Introduction
Tomato (Solanum lycopersicum L.) ranks fourth in production among the most important vegetables worldwide, and between 1999 and 2014 its global production increased by more than 56% (FAOSTAT, 2017). Among tomato pests, the tomato leaf miner (TLM), Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), is one of the most destructive, reducing tomato yield in many parts of the world. TLM, native to South America, is a serious threat in both greenhouse and open-field tomato production, and it can cause up to 80–100% crop loss (Desneux et al., Reference Desneux, Wajnberg, Wyckhuys, Burgio, Arpaia, Narvaez-Vasquez, Gonzalez-Cabrera, Ruescas, Tabone and Frandon2010). It is estimated that 84.9% of areas growing tomato throughout the world and 87.4% of total tomato production are directly threatened by TLM and either area already infested or may be in the near future (Desneux et al., Reference Desneux, Luna, Guillemaud and Urbaneja2011). A recent study revealed that TLM increased from infesting 3 to 60% of the tomato-cultivated area worldwide in 10 years and some of major tomato-producing areas, such as China, Mexico, and the USA which produce 42% of the world's tomatoes, are at high risk of being invaded by TLM (Biondi et al., Reference Biondi, Guedes, Wan and Desneux2018). Although TLM's primary host plant is tomato, other solanaceous plants such as potato, pepper, and eggplant are suitable hosts (Desneux et al., Reference Desneux, Wajnberg, Wyckhuys, Burgio, Arpaia, Narvaez-Vasquez, Gonzalez-Cabrera, Ruescas, Tabone and Frandon2010). Both yield and fruit quality can be significantly reduced by direct feeding of TLM, and secondary pathogens may enter through the mines and holes made by the pest (Santos et al., Reference Santos, Bueno, Vieira and Bueno2011). Although chemical insecticides are widely used to control TLM, research on its natural enemies and their application is crucial because of pest resistance (Roditakis et al., Reference Roditakis, Vasakis, Garcia-Vidal, del Rosario Martinez-Aguirre, Rison, Haxaire-Lutun, Nauen, Tsagkarakou and Bielza2018) and risk of pesticides residue in highly consumed vegetable, such as tomato.
Although the use of plant-feeding predators such as certain mirid bugs for biological pest control has traditionally been neglected due to a perceived risk of feeding on the crop (Castane et al., Reference Castane, Arno, Gabarra and Alomar2011), these predators have come to be recognized as useful biological control agents for greenhouse crops (Calvo et al., Reference Calvo, Bolckmans, Stansly and Urbaneja2009; Molla et al., Reference Molla, Gonzalez-Cabrera and Urbaneja2011). The zoophytophagus bug, Nesidiocoris tenuis (Reuter) (Heteroptera: Miridae), is an omnivorous predator widely used in integrated pest management (IPM) programs in both greenhouse and outdoor tomato crops (Urbaneja et al., Reference Urbaneja, Gonzalez-Cabrera, Arno and Gabarra2012; Urbaneja-Bernat et al., Reference Urbaneja-Bernat, Alonso, Tena, Bolckmans and Urbaneja2013). In some regions of the world, the inoculation of tomato seedlings with this mirid has been successfully used to control TLM and other pests in commercial greenhouses (Urbaneja et al., Reference Urbaneja, Gonzalez-Cabrera, Arno and Gabarra2012).
Trichogrammatid egg parasitoids are biological control agents that have been used successfully as inundative applications against agricultural pests, especially lepidopteran insects (Hassan, Reference Hassan1993; Wajnberg & Hassan, Reference Wajnberg and Hassan1994). Trichogramma (Hymenoptera: Trichogrammatidae) wasps are cheaply reared on alternative hosts, which allows them to be used in inundative releases in several crops (Parra & Zucchi, Reference Parra and Zucchi2004). Different species of these egg parasitoids are natively associated with TLM (Gabarra et al., Reference Gabarra, Arno, Lara, Verdu, Ribes, Beitia, Urbaneja, del Mar Tellez, Molla and Riudavets2014) and have been assessed for control of this pest. For instance, T. achaeae has high efficiency in TLM control where tomato damage is reduced for 91.74% when it was released in 30 adults per plant every 3–4 days under the greenhouse conditions (Cabello et al., Reference Cabello, Gallego, Vila, Soler, Del Pino, Carnero, Hernandez-Suarez and Polaszek2009). This wasp and two other species including T. evanescens and T. euproctidis are able to parasitize more than 25% of TLM eggs under the greenhouse conditions (Chailleux et al., Reference Chailleux, Desneux, Seguret, Khanh, Maignet and Tabone2012). T. brassicae Bezdenko is the most common Trichogramma species in Iran, and in many other countries it has been evaluated for control of various pests (Ebrahimi et al., Reference Ebrahimi, Pintureau and Shojai1998; Lundgren et al., Reference Lundgren, Heimpel and Bomgren2002). This species could be an effective biocontrol agent against TLM, especially when it combines with another agent, such as Bacillus thuringiensis (Alsaedi et al., Reference Alsaedi, Ashouri and Talaei-Hassanloui2017).
