Introduction
Parasitoid wasps frequently have been used as model organisms for the study of life history evolution (Godfray, Reference Godfray1994; Mackauer et al., Reference Mackauer, Sequeira, Otte, Detter, Bauer and Völkl1997; Strand, Reference Strand, Hochberg and Ives2000; Harvey, Reference Harvey2005). As larval parasitoids obtain resources for growth, development and survival from just a single host individual, parasitoid fitness is closely linked to the quality of the hosts attacked. One of the strongest patterns to emerge from studies on the life history strategies of parasitoids is that large body size confers greater fitness (Godfray, Reference Godfray1994) and closely correlates with the size of the host at parasitism (Charnov et al., Reference Charnov, Los-den-Hartogh, Jones and van den Assem1981; Waage, Reference Waage, Waage and Greathead1986). While this correlation holds true for most idiobiont parasitoids, it does not apply to most koinobiont parasitoids for which the host represents a potentially dynamic resource that may vary many times in size between parasitoid oviposition and host death (Mackauer & Sequeira, Reference Mackauer, Sequeira, Beckage, Thompson and Federici1993; Harvey, Reference Harvey2000). Hence, the future growth of, and total resources available to, the developing koinobiont larva can often depend on the host's age or stage of development, rather than on its size, at the time of oviposition (Sequeira & Mackauer, Reference Sequeira and Mackauer1992; Mackauer et al., Reference Mackauer, Sequeira, Otte, Detter, Bauer and Völkl1997).
Aphidiine wasps provide some of the best-studied examples of adaptive responses to variable host resources (see reviews in Mackauer et al., Reference Mackauer, Sequeira, Otte, Detter, Bauer and Völkl1997). Aphid hosts of aphidiine parasitoids can differ in size, age, behavior and physiological or chemical attributes; but size, instar or age invariably provide the main focus for studies of host quality and its effect on parasitoid fitness (Chau & Mackauer, Reference Chau and Mackauer2000; Cloutier et al., Reference Cloutier, Duperron, Tertuliano and McNeil2000; Colinet et al., Reference Colinet, Salin, Boivin and Hance2005). Additionally, in most studies, host size is confounded with instar or age, as these attributes of host aphid nymphs inevitably are related to each other. Thus, it remains unclear which cues koinobiont parasitoids, such as aphidiine wasps, exercise to assess host quality. Kouamé & Mackauer (Reference Kouamé and Mackauer1991) explored this question using a regimen of controlled starvation to provide variation in size and stage of development at the same age for pea aphids, Acyrthosiphon pisum (Harris), exposed to parasitism by Ephedrus californicus Baker. However, as the starved aphid hosts showed abnormal patterns of growth and development, it remains unclear whether the parasitoids were responding to variation in the nutritional status of the aphids rather than their variation in size relative to age. Li & Mills (Reference Li and Mills2004) recently evaluated the importance of host size, as compared to host instar, as an index of host quality. They manipulated temperature to produce black bean aphid hosts, Aphis fabae Scopoli, which varied in size at the same instar for parasitism by Aphidius transcaspicus Telenga. They found contrasting responses of the parasitoid to host size for aphids reared at two different temperatures. However, this specialist parasitoid is more typically associated with mealy aphids (Hyalopterus spp.) rather than A. fabae, and it remains unclear whether the responses of the parasitoid to host size and instar were truly representative or generated by an unusual host.
Here, we use a natural aphid host-aphidiine parasitoid system, the black bean aphid, A. fabae, and Lysiphlebus ambiguus Haliday. L. ambiguus is distributed throughout Europe (Ståry, Reference Ståry1970), Central Asia (Ståry, Reference Ståry1979), and eastern Asia (Chang & Youn, Reference Chang and Youn1983) and is a generalist parasitoid capable of using multiple aphid species as hosts (Anjum et al., Reference Anjum, Ashraf and Mahmood2002). Rearing temperature was used to provide cohorts of aphids that differed in size at the same instar, following the protocols of Li & Mills (Reference Li and Mills2004). We intended, by this approach, to evaluate whether this generalist aphidiine wasp would show a consistent response to host size for aphid cohorts raised at low and high temperatures, and how well the response to aphid size matched the suitability of the aphids for subsequent parasitoid fitness-related performances.
