Introduction
Solanum melongena L. var. serpentinum (Solanales: Solanaceae), commonly known as eggplant, aubergine or brinjal, is one of the most important vegetables in the world. The area sown with eggplant exceeds 2 million hectares with a production of nearly 33 million tonnes (FAO, 2013). This crop is attacked by several insect pests in greenhouses and in open fields that often requiring chemical control by growers to protect yield (Gapud and Canapi, Reference Gapud and Canapi1994).
Greenhouse whitefly, Trialeurodes vaporariorum Westwood (Hem.: Aleyrodidae), is one of the most destructive pests of greenhouse crops. Similar to other sap-sucking pests, it causes direct damage by sucking and indirect damage by producing honeydew, which affects photosynthetic capability (Martin, Reference Martin1987; Byrne et al., Reference Byrne, Bellows, Parella and Gerling1990). This pest is also known to be the vector of several plant viruses (Tzanetakis et al., Reference Tzanetakis, Halgren, Wintermantel, Keller and Martin2004). The destructive activity of T. vaporariorum has been primarily controlled by chemical pesticides belonging to the four major pesticide groups, but their negative effects, such as pest resistance and toxicity problems for humans and the environment, limit their use by farmers (Orden et al., Reference Orden, Patricio and Canoy1994; Saldo and Szpyrka, Reference Saldo and Szpyrka2009). Therefore, an alternative approach of pest control without depending on chemical insecticides would be essential.
Zoophytophagous insects refer to omnivorous natural enemies that feed on both prey and plant during their developmental stages (Castañé et al., Reference Castañé, Arnó, Gabarra and Alomar2011). Phytophagy has several benefits, including facilitating the establishment of these predators in the crop and allowing them to survive in a crop when prey is scarce or totally absent (Urbaneja et al., Reference Urbaneja, Tapia and Stansly2005; Puentes and Björkman Reference Puentes and Björkman2017). This is the case for some zoophytophagous mirid predators (Hemiptera: Miridae), such as Nesidiocoris tenuis (Reuter), which has been commercially released for several greenhouse pests such as whiteflies, spider mites, aphids, and the South American tomato pinworm (Calvo et al., Reference Calvo, Lorente, Stansly and Belda2012; Urbaneja et al., Reference Urbaneja, Gonzalez-Cabrera, Arno and Gabarra2012; Pérez-Hedo et al., Reference Pérez-Hedo, Suay, Alonso, Ruocco, Giorgini, Poncet and Urbaneja2017; Biondi et al., Reference Biondi, Guedes, Wan and Desneux2018). Previous studies showed that feeding and/or oviposition activity of N. tenuis trigger induce effective defense responses in plants against herbivores (Pappas et al., Reference Pappas, Steppuhn, Geuss, Topalidou, Zografou, Sabelis and Broufas2015; Naselli et al., Reference Naselli, Urbaneja, Siscaro, Jaques, Zappalà, Flors and Pérez-Hedo2016; Pappas et al., Reference Pappas, Steppuhn and Broufas2016; Bouagga et al., Reference Bouagga, Urbaneja, Rambla, Granell and Pérez-Hedo2018a, Reference Bouagga, Urbaneja, Rambla, Flors, Granell, Jaques and Pérez-Hedo2018b; Pérez-Hedo et al., Reference Pérez-Hedo, Arias-Sanguino and Urbaneja2018a, Reference Pérez-Hedo, Rambla, Granell and Urbaneja2018b).
It has been established that almost all plant species suffer from attack by herbivores and have unique defense responses against insect herbivory (War et al., Reference War, Paulraj, Ahmad, Buhroo, Hussain, Ignacimuthu and Sharma2012; Golizadeh et al., Reference Golizadeh, Abedi, Borzoui, Golikhajeh and Jafary2016). Phytohormones such as jasmonic acid (JA) and abscisic acid (ABA) are key regulators of direct and indirect defenses against insect herbivores (Kagale et al., Reference Kagale, Marimuthu, Thayumanavan, Nandakumar and Samiyappan2004; Holopainen et al., Reference Holopainen, Heijari, Nerg, Vuorinen and Kainulainen2009). Exogenous applications of these phytohormones on plants may enhance plant resistance through the accumulation of secondary metabolites, including isoflavonoids, terpenoids, phenolics, alkaloids, as well as defensive proteins (Poelman et al., Reference Poelman, Broekgaarden, van Loon and Dicke2008; Sharma et al., Reference Sharma, Sujana and Rao2009; Mouttet et al., Reference Mouttet, Kaplan, Bearez, Amiens-Desneux and Desneux2011).