Plausible effects of the simultaneous use of two natural enemies of TLM on tomato fruit quantity and quality have not been well studied. To this end, we assessed different temporal combinations of N. tenuis and T. brassicae on some important tomato fruits’ attributes looking for an efficient IPM program in favor of tomato final yield.
Materials and methods
Plant
Seeds of cherry tomato, which is becoming common tomato in Tehran, were cultivated in seedling trays (30 × 60 cm) containing peat moss until seedlings had three true leaves. Thereafter, four seedlings were planted directly into the greenhouse soil inside of gauze-covered metal-framed cages (1 × 1 × 1.5 m, 0.5 mm2 mesh size). Plants were allowed to grow to a height of 35–40 cm before starting the experiment. No pesticides or additional fertilizers were used.
Insects
Adults of TLM and N. tenuis were originally collected from tomato fields in the Varamin region (southeast of Tehran, Iran) in September 2015. Subsequently, they were reared on 50–60 cm-high cherry tomato in separate wooden framed cages (1 × 1 × 1 m, 1 mm2 mesh size) containing 4–6 pots under greenhouse conditions (27 ± 3°C, 55 ± 5% RH, and a 16:8 h L:D photoperiod). Cotton soaked in water–honey solution was placed in the cages to feed adult TLM insects and ad libitum eggs of E. kuehniella were placed on the tomato leaves to feed the N. tenuis predators. Eggs of E. kuehniella were obtained from a colony maintained in a growth chamber (25 ± 1°C, 60 ± 5% RH, and a 16:8 h L:D photoperiod) at the Department of Entomology at Tarbiat Modares University which were kept in plastic vials in refrigerator (4°C) no more than 1 month prior to use. The initial population of T. brassicae was obtained from the Iranian Research Institute of Plant Protection. Wasps were reared on the E. kuehniella eggs (about 500 eggs were glued to white cardboard strips (10 × 80 mm) using water–honey solution) during the study under growth chamber conditions.
Experimental design
The experiment was carried out at facilities of the Faculty of Agriculture at Tarbiat Modares University from April to November 2016, under controlled conditions in a plastic greenhouse (25 ± 3°C, 55 ± 5% RH) inside of cages in which tomato plants were established. There were two predatory bug treatments crossed by two parasitoid wasp release rates; treatments were (1, 2) one pair of predators per m2, either 10 days before or 10 days after pest establishment and (3, 4) ten or 30 female parasitoids released per week per m2 until the end of the experiment. Therefore, the ten treatments examined were (1) pest only, (2) pest + ten parasitoids weekly, (3) pest + 30 parasitoids weekly, (4) pest (first) + predator, (5) predator first + pest, (6) pest first + predator + ten parasitoids weekly, (7) pest first + predator + 30 parasitoids weekly, (8) predator first + pest + 10 parasitoids weekly, (9) predator first + pest + 30 parasitoids weekly, and (10) the control (without any additions of pests or natural enemies). Each treatment was replicated four times (for a total of 40 cages) in a randomized block design. One pair of 3-day-old adult predators, ten or 30 1-day-old mated female parasitoids and four pairs of 3-day-old pests (adult stage) were released into cages, as appropriate, to establish these treatments (sex ratio of pest and predator were 1:1). In the predator-in-first treatments, one pair of 3-day-old N. tenuis was released into the cage 10 days before releasing the pest and ad libitum eggs of E. kuehniella were provided on the tomato leaves as an alternative food. The pest was released in all cages in the same time.
Tomatoes’ yield trials
The ripe fruits were harvested from each cage and transferred to the laboratory in labeled plastic containers (10 × 15 × 7 cm) every other day. There, they were divided into undamaged and damaged (those with larval mines or holes) groups where they were counted and weighed using a Sartorius analytical scale (L 610 D).