Materials and methods
Insect cultures
The aphid parasitoid, L. ambiguous, was field collected from Zhenjiang city in eastern China in November 2005 from the black bean aphid, A. fabae, on broad beans, Vicia fabae. The parasitoid was maintained on black bean aphids in wooden cages (45×45×50 cm) covered by plastic organza under a naturally fluctuating temperature and photoperiod. We cultured broad bean plants in plastic pots (15 cm dia.) as host plants for the aphid colonies.
Experimental design
To obtain cohorts of aphids of the same size and age, we transferred adult virginoparae from the stock colonies to individual experimental seedlings for a period of 6 h. The adult aphids subsequently were returned to the stock colonies and the offspring produced were kept as a synchronous cohort. To obtain the same host instar with different body size, the aphid cohorts were produced and reared in growth chambers (L 16: D 8, 40–50% RH) at either 30±1°C or 15±1°C. The aphids developed through four instars to reach the adult stage at both temperatures, but at 30°C the aphid cohorts developed rapidly with small body size, whereas at 15°C they developed slowly with large body size. As the relative duration of the different instars was fairly constant at different temperatures (Tsitsipis & Mittler, Reference Tsitsipis and Mittler1976), the aphid cohorts at a certain age representative of a specific instar were transferred into an insectary room (≈23°C, 45% RH) for exposure to parasitism and subsequent rearing to adult emergence of the parasitoid offspring.
Experimental aphid cohorts consisted of 50 individuals of either 1st, 2nd, 3rd or 4th instars or apterous adults with any additional aphids from the cohort collected into 75% ethanol for later body size measurement. Excised broad bean leaves were wrapped at the base of petioles with wet cotton and parafilm to keep them fresh, then spread over the bottom of plastic food boxes (6×6×4 cm). Experimental cohorts of aphids were transferred with a soft brush onto the excised leaves in the box, and then one male and one female parasitoid (naïve wasps of 3–4 days old) were introduced into the box for a period of 3 h. Parasitoids used in the experiment were similar in size. Fifteen replicates were performed for each aphid instar and rearing temperature, and any replicates without mummy production were excluded from the analysis (see table 2 for final sample sizes for each experiment).
The body size of host aphids and parasitoids was estimated by measuring the length of the right hind tibia under a dissecting microscope (OLYMPUS-DP12) using the software of Motic Images Plus 2.0 with a measurement accuracy of 0.001 mm. Hind tibia length is a standard measure of size for parasitoids (Godfray, Reference Godfray1994), and in aphids it shows a strong non-linear relationship with aphid dry mass (Nicol & Mackauer, Reference Nicol and Mackauer1999). To assess the overall susceptibility of the aphid cohorts to parasitism by L. ambiguus, we used the number of mummies produced per replicate during the 3 h exposure period. Although this measure does not distinguish between the acceptability of aphid cohorts for oviposition and their suitability for supporting successful parasitoid development (survivorship), it does provide a useful measure of the net effect of the different aphid instars on the reproductive success of parasitoid (Li & Mills, Reference Li and Mills2004; Henry et al., Reference Henry, Gillespie and Roitberg2005). The development time of the parasitoid progeny was defined as the period from the release of parent females into the experimental boxes to the emergence of the adult progeny, averaged across all individuals within each of the replicate boxes. Sex ratios were defined as the proportion of females among the parasitoid progeny.