Much work has focused on the effects of synthetic hormones on plant resistance against insect pests. For example, Liu et al. (Reference Liu, Du, Ding, Zhou, Xie and Wu2017) reported a significant decrease of deposited eggs by Nilaparvata lugens (Stål) (Hemiptera: Delphacidae) when the rice plants were treated with ABA. Nouri–Ganbalani et al. (Reference Nouri-Ganbalani, Borzoui, Shahnavazi and Nouri2018) reported that population parameters of Plutella xylostella (L.) (Lepidoptera: Plutellidae) were negatively affected by exogenous applications of JA to canola.
Polyphenol oxidase (PPO) and peroxidase (POD) are enzymatic antioxidants that are activated in plants by insect feeding, mechanical wounding, or hormones (War et al., Reference War, Paulraj, Ahmad, Buhroo, Hussain, Ignacimuthu and Sharma2012 & references therein) and play a main role in host–herbivore interactions (Usha Rani and Jyothsna, Reference Usha Rani and Jyothsna2010; Soffan et al., Reference Soffan, Alghamdi and Aldawood2014). Phenylalanine ammonia lyase (PAL) is another key enzyme involved in plant defense against diseases and insects through phenylpropanoid pathway (Tonnessen et al., Reference Tonnessen, Manosalva, Lang, Baraoidan, Bordeos, Mauleon, Oard, Hulbert, Leung and Leach2015). Previous studies have shown that exogenous hormones can elevate the activities of defensive enzymes, such as PPO, POD, and PAL, and reduce the performance of herbivores (Sinha et al., Reference Sinha, Balasaraswathi, Selvaraju and Shanmugasundaram2005; Rani and Jyothsna, Reference Rani and Jyothsna2010; Punithavalli et al., Reference Punithavalli, Muthukrishnan and Rajkuma2013; Ye et al., Reference Ye, Song, Long, Wang, Baerson, Pan, Zhu-Salzman, Xie, Cai and Luo2013; Han et al., Reference Han, Li, Gong, Yang, Wen and Hou2016; Lv et al., Reference Lv, Kong, Liu, Lu, Zhang, Liu, Ji, Zhu, Su and Gao2017).
Despite this abundance of studies (as cited above), there exists a lack of research characterizing the effects of JA, ABA, and prior herbivory induced resistance in eggplant. The focus of this study was on the effects of stimulation via JA, ABA, and prior herbivory on the eggplant inducer-associated defense chemistry and on the performance of T. vaporariorum. Our findings provide an insight into the complex chemical plant–herbivore interactions and hence can help to optimize the production of new crops.
Materials and methods
Chemicals
All chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Plants
Seeds of eggplant were obtained from the Plant and Seed Improvement Research Institute (Karaj, Iran). Plants were sown in 10 cm diameter plastic pots filled with a mixture of Coco peat (50%), perlite (40%), and peat moss (10%) and in the research greenhouse of the Vali-e-Asr University of Rafsanjan (Kerman, Iran), set at 26°C with natural daylight. The pots were arranged in randomized block design and protected by 100-mesh muslin to prevent insect infestation. Six-leaf plants were used for the experiments. The eggplant seedlings were fertilized by Hoagland fertilizer once every two weeks.
Insects
The initial population of the greenhouse whitefly originated from cultures maintained in the laboratory of the Isfahan Center for Research of Agricultural Science and Natural Resources (Isfahan, Iran), in June 2016. In order to rear T. vaporariorum, adults were transferred to plants sown in plastic pots. The offspring were maintained inside a growth chamber that was set at 27 ± 2°C, 50 ± 5% R.H. with a photoperiod of 16:8 (L:D) hours. After 5 months, newly emerged adults were used for the experiments.
The initial population of N. tenuis originated from cultures maintained in the laboratory of the Iranian Research Institute of Plant Protection (Tehran, Iran), in July 2016. This predator was reared on eggplants sown in plastic pots and provided with T. vaporariorum nymphs and Ephestia kuehniella (Zeller) (Lep.: Pyralidae) frozen eggs as prey. Plants were kept inside a growth chamber that was set at 27 ± 2°C, 50 ± 5% R.H. with a photoperiod of 16:8 (L:D) hours. After 4 months, newly emerged adults were used for the experiments.
