Introduction
Invasive species – species whose introduction cause ecological, environmental, or economic impacts (Colautti & Richardson, Reference Colautti and Richardson2009) – are widely recognized as a primary threat to habitats. In light of increasing dominance of global trade in recent decades, the threat from invasive species continues to rise. Although a large number of species are being transported across biogeographical barriers every day through international trade, only a small fraction (<1%) of these species become invasive pests (Williamson, Reference Williamson1996). To accurately predict the outcome of a particular invasion, it is crucial to understand the factors that govern the invasion success of exotic species in a new environment.
Ants offer a unique opportunity to understand competitive mechanisms of invasion through their comprehensive life cycles, social structures, and ecological roles in the context of the environment and evolutionary process (Lach et al., Reference Lach, Parr and Abbott2010). Social organization of ants may increase competitiveness and result in ecological dominance of invasive ants in new habitat (Giraud et al., Reference Giraud, Pedersen and Keller2002; Holway et al., Reference Holway, Lach, Suarez, Tsutsui and Case2002). In inter-specific encounters, species with greater aggressiveness are usually predicted to have a competitive advantage (Obin & Vander Meer, Reference Obin and Vander Meer1989; Tsutsui et al., Reference Tsutsui, Suarez and Grosberg2003).
Two native (Solenopsis geminata Fabricius and Solenopsis xyloni McCook) and three exotic (black, Solenopsis richteri Forel; red, Solenopsis invicta Buren; and their hybrid, S. richteri × S. invicta) fire ant species (Hymenoptera: Formicidae) are currently found in the southern United States (US). Black imported fire ant invaded the US in 1910s, followed by the invasion of the red imported fire ant in late 1930s through Mobile, Alabama (Wilson, Reference Wilson1951, Reference Wilson1958). Although S. richteri invaded and established two decades earlier than S. invicta, its distribution is currently restricted to only few parts of Alabama, Mississippi, and Tennessee (Obin & Vander Meer, Reference Obin and Vander Meer1989; Streett et al., Reference Streett, Freeland and Vander Meer2006; Oliver et al., Reference Oliver, Vander Meer, Ochieng, Youssef, Pantaleoni, Mrema, Vail, Parkman, Valles, Haun and Powell2009). In contrast, S. invicta is widely distributed throughout the southeastern US and Puerto Rico (Callcott & Collins, Reference Callcott and Collins1996; Porter, Reference Porter2000; Callcott et al., Reference Callcott, Porter, Weeks, Graham, Johnson and Gilbert2011). This notorious global invasive pest has also invaded other regions of the world, including the Caribbean, Australia, New Zealand, Taiwan, and China (Ascunce et al., Reference Ascunce, Yang, Oakey, Calcaterra, Wu, Shih, Goudet, Ross and Shoemaker2011). The two exotic fire ant species were able to interbreed and their hybrid is currently occurring sympatrically with their parent species in northern Mississippi, Alabama, and Georgia (Diffie et al., Reference Diffie, Vander Meer and Bass1988; Vander Meer & Lofgren, Reference Vander Meer and Lofgren1988; Streett et al., Reference Streett, Freeland and Vander Meer2006). The restricted range of S. richteri has been attributed to displacement by S. invicta and continued range expansion of the hybrid (Callcott & Collins, Reference Callcott and Collins1996; Streett et al., Reference Streett, Freeland and Vander Meer2006). On the other hand, native fire ant species such as S. geminata, which used to be abundant in the southern US before invasion of the exotic fire ant species, are gradually losing their habitat and their range is now restricted to a few regions in Florida and Texas (Porter, Reference Porter1992; Wojcik, Reference Wojcik and Williams1994; Wetterer, Reference Wetterer2011).
