Introduction
In many potato producing areas around the world, defoliation by the Colorado potato beetle Leptinotarsa decemlineata (Say) can cause 40–100% yield losses in potato crops (Cingel et al., Reference Cingel, Savić, Lazarević, Ćosić, Raspor, Smigocki and Ninković2016). Chemical insecticides are extensively used to control the beetles and protect potato production. However, this leads to rapid development of insecticide resistance (Clements et al., Reference Clements, Schoville, Clements, Chapman and Groves2017; Crossley et al., Reference Crossley, Chen, Groves and Schoville2017). In order to efficiently reduce the damage of L. decemlineata, novel management strategies must be explored (Xu et al., Reference Xu, Meng, Shi, Deng, Guo and Li2017). RNA interference (RNAi) is a sequence-specific mRNA degradation process initiated by double-stranded RNA (dsRNA). It is being developed as a technology for pest management (Palli, Reference Palli2014; Wang & Jin, Reference Wang and Jin2017). In L. decemlineata, RNAi by the dietary introduction of bacterially expressed dsRNAs is able to effectively knockdown target genes (Meng et al., Reference Meng, Xu, Deng, Fu, Guo and Li2018a, Reference Meng, Xu, Deng, Fu, Guo and Lib, Xu et al., Reference Xu, Meng, Deng, Guo and Li2018). Moreover, a dsRNA spraying method, the spray-induced gene silencing, has been proven to be effective to decrease the defoliation by L. decemlineata larvae on potato plant in the glass room (San Miguel & Scott, Reference San Miguel and Scott2016). These demonstrate that RNAi can be developed against L. decemlineata.
However, RNAi efficiency is variable among life stages (Scott et al., Reference Scott, Michel, Bartholomay, Siegfried, Hunter, Smagghe, Zhu and Douglas2013). In Apis mellifera, for example, when a vitellogenin-dsRNA is injected at the preblastoderm stage, 15% workers have strongly reduced levels of vitellogenin mRNA. In contrast, 96% individuals show the knockdown phenotype when dsRNA is introduced by intra-abdominal injection in newly emerged bees (Amdam et al., Reference Amdam, Simões, Guidugli, Norberg and Omholt2003). In L. decemlineata, dsRNA from a housekeeping gene S-adenosyl-L-homocysteine hydrolase (LdSAHase) causes lethality, inhibits growth and impairs pupation in an instar-dependent manner: the young larvae are more susceptible to dsRNA than the old ones (Guo et al., Reference Guo, Fu, Yang, Li and Li2015). Similarly, knockdown of a chitin synthase gene LdChSB causes more serious defects and more mortality in the young larvae (Shi et al., Reference Shi, Mu, Chen, Guo and Li2016).
However, the first- to fourth (final)-instar L. decemlineata larvae frequently occur simultaneously in the potato field (Guo et al., Reference Guo, Tan and Zhang2013). Depending on climate, L. decemlineata beetles have several generations annually. Moreover, the females often lay eggs continuously for months. The generation overlapping makes young and old larvae, as well as adults, simultaneously feed foliage in the same potato field. Therefore, an RNAi-mediated management approach must be effective to both young and old larvae, and even to the adults. In the present paper, therefore, we intended to find attractive candidate genes for RNAi effective against third and final instar larvae.