Experiments
Experiments were conducted during autumn and winter of 2016 on eggplant inside a growth chamber that was set at 27 ± 2°C, 50 ± 5% R.H. with a photoperiod of 16:8 (L:D) hours.
JA and ABA preparation
Synthetic JA was first dissolved in 1 ml of acetone and then diluted in an appropriate volume of water until it reached a concentration of 1.5 mM. JA solution was sprayed on six-leaf plants (Qiu et al., Reference Qiu, Harvey, Raaijmakers, Vet and van Dam2009). Control solution was acetone dissolved in water without JA. Synthetic ABA was first dissolved in 1 ml of NaOH (1 M) and then diluted in an appropriate volume of water until it reached a concentration of 100 µM. ABA solution was sprayed on six-leaf plants (Wang et al., Reference Wang, Yang, Guo, Fang, Zhou and Gu2015). Control solution was NaOH dissolved in water without ABA. For foliar application, plants were treated by the spraying of the solutions on expanded leaves. For root application, the solutions were injected into the soil around the root with a plastic syringe.
Plant treatments
Ten treatments were compared: (i) control, (ii) root JA application (RJA), (iii) leaf JA application (LJA), (iv) root ABA application (RABA), (v) leaf ABA application (LABA), (vi) prior feeding by N. tenuis, (vii) RJA + N. tenuis, (viii) LJA + N. tenuis, (ix) RABA + N. tenuis, and (x) LABA + N. tenuis.
For JA treatment, roots or leaves of 20 eggplants were separately treated with 10 ml of JA solution (10 plants for each root and leaf treatment). Ten plants were treated with 10 ml of control solution. For ABA treatment, roots or leaves of 20 eggplants were separately treated with 10 ml of ABA solution (10 plants for each root and leaf treatment). Ten plants were treated with 10 ml of control solution. For prior feeding by N. tenuis treatment, newly emerged adults of N. tenuis (>24 h old with undefined sex) that had been starved for 4 h were transferred on top leaves of 10 caged eggplants at the rate of 25 insects per plant. Control plants were not manipulated. For hormone plus prior feeding by N. tenuis treatment, roots or leaves of 20 eggplants were separately treated with 10 ml of each hormone (10 plants for each root and leaf treatment). After 1 h, N. tenuis adults (>24 h old) that had been starved for 4 h were transferred on top leaves of caged eggplants at the rate of 25 insects per plant. Ten plants were treated with 10 ml of each control solution, separately. To prevent the inductive effects of treated plants on control plants, the plants of each group were separated by using different cages. In all cases, bioassays and extractions were performed 3 days after the treatment.
Development and survival of immature stages
Initially, ten female–male pairs (10 males and 10 females) of T. vaporariorum adults (4-day old) from the stock colony were transferred onto 10 caged eggplants. Females were allowed to deposit eggs for 24 h; after that, one egg per leaf and a total of five eggs per plant were randomly selected and the remaining eggs were removed by a needle. Experiments were started with 50 eggs for all treatments. These eggs and nymphs that came from them were observed daily, and the duration of egg, nymphal stage, and pharate adult stage was recorded.
To study the cumulative percent mortality of T. vaporariorum immatures, 50 newly deposited eggs (<24 h) per plant were selected, as described above. Mortality was recorded daily until the immature stages completed their development or died. The experiment was replicated five times for each treatment or control.
Adult longevity and fecundity
After the pharate adult became an adult, one pair of male and female adults was transferred onto each rearing container (diameter 5 cm, depth 10 cm) attached to one leaf of the same treatment. The number of pairs of tested adults for each control and treated plant depended on their survival from the ‘Development of immature stages’ section and ranged from 9 to 20 couples. The adult longevity, as well as the reproduction of females, was recorded daily until the death of all individuals. This experiment was performed for all control and treated plants.
Oviposition free choice assay
To test T. vaporariorum free-choice between control and treated plants, eggplant used in this experiment were matched for size and number of leaves. The pots of control and each treatment were arranged randomly in a 100 × 50 × 50 cm cage covered with a 50 mesh muslin screen net and maintained in a growth chamber. For each treatment, we collected one hundred fifty female–male pairs (150 males and 150 females) of T. vaporariorum adults (4-day old) from the stock colony and released inside each metal cage. Then, the adults were allowed to mate and oviposit freely between the plants for 2 days. The number of females per plant, the number of females per cm2 of leaf and the number of eggs deposited on each plant were counted at 48 h after release. The experiment was replicated five times for each treatment or control.