Biological, ecological, and genetic factors may account for population displacement of native fire ant species in southern US. The ecological dominance of S. invicta in invaded regions has been attributed to its superior competitive ability (Jones & Phillips, Reference Jones and Phillips1987; Tschinkel, Reference Tschinkel2006). However, the exact mechanisms that mediate this competitive advantage remain unclear. Highly effective chemical defense and pheromonal communication may contribute to the competitive success of the exotic fire ants. One example would be the use of alarm pheromones to alert nestmates for colony defense against threats and intrusion, such as robbery, predation, parasitism, and competition (Vander Meer & Morel, Reference Vander Meer, Morel, Vander Meer, Breed, Espelie and Winston1998; Mizunami et al., Reference Mizunami, Yamagata and Nishino2010). Several glands in ants are responsible for biosynthesis of chemical stimuli eliciting alarm behavior, including the mandibular gland, Dufour's gland, and anal glands (Blum, Reference Blum1969, Reference Blum, Kerkut and Gilbert1985). The chemical components of alarm pheromones have been reported for a large number of ant species (Attygalle & Morgan, Reference Attygalle and Morgan1984). Recently, 2-ethyl-3,6-dimethylpyrazine (2E36DMP) was identified as an alarm pheromone component from the mandibular gland of S. invicta (Vander Meer et al., Reference Vander Meer, Preston and Choi2010). This chemical has been reported as a trail pheromone component from the venom gland of a number of ant species in the genera Atta, Manica, Messor, Myrmica, Pheidole, Pogonomyrmex, and Tetramorium (Morgan, Reference Morgan2009). Although alarm pheromone is known to signal danger and trigger a high state of alert among nestmates, its contribution to the invasion success and ecological dominance of Solenopsis fire ants has not been well studied. Thus, the exotic fire ant complex in southern US presents a good model system for investigating the mechanisms of inter-specific competition, in particular the connection between alarm pheromone quantity and its competitive superiority.
The aims of this study were to quantify and compare (1) variation in the amount of alarm pheromone among castes of S. invicta, and (2) variation in the amount of alarm pheromone among different fire ant species. Based on the range expansion and wider distribution of the exotic fire ants relative to S. geminata, we hypothesized that S. invicta, S. richteri, and hybrids will have greater pyrazine content than S. geminata. We reasoned that an ant species with greater amount of 2E36DMP would have a greater recruitment ability that would favor the species in inter-specific encounters, ultimately conferring a competitive advantage to the species.
Materials and methods
Fire ants
In May 2015, S. richteri colonies were collected from Northern Mississippi and Western Tennessee, S. invicta colonies from Alabama (Auburn University campus), and hybrid fire ant colonies from northern Alabama and northeastern Mississippi where hybrids are known to occur almost exclusively (supplementary table S1). Solenopsis geminata colonies were collected from Central Florida. At least five colonies of each species were obtained. Mounds were removed from the ground by excavation and the ants were maintained in 25 litre plastic containers coated with Fluon® to prevent escape. All colonies were kept in the laboratory at 25 ± 2 °C, 70 ± 5% r.h. and fed with 10% sugar solution and house crickets. Species identifications were confirmed using specific morphological characters, such as presence of median frontal streak (Pitts et al., Reference Pitts, McHugh and Ross2005), as well as gas chromatography (GC) analysis for the exotic species (S. richteri, S. invicta, and the hybrid) using both alkaloid and hydrocarbon component profiles as previously reported (Fadamiro et al., Reference Fadamiro, He and Chen2009; Chen et al., Reference Chen, Hu and Fadamiro2010).
Pheromone extraction
In previous studies, preparation of a sample of ant alarm pheromone components for qualitative analysis was conducted by dissecting mandibular glands or whole mandibles from individual ants under a microscope, followed by immersion in an organic solvent such as hexane (Hughes et al., Reference Hughes, Howse and Goulson2001; Showalter et al., Reference Showalter, Troyer, Aklu, Jang and Siderhurst2010; Vander Meer et al., Reference Vander Meer, Preston and Choi2010). This process however, is extremely time-consuming. Here we utilized a simpler method by mass-extracting whole ants followed by gas chromatography-mass spectrometry (GC-MS) analysis under single ion monitoring (SIM) mode by monitoring several characteristic ions.