The first undamaged fruit from each cage was used for quality testing. The percentage of water was assessed by weighing the fruits before and after 24 h in the oven at 80°C. All of other quality parameters were assayed by spectrophotometry using Epoch Microplate Spectrophotometer (BioTek, Winooski, VT, USA). The phenol-sulfuric acid method was used for total carbohydrates estimation with glucose solution as the standard (Dubois et al., Reference Dubois, Gilles, Hamilton, Rebers and Smith1956). To determine the amount of protein in fruits, the Bradford method was applied using bovine serum albumin as the standard (Bradford, Reference Bradford1976). The method of Roe & Kuether (Reference Roe and Kuether1943) was used to estimate ascorbic acid content. In this method, ascorbate is converted to dehydroascorbate and reacts with 2, 4-dinitrophenyl hydrazine to form osazones, which dissolve in sulfuric acid to give an orange colored solution whose absorbance can be measured spectrophotometrically at 540 nm. The ascorbic acid standard curve was created to determine the level of ascorbic acid in fruits. Lycopene and β-carotene levels in undamaged tomatoes were obtained using the method of Bicanic et al. (Reference Bicanic, Swarts, Luterotti, Pietraperzia, Doka and de Rooij2004). In this method, fruit was homogenized by adding a solvent mixture (hexane:acetone:ethanol, 2:1:1) and shaking the samples for 10 min. The values of these two parameters were measured by samples absorbance at 502 nm (extinction coefficient = 3150 dL g−1 cm−1) for lycopene and 475 nm (extinction coefficient = 2049 dL g−1 cm−1) for β-carotene using equation (1):
where A, ε, B, and C are absorbance, extinction coefficient, length of cell (1 cm), and concentration of parameter, respectively. Anthocyanin content was measured by Wagner (Reference Wagner1979) method and acidified methanol solution (methanol: chloridric acid, 99:1) was used for homogenization. After 24 h of darkness and centrifugation at 4000 g, anthocyanin content was obtained using the absorbance of supernatant at 550 nm, extinction coefficient (33,000 cm2 mol−1) and the molecular weight of anthocyanin (207.252 g mol−1). The Krizek et al. (Reference Krizek, Britz and Mirecki1998) method and acidified ethanol solution (ethanol:glacial acetic acid, 99:1) were used to determine the flavonoid content of tomatoes. After centrifugation at 4000 g, extracts were placed in a warm water bath (80°C) for 10 min. Flavonoid content was estimated using the absorbance at 300 nm, extinction coefficient (33,000 cm2 mol−1), and the average molecular weight of flavonoid (286.909 g mol−1). Samples of 0.05 g were used for homogenizing (in 10 ml acetone 80%) and measuring chlorophylls and carotenoid by equations 2–5 (Arnon, Reference Arnon1949; Lichtenthaler, Reference Lichtenthaler1987):
 $${\rm Carotenoid} = \displaystyle{\matrix{{[1000 \times A_{480}-((1.8 \times {\rm Chlorophyl}{\rm l}_a)}\cr {-(85.02 \times {\rm Chlorophyl}{\rm l}_b))]}} \over {198}}$$
$${\rm Carotenoid} = \displaystyle{\matrix{{[1000 \times A_{480}-((1.8 \times {\rm Chlorophyl}{\rm l}_a)}\cr {-(85.02 \times {\rm Chlorophyl}{\rm l}_b))]}} \over {198}}$$where A 663, A 645, A 480, a, and W are absorbance at 663 nm, absorbance at 645 nm, absorbance at 480 nm, length of cell (1 cm), and fresh weight of sample (mg), respectively. The amount of phenolic compounds was determined with the Seevers & Daly (Reference Seevers and Daly1970) method using the gallic acid standard curve. According to this method, homogenized samples (0.1 g fruit in 5 ml ethanol 95%) were placed in darkness for 72 h. After adding 1 ml of ethanol (95%), 3 ml of distilled water, 0.5 ml of folin reagent (50%), and 1 ml of sodium carbonate (5%) to the 1 ml of sample supernatant, absorbance at 725 nm was read. The weekly population dynamic of the pest and predator were also recorded in each treatment which is preparing to publish.
Data analysis
Before analysis, homoscedasticity was checked using Levene's test and homogeneity of variance was found among the treatments. Data were also checked for normality using the Kolmogorov–Smirnov test and non-normal data were normalized using square root (total number of fruits, total yield, protein, β-carotene, flavonoid) and logarithmic transformations (undamaged yield, damaged yield, chlorophyll a). The percentage data were arcsine square root-transformed before analysis. All quantity and quality parameters were subjected to one-way analysis of variance, followed by a Tukey's test (α = 0.05) to separate means using IBM SPSS software (SPSS, 2011). Contrast (t-test) analysis was conducted on data to determine whether there were any significant differences between predator-in-first vs. predator-after-pest, predator only vs. predator + parasitoid, parasitoid only vs. predator + parasitoid, predator only vs. parasitoid only, and ten parasitoid weekly vs. 30 parasitoid weekly. Pearson correlation was used to evaluate the strength of the relationship between percentages of damaged fruits and tomato quality parameters.