Leaf chemistry
Concurrent with the development experiments, the 3rd day after treatment, foliar samples of the control and treated eggplants were collected for chemical analyses. Plants were treated with inducers when they had six expanded leaves. The sixth leaf was used to measure the phenolic content and chlorophyll as well as the activities of PPO, POD, and PAL. All assays were performed three times for each treatment.
Extraction of plants and enzyme activity assay
For extraction, 500 mg of leaves was homogenized, using a tissue grinder, with 50 mM potassium phosphate buffer (pH 7.0 containing 1 mM ethylenediaminetetraacetic acid, 1% (w/v) soluble polyvinylpyrrolidone, and 1 mM phenylmethylsulfonyl fluoride (PMSF)) and with liquid nitrogen for 1 min. The mixture was centrifuged at 20,000 × g for 20 min at 4°C, and the supernatant was stored in aliquots at −20°C. Protein concentrations of the leaf extract were measured using bovine serum albumin (Roche Co., Germany) (Bradford, Reference Bradford1976).
PPO activity was assayed according to the method reported by Stout et al. (Reference Stout, Fidantsef, Duffey and Bostock1999) to calculate the rate of formation of melanin-like material from catechol. The increase in absorbance for 3 min at 420 nm was recorded as the measure of PPO activity. POD activity was assayed according to the method reported by Plewa et al. (Reference Plewa, Smith and Wagner1991) to calculate the rate of the oxidation of guaiacol to tetraguaiacol. The increase in absorbance for 3 min at 470 nm was recorded as the measure of POD activity. PAL activity was assayed according to the method reported by D´cunha et al. (Reference D´cunha, Satyanarayan and Nair1996) to calculate the rate of cinnamic acid production. The increase in absorbance for 3 min at 260 nm was recorded as the measure of PAL activity. Enzyme activities were expressed as unit per mg protein.
Total phenolic content was assayed according to the Folin–Ciocalteu method reported by Gao et al. (Reference Gao, Ohlander, Jeppsson, Björk and Trajkovski2000) and quantified by gallic acid (GA) as standard. Results were expressed as mg GA per gram of the fresh weight. Chlorophyll content was assessed using a method reported by Sumanta et al. (Reference Sumanta, Haque, Nishika and Suprakash2014), where the absorbencies were read at 400–700 nm. It was reported that chlorophyll A and chlorophyll B showed the maximum absorbance at 663 and 645 nm, respectively. Results were expressed as mg per gram of the fresh weight.
Data analysis
All data calculated for each study were examined for normality using Kolmogorov–Smirnov test (PROC GLM; SAS Institute, 2011). Controls of different treatments were compared and, because there was no statistically significant difference, the mean of controls was used in the statistical analysis. One-way ANOVA, based on a completely-randomized design, was used to compare the effects of induced resistance on the biological aspects and oviposition preference of T. vaporariorum, and also the biochemical analyses of eggplants. The statistical differences among means were compared at P < 0.05 and Tukey's HSD method using SAS 9.3 software (PROC GLM, SAS Institute, 2011).
Results
Growth rates and mortality of immature stages
The incubation period was not statistically significant in the insects reared on the control and treated plants (F = 0.45; df = 9, 472; P = 0.9073; table 1). The shortest nymphal period was detected on control plants (9.84 days), and the longest on RJA + N. tenuis (11.53 days) and RABA + N. tenuis (11.64 days) (F = 9.70; df = 9, 408; P < 0.0001; table 1). The pharate adult period was the shortest in the insects reared on control plants (3.68 days), and was the longest in the insects reared on RABA + N. tenuis (4.29 days) (F = 2.38; df = 9, 377; P = 0.0125; table 1). T. vaporariorum exhibited the shortest developmental time of immature stages (from egg to adult stage) on control plants (19.44 days), while the longest one on RABA + N. tenuis (21.62 days) and RJA + N. tenuis (21.75 days) (F = 8.14; df = 9, 377; P < 0.0001; table 1).