From each colony, we removed 0.5 g of ants of each of three different castes (workers for all four fire ant species, male alates, and female alates for S. invicta only), they were freeze killed (kept in a −20 °C freezer for ≥20 min), and then immersed in 2 ml of hexane (in a 4 ml vial) at room temperature. The vial was tightly capped during extraction to prevent evaporation. The number of ants in each sample was counted after extraction for calculation of the mean weight of an individual ant of each caste (workers = 0.7 ± 0.08 mg ant−1, male alates = 7.1 ± 0.07 mg ant−1, female alates = 12.4 ± 0.17 mg ant−1). After given extraction times (see below), the extract was transferred into a new 4 ml vial, and dried over anhydrous sodium sulfate (0.5 g) for 12 h. The extract was then transferred to a 2 ml vial and finally concentrated to 1 ml under a mild stream of N2. All samples were kept in a refrigerator at 4 °C until used.
Experiment 1: The effect of soaking time on extraction efficiency of the 2E36DMP from whole ant bodies was determined. To avoid inter-colony variation, only one colony of S. invicta was subjected to six soaking periods of 2, 6, 12, 24, 48, or 72 h. Each treatment was repeated five times.
Experiment 2: This experiment was used to detect caste-specific variations in the amount of 2E36DMP produced by workers, male alates, and female alates. All five colonies of S. invicta were used for extraction and each colony was considered as a replicate (N = 5). The extraction time was 48 h (as based on results from experiment 1).
Experiment 3: Variation in the amount of 2E36DMP in worker ants from four different fire ant species (S. richteri, S. invicta, S. richteri × S. invicta, and S. geminata) was evaluated using five colonies of each species and an extraction time of 48 h.
Coupled GC-MS
The alarm pheromone component, 2E36DMP, was synthesized as described in Fang & Cadwallader (Reference Fang and Cadwallader2013). We added EtMgBr (0.7 g, 5.26 mmol) dropwise at 0 °C under N2 atmosphere to a mixture of 2-chloro-3,6-dimethylpyrazine (0.5 g, 3.5 mmol) and ferric acetate (0.13 g, 0.7 mmol) in N-methyl-2-pyrrolidone (10 ml). The reaction mixture was stirred at 0 °C for 2 h. While the liquid chromatography-mass spectrometry analysis showed that the reaction was completed, the mixture was quenched with EtOAc and filtered. The organic phase was washed with saturated brine (15 ml × 4), dried with anhydrous Na2SO4, filtered and concentrated in vacuum. The residue was purified by silica gel chromatography (hexane/EtOAc = 30 : 1) to give 2E36DMP (0.15 g, 1.1 mmol, 31.4% yield) as a colorless liquid. 1HNMR (400 MHz, CDCl3): δ 8.12 (s, 1H), 2.77 (q, J = 7.3 Hz, 2H), 2.49 (s, 3H), 2.45 (s, 3H), 1.24 (t, J = 7.6 Hz, 3H). Synthetic 2E36DMP was used as standard for quantitative analysis. A stock solution of standard compound (2000 ng µl−1) was prepared and then diluted to obtain a series of concentrations: 1.5625, 3.125, 6.25, 12.5, 25, 50, 100, and 200 pg µl−1. The standard compound solutions and fire ant extracts were analyzed by GC-MS using an Agilent 7890A GC coupled to a 5975C Mass Selective Detector, with an HP-5 ms capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness). Helium was used as a carrier gas at a flow rate of 1 ml min−1. Injections (1 µl) were made in the splitless mode at an injector temperature of 270 °C with a GC autosampler. The GC oven temperature was programmed from 50 to 100 °C at 5 °C min−1, then to 270 °C at 10 °C min−1, and held for 11 min. Total run time was 40 min. The transfer line temperature was set at 280 °C. Mass spectra were obtained using electron impact (70 eV) in SIM mode.