Statistical analysis
The dependence of aphid size on age at the two rearing temperatures was analyzed by linear regression based on the hind tibia length of two aphids from each of the 15 cohorts of aphids set up for exposure to parasitism by L. ambiguus. Separate two-way ANOVAs, with temperature and aphid instar as factors, were performed to test for variation in size of host aphids exposed to parasitism and for possible variation in the susceptibility of particular aphid cohorts to parasitism by L. ambiguus. A t-test was used to compare the size of host aphids between temperatures within each instar, using Bonferroni correction to adjust significance levels for multiple tests (Quinn & Keough, Reference Quinn and Keough2004). The dependence of various measures of parasitoid performance on aphid size was analyzed by regression, based on mean values from the parasitoid progeny within each replicate. Prior to analysis, emergence rates and sex ratios were transformed by arcsin square root, while hind tibia length and development time were log-transformed, to normalize the distributions. The data were first tested for departure from linearity using ANOVA, based on a comparison of linear and second order polynomial regression models, before fitting each linear model (Zar, Reference Zar1999). Data analyses were performed using Minitab 14.0 statistical software (Eddison, Reference Eddison2000).
Results
The black bean aphid, A. fabae, was significantly larger in size with slower development at 15°C than at 30°C within each instar (t-test, P<0.001; table 1). Thus, the development time from 1st instar through adult took more than twice as long at 15°C compared to 30°C. Body size, as estimated by hind tibia length, increased with age but at a significantly lower rate at 15°C compared to 30°C (15°C: y=0.05+0.14x, R 2=0.968, F 1,148=4465.84, P<0.001; 30°C: y=0.08+0.05x, R 2=0.965, F 1,148=4098.53, P<0.001; comparison of slopes: t=10.83, df=296, P<0.001).
Table 1. Comparison of the instar, size (mean hind tibia length (HTL)), and median age of the cohorts of Aphis fabae reared at 15°C and 30°C for exposure to parasitism by Lysiphlebus ambiguus.
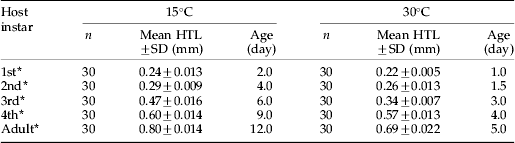
* An unpaired t-test showed a significant difference in host aphid size between temperatures within each instar (1st instar: t=4.64, df=58, P<0.001; 2nd instar: t=9.06, df=58, P<0.001; 3rd instar: t=3.08, df=58, P<0.001; 4th instar: t=5.37, df=58, P<0.001; Adult: t=2.01, df=58, P<0.001).
The number of mummies produced did not differ significantly between temperatures, but a significant difference was detected between aphid instars and there was a significant interaction (table 2; two-way ANOVA, temperature: F 1,114=2.58, P=0.11; instar: F 4,114=15.12, P<0.001; interaction: F 4,114=3.01, P=0.02). More mummies were produced from younger instars (1st instar for 15°C, 1st and 2nd instars for 30°C) than from older instars (adult for 15°C, 4th instar and adult for 30°C). This suggests that the younger aphid nymphs were, to some extent, more susceptible to parasitism than the older nymphs and adults at both temperatures, although the interaction arises from a differential response to 2nd instars. The emergence rate of adult progeny from mummies did not differ among aphid instars at 15°C, but more adults emerged from mummies produced from attacks on younger instars than from those produced from attacks on older instars at 30°C (F 4,58=9.77, P<0.001; table 2).
Table 2. The overall susceptibility (number of mummies produced) and suitability (emergence rate of the parasitoids) of the 50 aphids in each cohort exposed to parasitism by Lysiphlebus ambiguous.
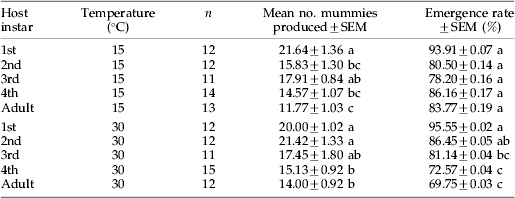
Different letters indicate significant differences between instars at rearing temperature of either 15°C or 30°C (Tukey test, P<0.05).