Table 1. Mean (±SE) duration (days) of immature stages of T. vaporariorum fed on eggplants treated with JA, ABA, and/or N. tenuis a

a The roots or leaves of eggplants were treated with JA, ABA (1.5 mM for 3 days), and/or N. tenuis (25 adults for 3 days) prior to determining life history of T. vaporariorum. Mean values followed by different letters in the same column are significantly different (Tukey's test, P < 0.05).
Nymphs (F = 38.62; df = 9, 40; P < 0.0001) and pharate adults (F = 27.11; df = 9, 40; P < 0.0001) had significantly lower percent mortality on control (6.8 and 9.8%), and the higher on RABA + N. tenuis treatment (27.6 and 38.0%; fig. 1).
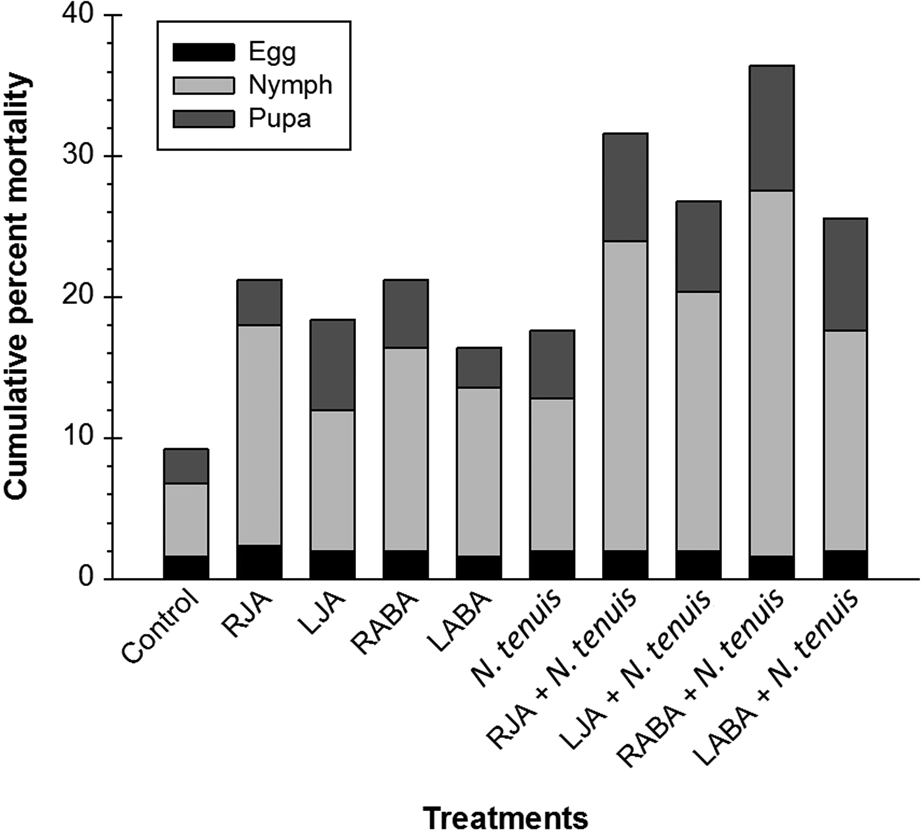
Figure 1. Mean (±SE) cumulative percent mortality of T. vaporariorum fed on eggplants treated with JA, ABA, and/or N. tenuis. Each point is average of five replications.
Adult longevity and fecundity
Different treatments showed a significant effect on the longevity of male (F = 7.55; df = 9, 203; P < 0.0001) and female (F = 8.87; df = 9, 164; P < 0.0001), which was the longest on control (13.33 and 17.36 days, respectively). Also, the shortest longevity of male and female was recorded on RJA + N. tenuis (10.10 and 13.52 days, respectively) (table 2).
Table 2. Mean (±SE) adult longevity and fecundity of T. vaporariorum fed on eggplants treated with JA, ABA, and/or N. tenuis a

a The roots or leaves of eggplants were treated with JA, ABA (1.5 mM for 3 days), and/or N. tenuis (25 adults for 3 days) prior to determining life history of T. vaporariorum. Mean values followed by different letters in the same column are significantly different (Tukey's test, P < 0.05).
Except for LABA, N. tenuis and LJA + N. tenuis, other treatments significantly reduced the fecundity of the females when compared with control plants (F = 4.89; df = 9, 164; P < 0.0001; table 2). The data showed that the highest number of deposited eggs was recorded for females fed on control plants (149.1 eggs per female), while the lowest was on RJA + N. tenuis treatment (118.2 eggs per female) (table 2).
