reference Hilfiker-Kleiner, Dübendorfer, Hilfiker and Nöthiger1994). Following these procedures, what we suggest is that A-females had a standard sex determining system (females XX and males XY) and B-males were homozygous for any M factor; C-males would be heterozygous for every M factor. The possibility of hybridizing different strains of one species it is an important aspect to consider, as individuals with better qualities for the artificial rearing could be obtained. Some authors suggest that the colonies maintained in laboratory conditions should be periodically refreshed with wild specimens to avoid their aging or deterioration (van Lenteren, Reference Van Lenteren and van Lenteren2003). Regarding our results, we suggest that previous studies should be done before performing this practice.
Summarizing, the results provided in this study show that different strains of M. domestica present differences in their biological and morphological parameters; some due to their domestication level, others due to their particular genetic variability. The main differences between strains of housefly are: size, egg production and developmental time; these differences can optimize the mass rearing of this species. Hybridizing strains of M. domestica was possible and the resulting hybrids present differences with their parent strains. In some cases, when hibridizing strains, in the resulting generations, can appear abnormal sex ratios, due to the different sexual determination mechanisms of this species.
Acknowledgements
This study was funded by the project LIFE-ECODIPTERA (LIFE05-ENV/E/000302) and partially by GV/2011/039 (Generalitat Valenciana) y GRE09-27 (University of Alicante) projects. We are very thankful to Dr Gabrielle Beans for her language and content corrections.