Statistical analysis
A standard curve was generated by linear regression analysis. The concentrations of 2E36DMP in fire ant samples were calculated against the standard curve. The absolute amounts of 2E36DMP were analyzed using a one-way analysis of variance followed by Tukey's honestly significant difference (HSD) comparison test to establish significant effects of soaking time, caste, and species. All analyses were performed using SPSS 13.0.
Results
GC-MS analysis
GC-MS analysis of synthetic sample showed that the retention time of 2E36DMP was 11.086 min in total ionization chromatography (TIC) mode (fig. 1a). The high abundant characteristic fragment ions of m/z 135, 136 from the standard compound in full scan mode (fig. 1b) were chosen as the monitoring ions for the SIM mode. Thus, ions 135 and 136 determined from the full scan mode were used as target ions for construction of a calibration curve of the standard and quantitation of alarm pheromone component in the fire ant samples. The chromatographic peak of the alarm pheromone component in the samples was determined by matching the retention time of the standard compound (fig. 1c–f). A regression equation was obtained by the external standard method, A = 110.45 C – 62.82, where A is the peak area and C is the concentration of the standard compound (pg µl−1).
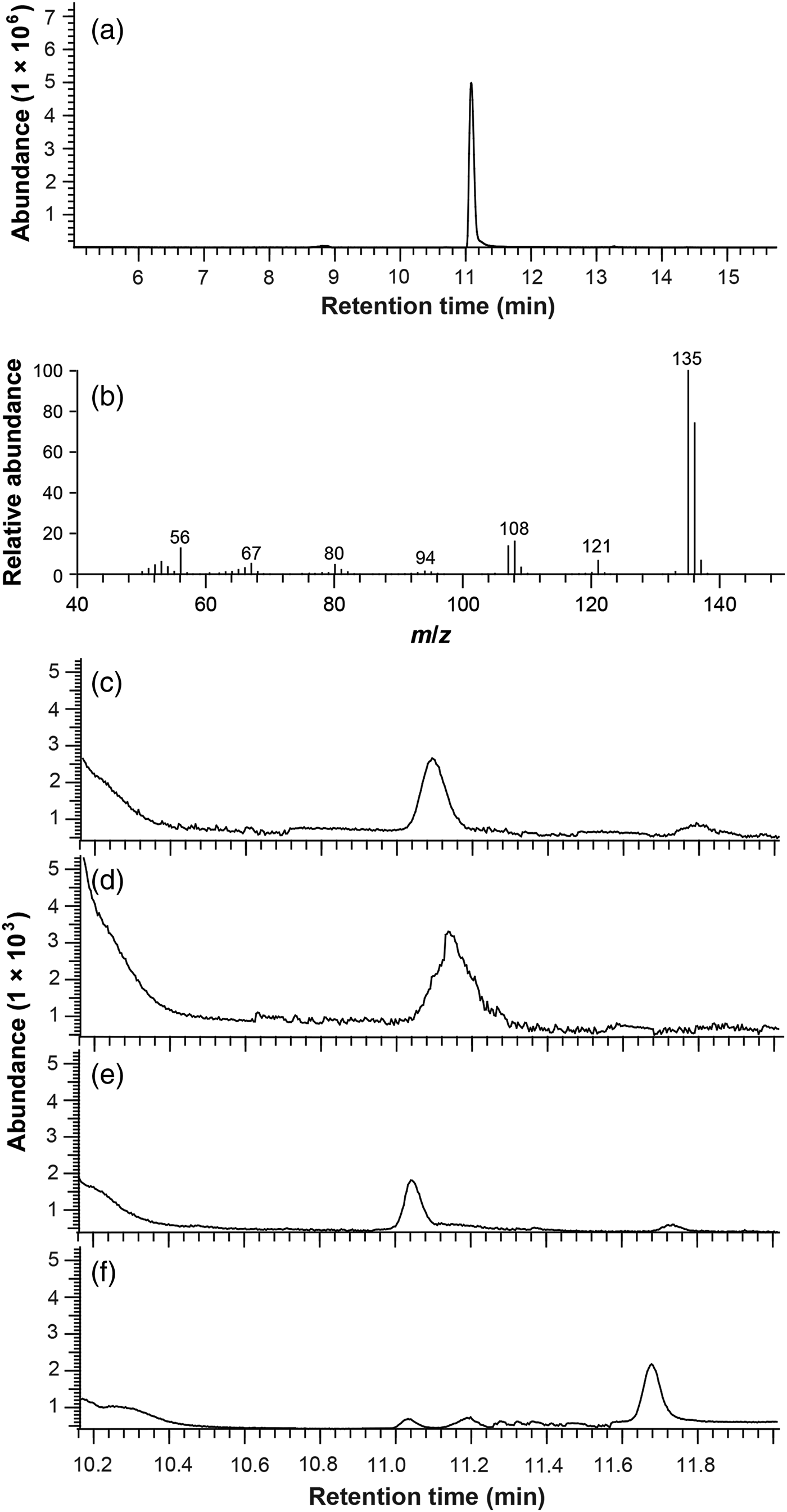