Oviposition free choice assay
The number of T. vaporariorum females attracted to each treatment indicated that there was a significant difference among different treatments within 48 h (F = 138.44; df = 9, 40; P < 0.0001 for adults per plant and F = 22.92; df = 9, 40; P < 0.0001 for adults per cm2 of leaf; table 3). The whitefly adults were mostly attracted to control plants (77.8 adults per plant and 2.08 adults per cm2 of leaf), least to RJA + N. tenuis (7.0 adults per plant and 0.15 adults per cm2 of leaf), and RABA + N. tenuis (7.6 adults per plant and 0.16 adults per cm2 of leaf), and intermediately to other treatments. Also, the numbers of deposited eggs by T. vaporariorum significantly differed on control plants in comparison with treated plants, although, there was no significance in deposited eggs among different treatments (F = 24.14; df = 9, 40; P < 0.0001; table 3). Females laid 3.6 to 9.9-fold more eggs per cm2 of leaf on control plants when compared to treated plants.
Table 3. Oviposition preference (n = 5) of T. vaporariorum fed on eggplants treated with JA, ABA, and/or N. tenuis a

a The roots or leaves of eggplants were treated with JA, ABA (1.5 mM for 3 days), and/or N. tenuis (25 adults for 3 days) prior to determining ovipositional preference of T. vaporariorum. Mean values followed by different letters in the same column are significantly different (Tukey's test, P < 0.05).
Leaf chemistry of control and treated plants
Eggplants treated with different treatments showed the significant differences in the PPO (F = 7.68; df = 9, 20; P < 0.0001), POD (F = 6.70; df = 9, 20; P = 0.0002), and PAL (F = 19.55; df = 9, 20; P < 0.0001) activities compared to control plants (fig. 2). The PPO activity was higher in RABA + N. tenuis-treated plants (0.46 unit mg−1 protein), whereas the lowest activity was recorded in control plants (0.18 unit mg−1 protein; fig. 2a). The POD activity was significantly lower in control plants (0.18 unit mg−1 protein) when compared to treated plants, however, there was no difference in POD activity between different treatments (fig. 2b). In eggplants, exposure to both hormone and N. tenuis resulted in an increase in PAL activity, however, a statistically significant difference was not found in individual treatments (fig. 2c).
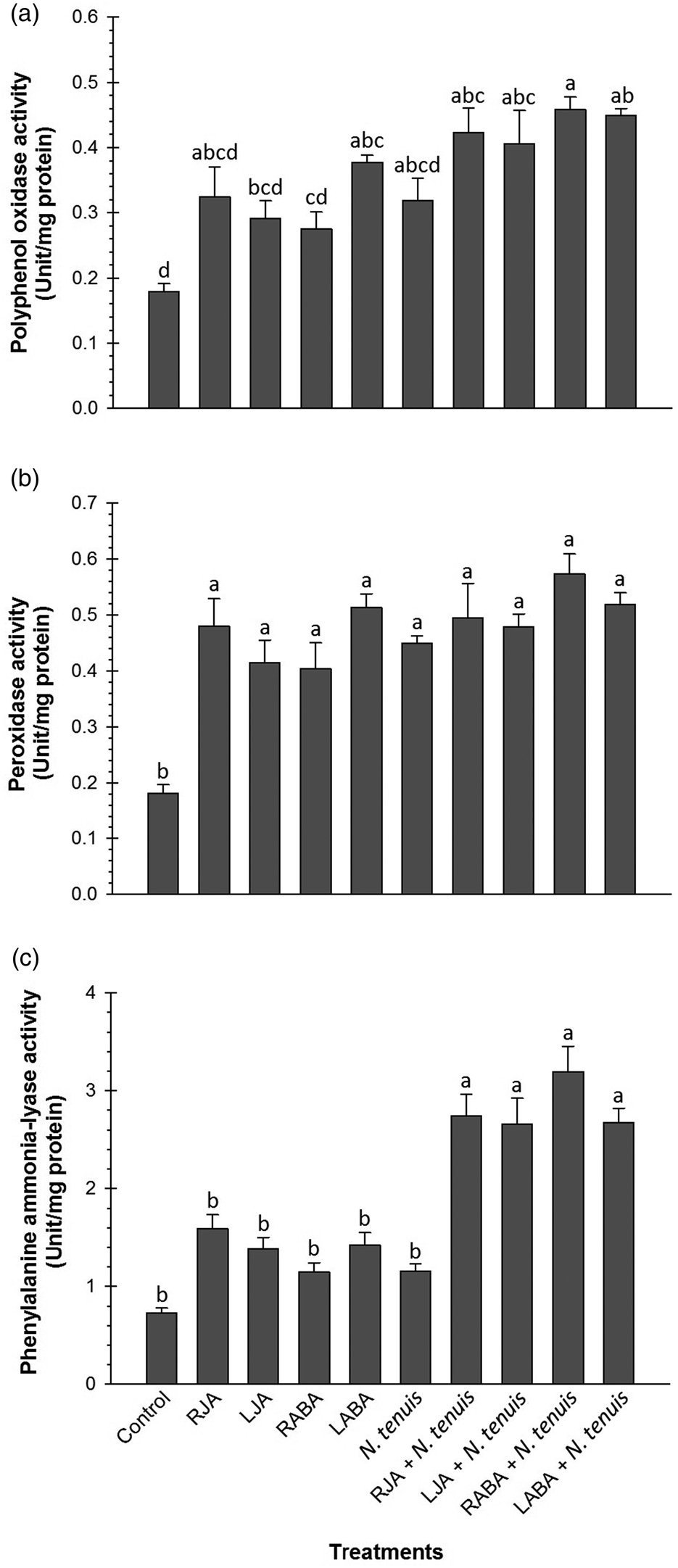
Figure 2. Effect of applying JA, ABA, and/or N. tenuis on the POD, PPO, and PAL activity in leaves of eggplants. Each point is average of three replications. Mean values followed by different letters are significantly different (Tukey's test, P < 0.05).