Results
Tomato yield
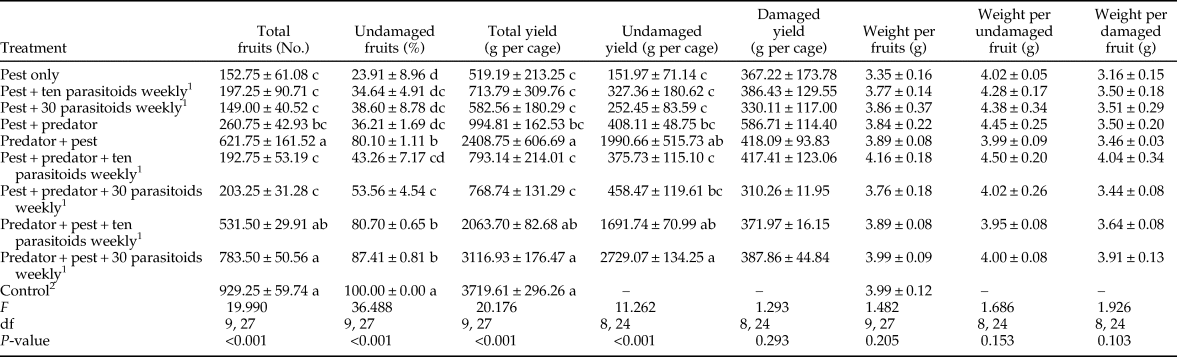
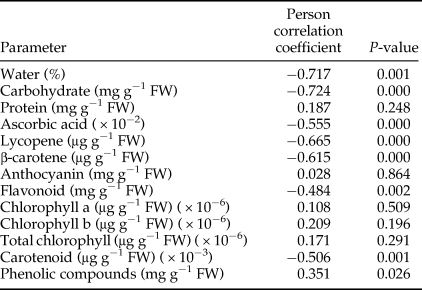
The total number of fruits, percentage of undamaged fruits, and total and undamaged yield were strongly affected by the treatments (table 1). While there was no significant difference in the total number of fruits between the control (without any additions of pests or natural enemies) and predator-in-first treatments, these treatments were significantly different from the others. The lowest number of fruits was for the pest + 30 parasitoids weekly treatment, which was not significantly different from the pest only treatment. The control treatment had the highest percentage of undamaged fruits (100%) and this was statistically different from all other treatments. After the control treatment, the highest percentage of undamaged fruits was in predator-in-first treatments, while the lowest percentage was in the pest only treatment. Total yield differed significantly among treatments and ranged from 519.19 g per cage in the pest only treatment to 3719.61 g per cage in the control (no pest) treatment. There were no significant differences between the control treatment and treatments in which the predator was released before pest establishment. The highest undamaged yield was for the predator + pest + 30 parasitoids weekly treatment, but this was not significantly different from other predator-in-first treatments.
Table 1. Tomato quantity parameters (mean ± SE) after feeding activity of Tuta absoluta and release of up to two natural enemies (Nesidiocoris tenuis and Trichogramma brassicae).
1 Predatory bug and parasitoid wasp were used at two deployment times (10 days before and after pest establishment) and two densities (ten and 30 female parasitoids per week), respectively. Means with the same letter were not significantly different within columns (Tukey's test, α = 0.05).
2 This treatment had neither pest nor natural enemy.
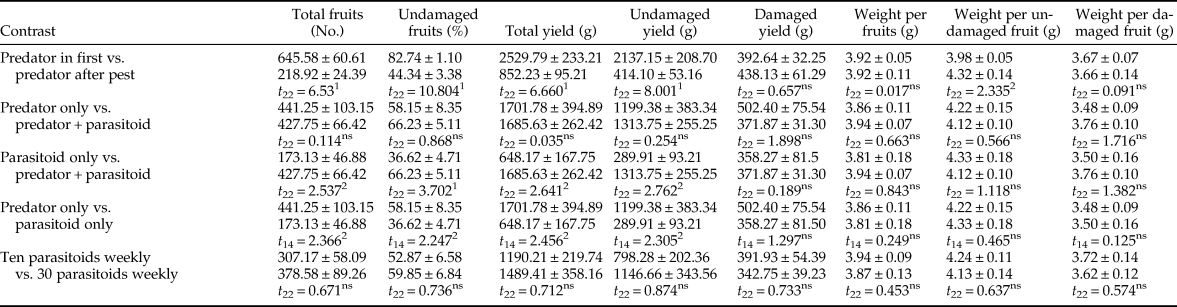
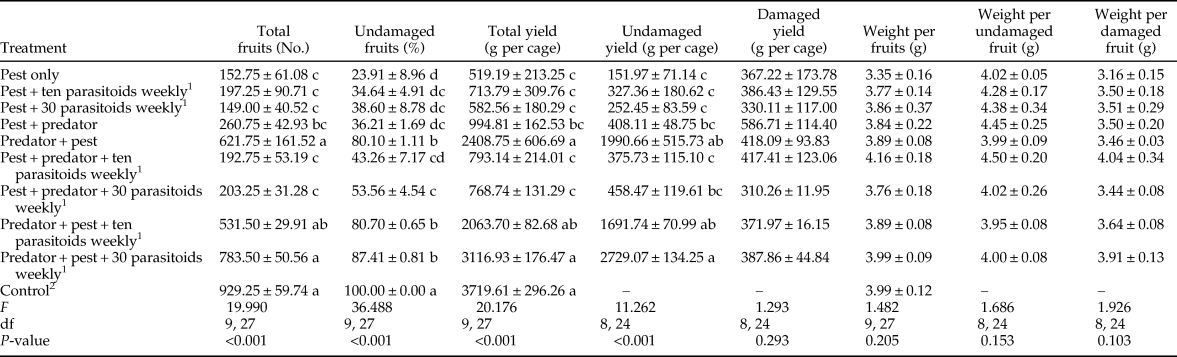