Use of a second-order polynomial regression confirmed that there was no departure from linearity for any of the fitness correlates used in relation to aphid size (P<0.05 in all cases). The proportion of female parasitoid progeny generally increased with aphid size, from 0.61 (±0.06 SEM) in 1st instars to 0.82 (±0.02 SEM) in adult aphids at the time of parasitism, for cohorts raised at 15°C (fig. 1a). In contrast, for cohorts raised at 30°C, the sex ratio declined steadily with aphid size from 0.83 (±0.03 SEM) in 1st instar aphids to 0.63 (±0.02 SEM) in adult aphids (fig. 1b). Development time increased with aphid size at the time of parasitism for cohorts raised at 15°C from 10.19 (±0.12 SEM) days in 1st instar aphids to 11.61 (±0.15 SEM) days in adult aphids (fig. 1c). However, there was no variation in parasitoid development time with aphid size at the time of parasitism for aphid cohorts raised at 30°C(F=1.121, P=0.31), with an average development time of 10.88 (±0.10 SEM, n=62) days (fig. 1d).

Fig. 1. Mean progeny sex ratio at (a) 15°C and at (b) 30°C, development time at (c) 15°C and (d) 30°C, female hind tibia length at (e) 15°C and (f) 30°C, and male hind tibia length at (g) 15°C and (h) 30°C for Lysiphlebus ambiguus in relation to the size of Aphis fabae hosts at the time of parasitism. Linear regression analysis: (a) 15°C: y=0.53+0.23x, R 2=0.30, F 1,60=26.15, P<0.001; (b) 30°C: y=0.85−0.21x, R 2=0.44, F 1,60=46.06, P<0.001; (c) 15°C: y=9.66+1.26x, R 2=0.53, F 1,60=67.46, P<0.001; (d) 30°C: y=10.88±0.09 (SEM), F 1,60=1.22, P=0.31; (e) 15°C: y=0.44+1.26x, R 2=0.47, F 1,60=53.03, P<0.001; (f) 30°C: y=0.55−1.37x, R 2=0.50, F 1,60=60.05, P<0.001; (g) 15°C: y=0.41+1.34x, R 2=0.68, F 1,60=124.54, P<0.001; (h) 30°C: y=0.49−1.19x, R 2=0.22, F 1,60=16.47, P<0.001.
The body size of both female and male progeny increased with aphid size at the time of parasitism for the aphid cohorts raised at 15°C, from 0.47 (±0.009 SEM) mm in 1st instar aphids to 0.54 (±0.003 SEM) mm in adult aphids for females, and from 0.43 (±0.009 SEM) to 0.51 (±0.004 SEM) mm for males (fig. 1e,g). However, the body sizes of both female and male progeny decreased in relation to host size at the time of parasitism for cohorts raised at 30°C, from 0.52 (±0.008 SEM) mm in 1st instar aphids to 0.44 (±0.008 SEM) mm in adult aphids for females, and from 0.47 (±0.012 SEM) to 0.43 (±0.005 SEM) for males (fig. 1f,h).
Discussion
Cohorts of A. fabae raised at 15°C and 30°C and exposed to individual female L. ambiguus in no-choice tests were successfully parasitized in all host stages from 1st instar nymphs to adults. However, younger and smaller aphids were more susceptible to parasitism than older and larger nymphs or adults, as measured by the number of mummies produced, an overall measure of the effect of host characteristics on the success of parasitism (table 2). This finding is different from that revealed in a similar study, where no consistent pattern across the aphid instars in mummy production was demonstrated (Li & Mills, Reference Li and Mills2004). Several species of aphidiine parasitoids have been shown to selectively parasitize younger and/or smaller aphid instars, as later instars show more vigorous behavioral defenses than earlier instars (Hofsvang & Hagvar, Reference Hofsvang and Hagvar1991; Kouamé & Mackauer, Reference Kouamé and Mackauer1991; Chau & Mackauer, Reference Chau and Mackauer2000; Perdikis et al., Reference Perdikis, Lykouressis, Garantonakis and Iatrou2004). Although later instar A. fabae were seen to kick away female L. ambiguus at oviposition, the defensive behaviors of the aphid cohorts were not rigorously assessed during this study. There was also a surprising difference in the relative susceptibility of 2nd instar aphid hosts at the two rearing temperatures, being higher than expected for the cohort raised at 30°C and lower than expected for the cohort raised at 15°C, although the reason for this remains unclear.