Fig. 1. GC-MS analyses of authentic compound and worker samples from different fire ant species. (a) GC profile of authentic sample in full scan mode. (b) Mass spectra of 2E36DMP. (c–f) GC profiles of worker samples from Solenopsis richteri, Solenopsis invicta, S. richteri × S. invicta, and Solenopsis geminata, respectively.
Effect of soaking time on extraction efficiency
Increasing soaking time periods from 2 to 48 h lead to a linear increase in the amount of extracted alarm pheromone component. When the extraction time was extended to 72 h, the concentration of alarm pheromone component increased slightly but with no significant difference between 48 and 72 h (fig. 2). Thus, 48 h was chosen in the subsequent experiments as the optimal soaking time for extracting fire ant alarm pheromone component.
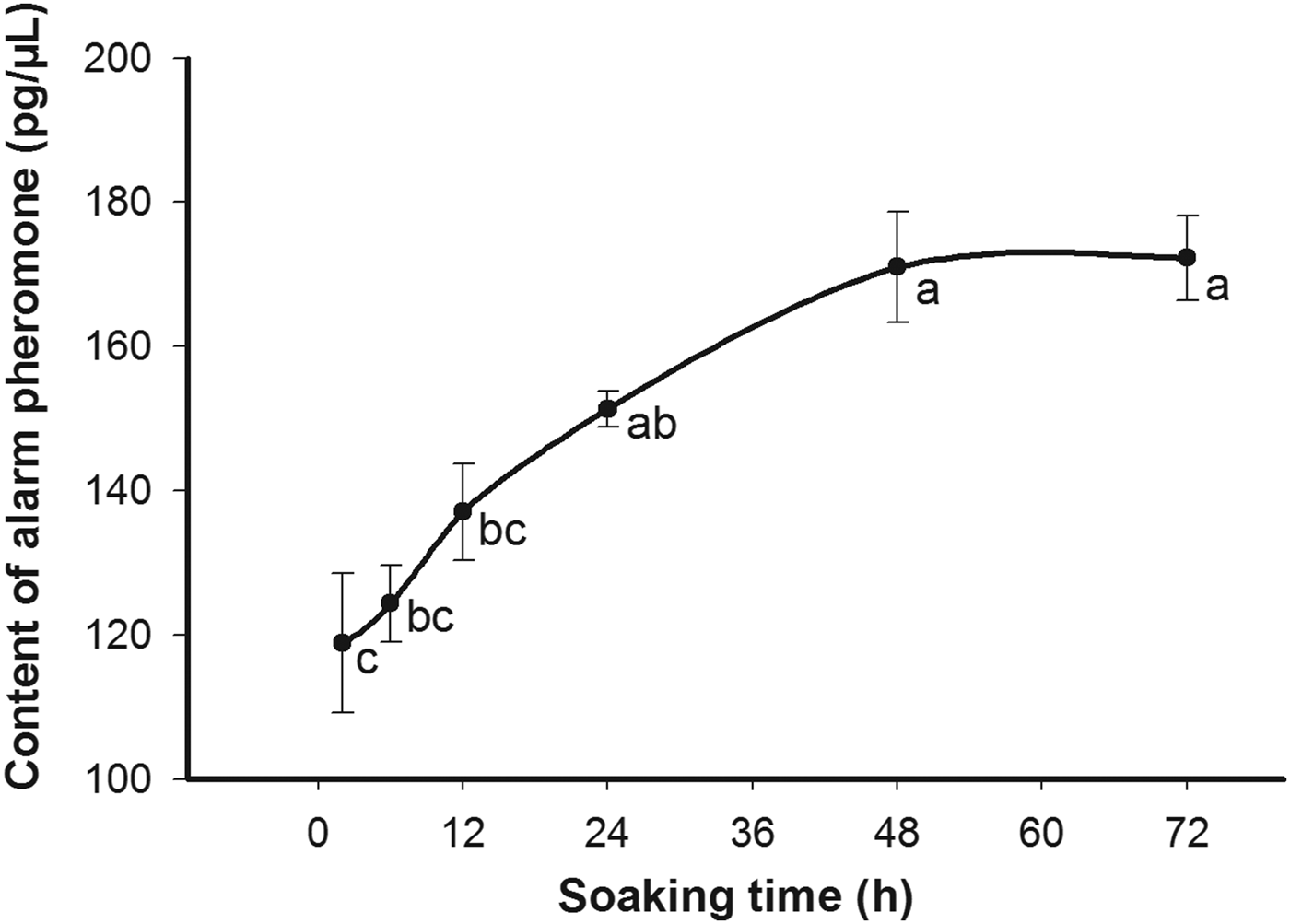
Fig. 2. Amounts of alarm pheromone in Solenopsis invicta workers as quantitated using whole body extracts at 2, 6, 12, 24, 48, and 72 h soaking times. Significant differences (P < 0.05) among treatments are indicated by different letters on bars. Values are mean ± standard error from five replicates.
Pheromone component concentration among S. invicta castes
The three castes produced significantly different pyrazine quantities from each other, with workers (360.8 ± 32.23 ng g−1) having the most, followed by male alates (134.8 ± 13.18 ng g−1) and female alates (58.2 ± 4.17 ng g−1) (fig. 3a). Individual