Contrast analysis found significant differences in the total number of fruits, percentage of undamaged fruits, total yield, and undamaged yield between predator-in-first and predator-after-pest treatments (table 2). All of these parameters were considerably higher in the predator-in-first treatments than in the predator-after-pest treatments. There were no significant differences in fruit quantity parameters between predator-only vs. predator + parasitoid, or between the ten parasitoids vs. 30 parasitoids per week treatments (table 2). The t-test analysis indicated that the total number of fruits, the percentage of undamaged fruits, total yield, and undamaged yield were all significantly greater in parasitoid + predator and predator only treatments than in parasitoid only treatments.
Table 2. Contrast analysis of tomato quantity parameters (mean ± SE) after feeding activity of Tuta absoluta and release of up to two natural enemies (Nesidiocoris tenuis and Trichogramma brassicae).
ns, Not significant.
Initial treatments are presented in Table 1.
1 Mean significant at 99%, (t-test).
2 Mean significant at 95%, (t-test).
Tomato quality
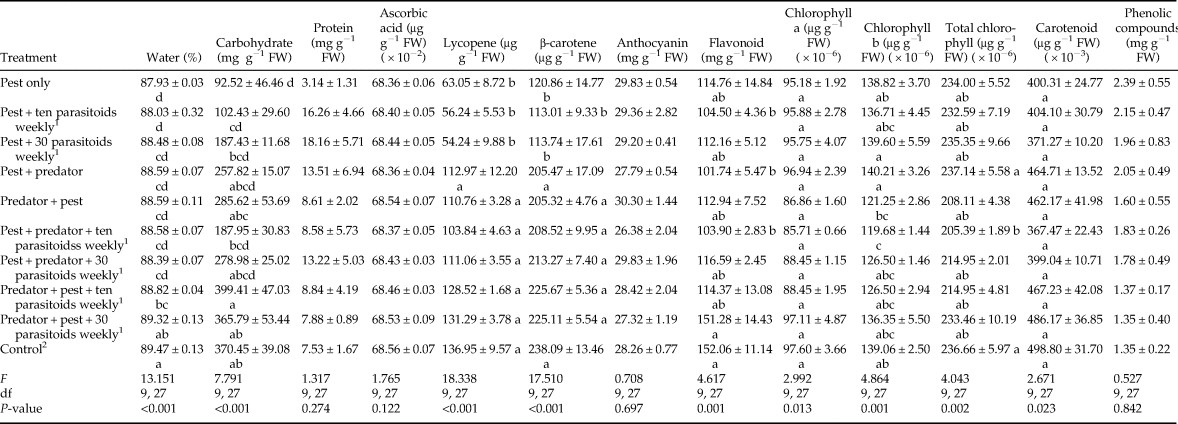
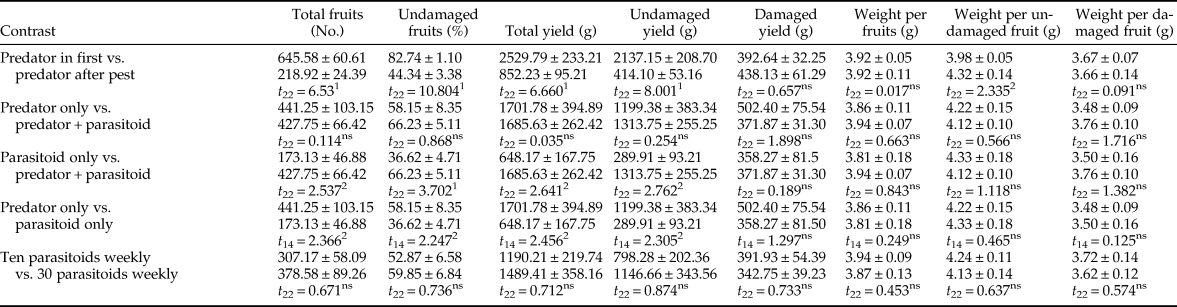
Assessment of tomato quality found that some parameters, including protein, ascorbic acid, anthocyanin, and phenolic compound content did not differ significantly among treatments (table 3). The highest and lowest percentage of fruit water belonged to the control and pest only treatments, respectively. The highest and lowest amounts of carbohydrates occurred in the predator + pest + 10 parasitoids weekly and pest only treatments, respectively, although a clear trend was not observed among the treatments. The amounts of lycopene and β-carotene were statistically identical in the control treatment and other predator treatments (P = 0.088 and P = 0.805, respectively), but they were significantly different from the pest only and pest + parasitoid treatments (table 3). Although no clear trend was observed in flavonoid content, there was no significant difference among the control and other predator-in-first treatments. Despite significant differences among treatments in the content of carotenoid and chlorophyll a, all treatments were classified in one group by Tukey's test. The lowest amounts of chlorophyll b and total chlorophyll belonged to pest + predator + ten parasitoids weekly treatment.