Host quality is known to influence sex allocation in numerous parasitoids where male eggs are allocated to lower quality hosts and female eggs to higher quality hosts (Charnov & Skinner, Reference Charnov and Skinner1985; King, Reference King, Wrensch and Ebert1993). While host size is a reliable indicator of host quality at the time of oviposition for idiobiont parasitoids, it is less indicative for koinobiont parasitoids as host size provides an uncertain measure of future resources for progeny development (Waage, Reference Waage, Waage and Greathead1986; Sequeira & Mackauer, Reference Sequeira and Mackauer1992; Mackauer et al., Reference Mackauer, Sequeira, Otte, Detter, Bauer and Völkl1997; Harvey, Reference Harvey2000). Solitary koinobiont parasitoids are more likely to evolve host use strategies that increase the predictability of the quantity of host resources that will be available to their progeny (Strand, Reference Strand, Hochberg and Ives2000), so that they can respond to variation in host quality by allocating female eggs to high quality hosts. However, some aphidiine parasitoids have been shown to allocate female eggs in relation to host aphid size or host instar at the time of oviposition in both non-choice and choice situations (Singh & Pandey, Reference Singh and Pandey1997; Pandey & Singh, Reference Pandey and Singh1999; Cloutier et al., Reference Cloutier, Duperron, Tertuliano and McNeil2000; Chau & Mackauer, Reference Chau and Mackauer2001). This suggests that, unlike other koinobionts, aphidiines support the host size model that is characteristic of idiobionts (King, Reference King, Wrensch and Ebert1993; Harvey, Reference Harvey2005). This was indeed the case for our study of the L. ambiguus – A. fabae system for aphid cohorts reared at 15°C, but a contrasting oviposition strategy was observed for aphid cohorts raised at 30°C where more females were produced from hosts attacked as smaller instars. Assuming, first of all, that this is a sex allocation response, it would appear that the very small early instars of aphids raised at 30°C were estimated by female L. ambiguus to be high quality hosts, whereas the same early instars of larger aphids raised at 15°C were estimated to be of poor quality. However, differential mortality during development between male and female progeny can transform the primary sex ratio allocated by ovipositing female parasitoids (King, Reference King, Wrensch and Ebert1993). As female parasitoids are on average larger than males, they likely require more resources to complete development and would be more prone to mortality under stress, such as in hosts of poor quality (Hurlbutt, Reference Hurlbutt1987).
There are several lines of evidence suggesting that differential mortality during development influenced the observed secondary sex ratio in relation to aphid instar for aphid cohorts raised at 30°C. Firstly, there was a significant decline in mummy production from early to later instar aphid cohorts, although this was also apparent at 15°C and so may be partly due to aphid behavioral defense rather than differential mortality (table 2). Secondly, adult emergence rate, a measure of host suitability, clearly decreased from early to later instar aphid cohorts at 30°C in contrast to a more constant emergence rate at 15°C (table 2). Thirdly, female body size at 30°C was more affected by the successive host instars than was the case for males (as indicated by the steeper slope of the linear regression in fig. 1f,h). On the other hand, there is corresponding evidence suggesting that L. ambiguus also responded to the aphid cohorts through manipulation of primary sex ratios. For example, the sex ratio of parasitoid progeny emerging from attack of 1st instar hosts raised at 30°C was 85% female as compared to 65% for 1st instar hosts raised at 15°C (fig. 1a,b), a response that was also found by Li & Mills (Reference Li and Mills2004) for A. transcaspicus. Similarly, Cloutier et al. (Reference Cloutier, Lévesque, Eaves and Mackauer1991) have demonstrated that for E. californicus changes in the secondary sex ratio with successive aphid instars is due to manipulation of the primary sex ratio rather than differential mortality.