workers contained significantly less 2E36DMP (307.0 ± 30.39 pg ant−1) than individual male alates (973.1 ± 104.97 pg ant−1) and female alates (717.4 ± 49.63 pg ant−1), but there was no significant difference in the content of 2E36DMP between male and female alates (fig. 3b).

Fig. 3. Amounts of alarm pheromone in different castes of Solenopsis invicta (workers, male alates, and female alates). (a) This figure indicates the amount of alarm pheromone per gram of extracted ant bodies. (b) This figure indicates the amount of alarm pheromone per an individual ant. Significant differences (P < 0.05) among treatments are indicated by different letters on bars. Values are mean ± standard error from five replicates.
Pheromone component concentration among different fire ant species
The four species produced significantly different amounts of pheromone component (fig. 4, F = 54.66, df = 3, P < 0.0001). Solenopsis invicta (329.6 ± 17.27 ng g−1) had significantly more 2E36DMP than the hybrid (139.1 ± 9.43 ng g−1). The pyrazine content in S. richteri (261.0 ± 26.32 ng g−1) seemed intermediate to those in S. invicta and the hybrid. The difference was significant between S. richteri and hybrid but not between S. richteri and S. invicta (fig. 4). The amount of 2E36DMP in the native fire ant S. geminata (28.5 ± 9.43 ng g−1) was significantly lower than that for any of the three exotic fire ant species.