Table 3. Tomato quality parameters (mean ± SE) after feeding activity of Tuta absoluta and release of up to two natural enemies (Nesidiocoris tenuis and Trichogramma brassicae).
1 Predatory bug and parasitoid wasp were used at two deployment times (10 days before and after pest establishment) and two densities (ten and 30 female parasitoids per week), respectively. Means with the same letter were not significantly different within columns (Tukey's test, α = 0.05).
2 This treatment had neither pest nor natural enemy.
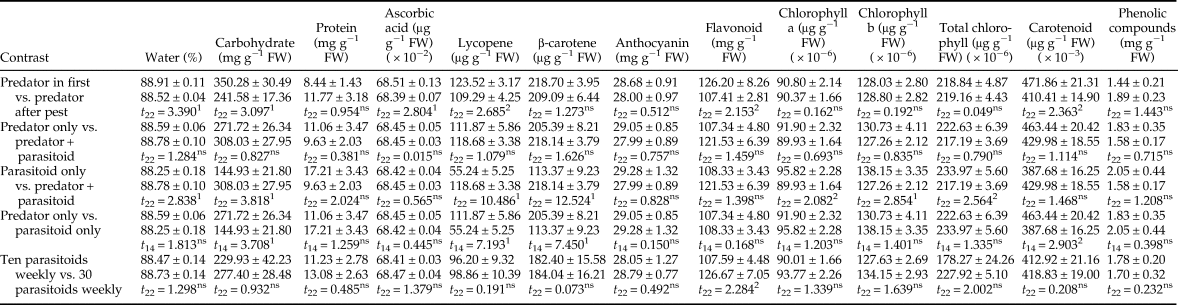
Contrast analysis showed the main effects of treatments on tomato fruit quality (table 4). The t-test analysis indicated that the predator-in-first treatments had higher water, carbohydrate, ascorbic acid, lycopene, flavonoid, and carotenoid content than treatments where predators were introduced after pest establishment. Whether predators were used alone or in combination with parasitoids had no significant difference on tomato quality parameters (P > 0.118). No significant effect was observed in protein, vitamin C, anthocyanin, flavonoids, carotenoids, and phenolic compound content between parasitoid only and predator + parasitoid treatments (P > 0.055), but all other parameters except chlorophyll were significantly higher in treatments using both of the natural enemies compared with ones with parasitoids alone (P < 0.018). There were also significant differences between predator only and parasitoid only treatments in the carbohydrate, lycopene, β-carotene, and carotenoid content. Finally, number of parasitoids released per week had no significant effect on tomato quality parameters except flavonoid content.
Table 4. Contrast analysis of tomato quality parameters (mean ± SE) after feeding activity of Tuta absoluta and release of up to two natural enemies (Nesidiocoris tenuis and Trichogramma brassicae).
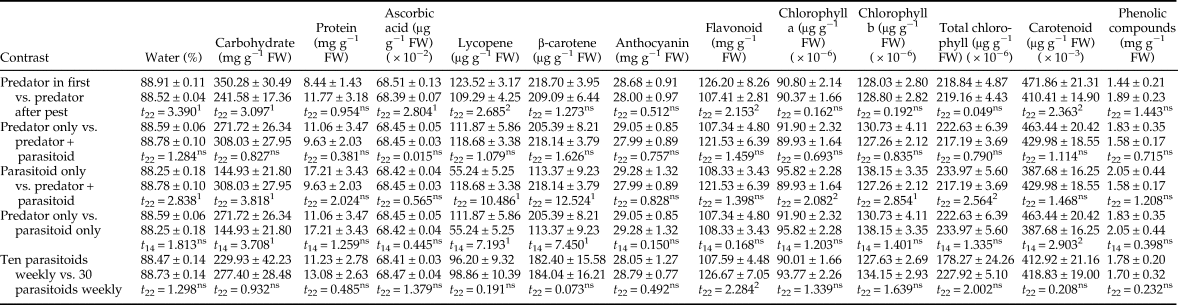
ns, Not significant
Initial treatments are presented in Table 1.