Under JA, ABA or N. tenuis treatments, total phenolic content was significantly higher in eggplants compared to the control plants (F = 47.59; df = 9, 20; P < 0.0001; fig. 3). The plants treated with both hormone and N. tenuis had relatively high amounts of total phenolic in comparison with plants treated with only one of the inducers. The highest total phenolic content was detected in plants treated with RABA + N. tenuis treatment (2.82 mg g−1 fresh weight), and the lowest on control plants (0.30 mg g−1 fresh weight).

Figure 3. Effect of applying JA, ABA, and/or N. tenuis on the phenolic content in leaves of eggplants. Each point is average of three replications. Mean values followed by different letters are significantly different (Tukey's test, P < 0.05).
Total chlorophyll content was equivalent for control and treated eggplants (F = 3.55; df = 9, 20; P = 0.0088; table 4). However, chlorophyll A content was significantly reduced in N. tenuis (0.60 unit mg−1 fresh weight), RJA + N. tenuis (0.66 unit mg−1 fresh weight), and RABA + N. tenuis (0.69 unit mg−1 fresh weight) (F = 2.68; df = 9, 20; P = 0.0320; table 4). By contrast, chlorophyll B content increased in LJA (0.74 unit mg−1 fresh weight) and LABA (0.73 unit mg−1 fresh weight) (F = 2.61; df = 9, 20; P = 0.0354; table 4).
Table 4. Leaf chlorophyll level of eggplants treated with JA, ABA, and/or N. tenuis a

a The roots or leaves of eggplants were treated with JA, ABA (1.5 mM for 3 days), and/or N. tenuis (25 adults for 3 days) prior to determining the chlorophyll level of leaves. Mean values followed by different letters in the same column are significantly different (Tukey's test, P < 0.05).
Discussion
The results of the present study showed that different treatments found to elicit higher mortality rates and longer developmental time in T. vaporariorum immature stages had a strong relationship with changes in enzyme activity. Levels of PPO, POD, and PAL were higher in the treated plants when compared to the control plants, and we noted that the activity of these enzymes was highest when eggplants were exposed to both hormone (in root application) and N. tenuis. Probably, the high activity of these enzymes reduces the plant quality for T. vaporariorum and has toxic effects on immatures (War et al., Reference War, Paulraj, Ahmad, Buhroo, Hussain, Ignacimuthu and Sharma2012). Our results are in agreement with those achieved by Soffan et al. (Reference Soffan, Alghamdi and Aldawood2014), who reported that Aphis craccivora Koch (Hem.: Aphididae) nymphs had the highest mortality and longer development on plants with increased levels of PPO and POD activity.