Fig. 4. Amount of alarm pheromone in workers of different fire ant species (Solenopsis richteri, Solenopsis invicta, S. richteri × S. invicta, and Solenopsis geminata). Significant differences (P < 0.05) among treatments are indicated by different letters on bars. Values are mean ± standard error from five replicates.
Discussion
Efficiency of extraction of alarm pheromone component related to soaking time
This study highlights the convenience and efficiency of the soaking method of extraction in analyzing fire ant alarm pheromone component, as evidenced by GC-MS analysis. Extraction is the first important step in the recovery and purification of active substrates from biological materials (Tan et al., Reference Tan, Tan and Ho2013). The objective of an extraction process should provide the maximum yield of substances which consist of high concentrations of target compounds in the extracts (Spigno et al., Reference Spigno, Tramelli and De Faveri2007). Moreover, the extraction method and the extraction time are two of the most important factors in the extraction process (Upadhya et al., Reference Upadhya, Pai and Hegde2015). Different methods have been used to extract ant alarm pheromones. For instance, crushed heads of two species of grass-cutting ants were extracted in dichloromethane (CH2Cl2) for 24 h (Hughes et al., Reference Hughes, Howse and Goulson2001); whole bodies, separated heads, and gasters of Wasmannia auropunctata workers were extracted for 5 min in CH2Cl2 (Showalter et al., Reference Showalter, Troyer, Aklu, Jang and Siderhurst2010); and dissected mandibles with mandibular glands, and heads without postpharyngeal glands and antennae of S. invicta were extracted in hexane at 4 °C overnight (Vander Meer et al., Reference Vander Meer, Preston and Choi2010). In this study, we soaked whole ants in hexane overnight at room temperature to later detect the alarm pheromone component in the crude extract reported by Vander Meer et al. (Reference Vander Meer, Preston and Choi2010). The resulting extraction efficiency is almost linear from 0 to 48 h tending to saturation level after 48 h, thus 48 h was selected for all extractions.
Purification of alarm pheromone component of fire ants is not an easy task because of high volatility and small quantities (Vander Meer et al., Reference Vander Meer, Preston and Choi2010). The results of this study indicate that an alarm pheromone component can be detected from whole ant extracts. This method is rapid and practical for preparation and analytical convenience, but further studies are needed to verify the feasibility of obtaining richer 2E36DMP fractions. It would also be interesting to try and detect the same compound from other ant extracts.
Relative amounts of alarm pheromone component among S. invicta castes
All ant castes use chemicals for communication (Attygalle & Morgan, Reference Attygalle and Morgan1984), and clearly we showed that the different castes produce greatly different amounts of alarm pheromone. By weight, workers had more pyrazine, but by capita workers had the least pyrazine. For a given overall biomass, a species may have more, smaller individuals or fewer, larger individuals, although smaller individuals do have slightly higher metabolic requirements (Calabi & Porter, Reference Calabi and Porter1989; Morrison, Reference Morrison2000). A similar principle can be applied to the different ant castes here. With the same mass, there are more workers than alates, thus they will add up to larger amounts of pheromone.