1 Mean significant at 99%, (t-test).
2 Mean significant at 95%, (t-test).
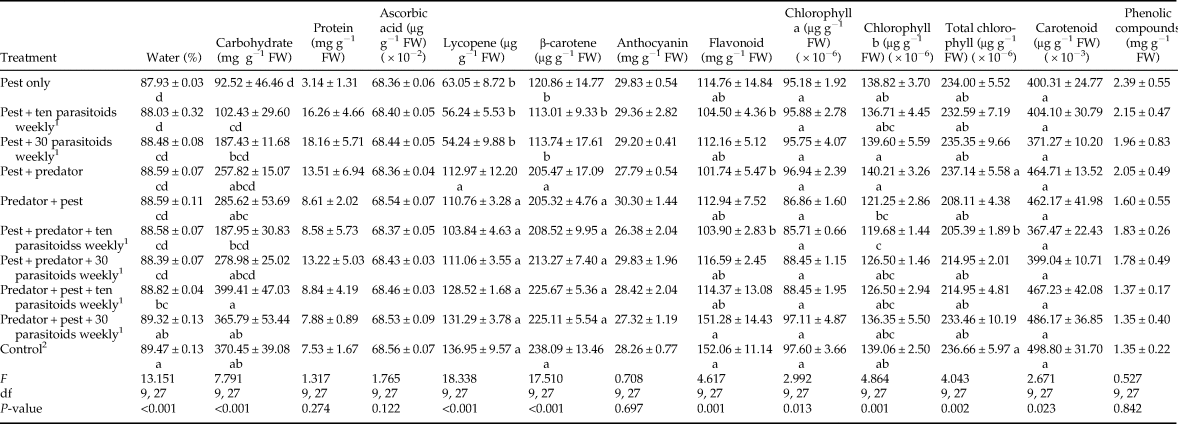
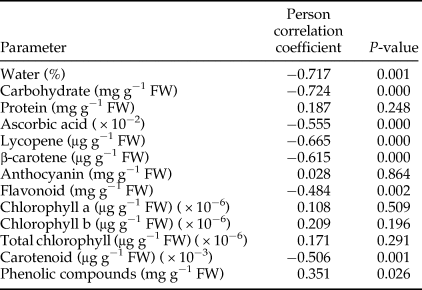
Protein, anthocyanin, and chlorophyll content were not correlated with the percentage of damaged fruits (table 5). Other parameters were significantly and inversely correlated with percentage of damaged fruits except phenolic compounds, which had a direct relationship with the percentage of damaged fruits. Pearson correlation also showed the percentage of water to be directly and significantly correlated with some parameters including carbohydrates (r = 0.579; P < 0.001), vitamin C (r = 0.342; P = 0.031), lycopene (r = 0.681; P < 0.001), β-carotene (r = 0.627; P < 0.001), flavonoids (r = 0.533; P < 0.001), and carotenoid (r = 0.470; P = 0.002).
Table 5. Correlation between percentage of damaged fruits and tomato quality parameters.
Discussion
In addition to the quantity, the quality of tomato fruits and their components such as carbohydrate, ascorbic acid, and lycopene were significantly affected by TLM attack. The plant photosynthesis decreases due to pest larval feeding on leaves mesophyll, which causes yield and quality losses. On the other side, plants respond to herbivores with producing some secondary metabolites and proteins that have toxic and repellent effects on the pest (Usha Rani & Jyothsna, Reference Usha Rani and Jyothsna2010; War et al., Reference War, Paulraj, War and Ignacimuthu2011). Some toxic chemicals such as anthocyanins and phenols are produced by attacked plants to kill or retard the development of herbivores as plant direct defense (Hanley et al., Reference Hanley, Lamont, Fairbanks and Rafferty2007). Releasing the volatiles to attract a pest's natural enemies and enhance their effectiveness is another defense method of plants against insects (Arimura et al., Reference Arimura, Matsui and Takabayashi2009). Production of these compounds takes energy and changes the normal plant mechanisms and enzymatic pathways.