For aphid cohorts raised at 15°C, the observed relationship between host size at oviposition and both parasitoid development time and adult body size in L. ambiguus is consistent with the most frequently reported empirical relationship for idiobiont parasitoids profiled by Harvey (Reference Harvey2005). As young aphid nymphs at 15°C were larger at the time of oviposition, there were already more resources for parasitoid development, and there was no need for the parasitoid larvae to slow down their development. This is in contrast to the pattern of development most frequently observed among koinobiont parasitoids that attack small, early-instar hosts (reviewed by Harvey, Reference Harvey2005). However, the parasitoid larvae required greater time to complete their development when oviposition occurred in older aphids, perhaps because of growing competition for resources with embryos of the host. Brough et al. (Reference Brough, Dixon and Kindlmann1990) showed that the allocation of nutritional resources to somatic and gonadal tissues changes when aphids (Megoura vicia) approach reproductive age.
Parasitoid development time and adult body size in relation to host size at oviposition was very different for aphids reared at 30°C. It is interesting to note that female parasitoid progeny from attack of 1st instar hosts at 30°C grew to a larger size than progeny from 1st instar hosts raised at 15°C (fig. 1e,f), indicating that the parasitoid larvae were able to compensate for smaller host size by extending their development time by 1–2 days (fig. 1c,d). As all parasitoids must consume some minimum amount of host resources in order to pupate (Strand, Reference Strand, Hochberg and Ives2000), this may have been a factor in the constant development time across all host instars reared at 30°C, even at the cost of smaller body size
Numerous studies have demonstrated that the obligate bacterial endosymbiont, Buchnera aphidicola, provides aphids with essential amino acids (Douglas, Reference Douglas1998). It has also been shown that the growth and development of parasitoid larvae is closely linked to the activity of the bacterial symbiont. For example, Pennacchio et al. (Reference Pennacchio, Fanti, Falabella, Digilio, Bisaccia and Tremblay1999) found that Aphidius ervi performed poorly in pea aphids experimentally deprived of their symbiont; Rahbé et al. (Reference Rahbé, Digilio, Febvay, Guillaud, Fanti and Pennacchio2002) showed that synthesis of essential amino acids by Buchnera is preserved or promoted in parasitized aphids; and Cloutier & Douglas (Reference Cloutier and Douglas2003) demonstrated that the number and biomass of mycetocytes were elevated in parasitized pea aphids. Bacterial symbiont activity in pea aphids can be arrested by high temperature (37°C) shock treatment, however, which consequently retards nymphal growth (Ohtaka & Ishikawa, Reference Ohtaka and Ishikawa1991).
In a study of Buchnera activity in A. fabae, Li & Li (Reference Li and Li2006) demonstrated a steady decline in the number of mycetocytes in 2nd and later instar aphids with a rise in temperature from 15°C to 35°C. Their observations also showed that the number of mycetocytes markedly increased from 1st instar through to adult at temperatures below 30°C; whereas, at 30°C and above, the number of mycetocytes increased from 1st to 3rd instar, but then declined to approximately one half the number observed in adult aphids at temperatures below 30°C. Thus, the 30°C temperature treatment in the current study is likely to have resulted in nutrient stress on the parasitoids during their development, mediated by abnormal functioning of the endosymbiont bacteria. In addition, the level of nutrient stress would have intensified with greater duration of rearing of the aphid cohorts at 30°C, particularly affecting the 4th instar and adult cohorts. Thus, we postulate that the reduced performance of L. ambiguus when parasitizing the successive instars of A. fabae was likely due to reduced endosymbiont activity for aphid cohorts raised at 30°C.
In conclusion, through manipulation of host rearing temperature, we have shown that at cooler temperatures the koinobiont parasitoid, L. ambiguous, responds to host size in the same way as an idiobiont parasitoid but that this response is compromised at higher temperatures. These results confirm the earlier observations by Li & Mills (Reference Li and Mills2004) based on A. transcaspicus. Our results also suggest that differential mortality during development is likely to have influenced the observed secondary sex ratio in relation to aphid instar for aphid cohorts raised at 30°C due to disruption of the activity of the host's primary endosymbiont and that increased development time cannot fully compensate for the reduced nutritional quality of aphids reared at higher temperatures.
Acknowledgements
We thank Gao Xiao-wen for help in field collection of the aphid parasitoid and referees for constructive reviews on the manuscript. This study was supported by the National Natural Science Fundation of China (NSFC, 30370237).