In our study, the longevity of adults was shorter and the fecundity lower when T. vaporariorum was reared on treated plants, especially RJA + N. tenuis and RABA + N. tenuis. Anti-nutritive compounds, such as phenolics, suppress the feeding by insect herbivore and/or reduce the plant digestibility, which in turn leads to a quantitative decrease of food intake with serious effects on its fitness (Chen et al., Reference Chen, Ni and Buntin2009). Increased POD and PAL activity, in our study, accumulates phenolic compounds and other metabolite compounds in damaged plants that play a key role in plant defense responses (La Camera et al., Reference La Camera, Gouzerh, Dhondt, Hoffmann, Fritig, Legrand and Heitz2004; Zhang et al., Reference Zhang, Hau and Zhang2008). Thus, a high amount of phenolic compounds is one of the primary characteristics that make a plant resistance to T. vaporariorum infestation. Duan et al. (Reference Duan, Yu, Bai, Zhu and Wang2014) reported a higher PAL activity in a cultivar resistant to Laodelphax striatellus Fallén (Hom.: Delphacidae) in comparison with a susceptible cultivar under non-infested conditions.
Our findings show that T. vaporariorum oviposition preference is most probably driven directly by the levels of phenolic compounds, resulting in over expression of POD and PAL (Li and Steffens, Reference Li and Steffens2002; Tonnessen et al., Reference Tonnessen, Manosalva, Lang, Baraoidan, Bordeos, Mauleon, Oard, Hulbert, Leung and Leach2015). Oviposition preference could also be related to chlorophyll content of leaves, especially chlorophyll A. Firdaus (Reference Firdaus2012) also found oviposition preferences of whitefly females for plants with a higher level of chlorophyll.
In general, the T. vaporariorum performance was lower on eggplants exposed to root induction compared to foliar induction. Tytgat et al. (Reference Tytgat, Verhoeven, Jansen, Raaijmakers, Bakx-Schotman, McIntyre, van der Putten, Biere and van Dam2013) and Papadopoulou et al. (Reference Papadopoulou, Maedicke, Grosser, van Dam and Martínez-Medina2018) reported that root and shoot JA induction elicits differential genes involved in the regulation and production of plant defense responses.
N. tenuis commonly appears in agricultural crops and natural vegetation in the Mediterranean region (Pérez–Hedo and Urbaneja Reference Pérez-Hedo, Urbaneja, Horowitz and Ishaaya2016). This predator has been introduced as an effective natural enemy of whitefly (Calvo et al., Reference Calvo, Bolckmans, Stansly and Urbaneja2009). Zoophytophagous predators, as a special case of natural enemies that feed on both plants and prey during the same developmental stage, can induce plant defenses (Halitschke et al., Reference Halitschke, Hamilton and Kessler2011; Pérez–Hedo et al., Reference Pérez-Hedo, Arias-Sanguino and Urbaneja2018a). In addition to the ability of N. tenuis to induce plant benefits directly by its entomophagy, our results also proved an indirect effect of this predator by its phytophagy through an increase in the antixenosis to T. vaporariorum.
In summary, results obtained in the present work showed that T. vaporariorum developed and reproduced on all treatments; however, the insects attained lower rates of development and survival on eggplants with greater levels of resistance, such as plants treated with RJA + N. tenuis and RABA + N. tenuis. Immature stages fed eggplant leaves developed slower on RJA + N. tenuis and RABA + N. tenuis, and fewer survived on these treatments. Correspondingly, female and male whiteflies from nymphs reared on RJA + N. tenuis lived for a shorter time, and when nymphs were fed on this and other treatments the females had lower fecundity in comparison with untreated plants. We hypothesize that the resistance of eggplants to T. vaporariorum was directly proportional to the level of enzyme activities and the amount of phenolic content of leaves. Our results are additional examples that provide evidence of the possible role of the POD, PPO, and PAL in mediating resistance to T. vaporariorum. Higher levels of these plant enzymes resulted in increased resistance of eggplants to this pest. However, complementary studies are crucial to identify the complexity of defense-signaling networks of plants and understand how they can be managed to impair the performance of herbivores.
Acknowledgements
The authors thank the Vali-e-Asr University of Rafsanjan (Rafsanjan, Iran), for cooperation by support for the experiment.