Based on our results, predator-in-first approach can dramatically prevent tomato quantity and quality losses where total yield and some quality attributes of tomato fruits increased when the predator was released before the pest establishment. These observations imply that in predator-in-first strategy with supplementary food (egg of E. kuehniella) to increase the predator population density to high enough, sufficient control of the pest damage can be achieved (Messelink et al., Reference Messelink, Bloemhard, Hoogerbrugge, Schelt, Ingegno and Tavella2015). Calvo et al. (Reference Calvo, Lorente, Stansly and Belda2012b) found that the pre-planting release of N. tenuis can considerably reduce population densities of TLM and whitefly on tomato. Another mirid predator, Macrolophus pygmaeus Rambur, also showed higher effectiveness when it was introduced before establishment of TLM rather than after (Trottin-Caudal et al., Reference Trottin-Caudal, Baffert, Leyre and Hulas2012). Indeed, it seems that higher performance in predator-in-first approach is common in zoophytophagous mirid predators (Lenfant et al., Reference Lenfant, Ridray and Schoen2000).
Cost-effective biological control is a fundamental component of IPM (Naranjo et al., Reference Naranjo, Ellsworth and Frisvold2015). Our results suggest that using 30 parasitoids per week did not significantly improve fruits quantity or quality over the treatment with just ten parasitoids. In addition, contrast analysis found no significant differences in quantity and quality parameters between predator only treatments or predators used in combination with parasitoids. These results imply that if predators are introduced before pest can become established, the use of parasitoids is not required for TLM management. Similarly, Calvo et al. (Reference Calvo, Lorente, Stansly and Belda2012b) found N. tenuis capable of significantly reducing TLM populations and the release of additional agents such as Trichogramma achaeae Nagaraja & Nagarkatti did not increase its effectiveness. This may be due to intraguild predation of N. tenuis on parasitized eggs of TLM (Cabello et al., Reference Cabello, Gallego, Fernandez, Gamez, Vila, Del Pino and Hernandez–Suarez2012). There are some evidences that N. tenuis and M. pygmaeus can use TLM eggs parasitized by trichogrammatid wasps as prey, thereby reducing the rate of parasitism under greenhouse conditions (Chailleux et al., Reference Chailleux, Bearez, Pizzol, Amiens-Desneux, Ramirez-Romero and Desneux2013; Cabello et al., Reference Cabello, Bonfil, Gallego, Fernandez, Gamez and Garay2015). Although the authors of these later studies speculated that the presence of both natural enemies (trichogrammatid wasp and predator) provided better control of TLM, their simultaneous application did not improve the quantity or quality of tomato fruits and thus is not economically advantageous.
We found that using parasitoids without predators failed to improve the quantity and quality of fruit above that of the pest only treatment, suggesting that T. brassicae cannot control TLM population in these densities. Although increasing T. brassicae density may lead better TLM control, low parasitoid efficacy may be due to its rearing history, unfavorable environmental conditions, or tomato cultivars unfavorable physical/chemical features to natural enemies. Previous studies have found that temperature, humidity, rearing history, and plant structure can strongly affect the searching behavior and performance of trichogrammatid wasps (Gingras & Boivin, Reference Gingras and Boivin2002; Moezipour et al., Reference Moezipour, Kafil and Allahyari2008; Cascone et al., Reference Cascone, Carpenito, Slotsbo, Iodice, Sorensen, Holmstrup and Guerrieri2015). However, some researchers believe that trichogrammatid wasps have high potential for TLM management in greenhouses (Do Thi Khanh et al., Reference Do Thi Khanh, Chailleux, Tiradon, Desneux, Colombel and Tabone2012; El-Arnaouty et al., Reference El-Arnaouty, Pizzol, Galal, Kortam, Afifi, Beyssat, Desneux, Biondi and Heikal2014), although our study did not find this to be the case with T. brassicae. Further tests with higher densities of T. brassicae under different environmental conditions and on different tomato cultivars are required to give a broader understanding of the potential effectiveness of this parasitoid.
This study showed that N. tenuis is able to establish on treated tomato when given supplementary food, and that its early establishment provides effective TLM control (even without the use of parasitoids) (unpublished data). Using just the one natural enemy reduces the complexity and costs of biological control of TLM. In addition, other tomato pests such as whiteflies and spider mites can also be well controlled by N. tenuis (Urbaneja et al., Reference Urbaneja, Tapia, Fernandez, Sanchez, Contreras and Bielza2003; Calvo et al., Reference Calvo, Lorente, Stansly and Belda2012b). The mass rearing of this predator and its use in a predator-in-first approach can be an effective method for tomato pest management, especially TLM. Future studies should explore the proper application density, an important issue for this predator due to the low activity of its phytophagy (Sanchez & Lacasa, Reference Sanchez and Lacasa2008; Calvo et al., Reference Calvo, Bolckmans and Belda2012a).
Acknowledgements
Financial and technical support for this research was provided by the Department of Entomology, Tarbiat Modares University, which is greatly appreciated. We are also grateful to Dr F. Ghanati from the Department of Plant Biology, Tarbiat Modares University, for her valuable assistance in tomato quality assessment.