Our data on pyrazine amounts in an individual ant from different castes were consistent with previous research (Vander Meer et al., Reference Vander Meer, Preston and Choi2010). Our finding that individual workers had significantly less pyrazine content than individual male and female alates is consistent with the findings of prior research on S. invicta (Vander Meer et al., Reference Vander Meer, Preston and Choi2010). Worker and alate castes were also found to differ in the concentration of alarm pheromones in Camponotus abdominalis (Blum et al., Reference Blum, Snelling, Duffield, Herman, Lloyd and Trager1988) and some other ant species (Brand et al., Reference Brand, Duffield, MacConnell, Blum and Fales1973; Lloyd et al., Reference Lloyd, Blum and Duffield1975; Do Nascimento et al., Reference Do Nascimento, Morgan, Billen, Schoeters, Della Lucia and Bento1993; Hernández et al., Reference Hernández, Cabrera and Jaffe1999). This leads to the question: If an alarm pheromone is predominantly used to recruit workers to defend a nest or resources, why do alate castes produce more alarm pheromone? A reasonable explanation would be that higher alarm pheromone concentrations in male and female sexuals indicate that it does not only serve a defense function but may also promote reproductive activity. In fact, Choi & Vander Meer (Reference Choi and Vander Meer2015) found that S. invicta male and female sexuals use mandibular gland secretions for mating flight initiation and during mating flights. Additionally, ant alarm pheromones could also play an important role in nestmate recognition (Hughes et al., Reference Hughes, Howse and Goulson2001). It is common for individual chemicals to serve different functions when they are emitted with other semiochemicals, or when emitted in different contexts. Further investigations on the functions of alarm pheromone in each caste will help to better understand its social role.
Alarm pheromone concentrations differ in workers of the exotic and native fire ants
Workers are normally by far more numerous than alates in ant colonies and therefore their behavior dictates colony biology (Buechel et al., Reference Buechel, Wurm and Keller2014). At the colony level, competitive ability may correlate more with the size of the group than with the size of the individuals (Buss, Reference Buss1981). Therefore, it makes more sense comparing the mean amount of workers based on their equivalent mass between species than between single ants. This view is supported by a series of laboratory behavioral observations that S. invicta would control more foraging territory than native counterparts given colonies of equivalent worker biomass, but not when colonies were equivalent in numbers of workers (Morrison, Reference Morrison2000). Our data showed that 2E36DMP could be detected in all chemical analyses of the tested fire ant species, suggesting that closely related Solenopsis fire ants may share the same alarm pheromone component. It has been in fact reported that the alarm pheromone in fire ants is not species-specific (Wilson, Reference Wilson1965; Blum Reference Blum1969), and that some species can respond to alarm pheromones produced by other species (Blum, Reference Blum1969; Hughes et al., Reference Hughes, Howse and Goulson2001). 2E36DMP and some pyrazine analogs were shown to trigger significant alarm response in S. invicta workers (Guan et al., Reference Guan, Lu, Liao, Wang and Chen2014; Sun et al., Reference Sun, Shao, Lu, Shi, Wang and Chen2017). The response of workers from other fire ant species to 2E36DMP and pyrazine analogs still awaits further investigation.
The concentration of the alarm pheromone component in workers of the two exotic fire ants and their hybrid was significantly higher than that in workers of the native S. geminata. This difference suggests a potential link between alarm pheromone production and invasion success, and may be a factor behind the displacement of the native fire ant species by exotic fire ant species (Fadamiro et al., Reference Fadamiro, He and Chen2009).
Chemical alarm communication particularly occurs among social insects where the number of individuals per colony is large (Wilson, Reference Wilson1962; Maschwitz, Reference Maschwitz1964). Solidarity is a strength that emphasizes the importance of teamwork. There is little doubt that efficient and organized teamwork contributes to the competitive advantages of social insects in their foraging, predation, defense, and reproduction. It is likely that alarm pheromones play an important role in the organization and coordination of ants to promote their success as an invasive taxon (Blum, Reference Blum and Beroza1970). A higher concentration of alarm pheromone component in the exotic fire ants may favor wider distributions, thus partially explaining their success as invasive species.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007485317001201.
Acknowledgements
The authors thank Dr Sanford D. Porter (Center for Medical, Agricultural and Veterinary Entomology, USDA-ARS) for providing Solenopsis geminata samples. This research was supported by the National Natural Science Foundation of China (Grant No. 31572315, 30970402).