Broad Complex (BrC) is an important metamorphic gene. It encodes a transcription factor which comprises a Bric-a-brac/Tramtrack/Broad complex (BTB) domain and an alternatively spliced C2H2 zinc finger domain (Z1–Z6) (DiBello et al., Reference DiBello, Withers, Bayer, Fristrom and Guild1991; Bayer et al., Reference Bayer, Holley and Fristrom1996; Nishita & Takiya, Reference Nishita and Takiya2004; Spokony & Restifo, Reference Spokony and Restifo2007; Piulachs et al., Reference Piulachs, Pagone and Bellés2010; Nagamine et al., Reference Nagamine, Kayukawa, Hoshizaki, Matsuo, Shinoda and Ishikawa2014). In holometabolous insects, BrC controls pupal commitment and morphogenesis, and prevents adult differentiation (Kiss et al., Reference Kiss, Bencze, Fodor, Szabad and Fristrom1976; Kiss et al., Reference Kiss, Beaton, Tardiff, Fristrom and Fristrom1988; Zhou et al., Reference Zhou, Hiruma, Shinoda and Riddiford1998; Zhou & Riddiford, Reference Zhou and Riddiford2002; Zhou et al., Reference Zhou, Zhou, Truman and Riddiford2004; Konopova & Jindra, Reference Konopova and Jindra2008; Parthasarathy et al., Reference Parthasarathy, Tan, Bai and Palli2008; Suzuki et al., Reference Suzuki, Truman and Riddiford2008; Daimon et al., Reference Daimon, Uchibori, Nakao, Sezutsu and Shinoda2015). In Manduca sexta, BrC expression is among the first molecular events underlying pupal commitment of both epidermis and wing discs (Zhou & Riddiford, Reference Zhou and Riddiford2001). In Drosophila melanogaster, loss-of-function mutation of BrC gene (nonpupariating (npr1) mutant) can cause failure of pupariation (Kiss et al., Reference Kiss, Beaton, Tardiff, Fristrom and Fristrom1988; DiBello et al., Reference DiBello, Withers, Bayer, Fristrom and Guild1991; Bayer et al., Reference Bayer, von Kalm and Fristrom1997). In Bombyx mori, BmBrC RNAi in imaginal discs and primordia inhibits metamorphosis (Uhlirova et al., Reference Uhlirova, Foy, Beaty, Olson, Riddiford and Jindra2003). Likewise, the majority of the TcBrC RNAi larvae in Tribolium castaneum arrest development at the end of the prepupal stage or just after the pupal molt (Konopova & Jindra, Reference Konopova and Jindra2008; Ureña et al., Reference Ureña, Chafino, Manjón, Franch-Marro and Martín2016). Comparable anti-development effects are also observed in the BrC RNAi Oncopeltus fasciatus nymphs (Erezyilmaz et al., Reference Erezyilmaz, Riddiford and Truman2006) and Chrysopa perla larvae (Konopova & Jindra, Reference Konopova and Jindra2008). It appears that BrC is a potential gene for RNAi against the fourth-instar L. decemlineata larvae.
In the work presented here, we identified five putative BrC cDNAs (LdBrC-Z1, LdBrC-Z2, LdBrC-Z3, LdBrC-Z4, and LdBrC-Z6) in L. decemlineata. We found that knockdown of all these LdBrC isoforms caused larval mortalities in resultant larvae. Our results imply that LdBrC gene is a potential target for a dsRNA-based control method to the final instar larvae in L. decemlineata.
Materials and methods
Insects and chemicals
The L. decemlineata beetles were kept in an insectary according to a previously described method (Shi et al., Reference Shi, Guo, Wan, Zhou, Ren, Tursun, Fu and Li2013), with potato foliage at the vegetative growth or young tuber stages in order to assure sufficient nutrition.
Ecdysteroid 20-hydroxyecdysone (20E) (Sigma-Aldrich, USA), a juvenile hormone (JH) analogue methoprene [isopropyl (2E, 4E, 7S)-11-methoxy-3, 7, 11-trimethyldodeca-2, 4-dienoate] (Shanghai Kewelchem Company, Shanghai, China), were purified by reversed-phase high-performance liquid chromatography or high-performance liquid chromatography before experiments.
Molecular cloning
The putative LdBrC isoforms were obtained from the genome (Schoville et al., Reference Schoville, Chen, Andersson, Benoit, Bhandari, Bowsher, Brevik, Cappelle, Chen, Childers, Childers, Christiaens, Clements, Didion, Elpidina, Engsontia, Friedrich, García-Robles, Gibbs, Goswami, Grapputo, Gruden, Grynberg, Henrissat, Jennings, Jones, Kalsi, Khan, Kumar, Li, Lombard, Ma, Martynov, Miller, Mitchell, Munoz-Torres, Muszewska, Oppert, Palli, Panfilio, Pauchet, Perkin, Petek, Poelchau, Record, Rinehart, Robertson, Rosendale, Ruiz-Arroyo, Smagghe, Szendrei, Thomas, Torson, Vargas Jentzsch, Weirauch, Yates, Yocum, Yoon and Richards2018) and transcriptome data (Shi et al., Reference Shi, Guo, Wan, Zhou, Ren, Tursun, Fu and Li2013) of L. decemlineata. The correctness of the sequences was substantiated by polymerase chain reaction (PCR) using primers in table S1. The full-length cDNAs were obtained by 5′- and/or 3′-RACE, using SMARTer RACE kit (Takara Bio.), with specific primers listed in table S1. After obtaining full-length cDNAs, primer pairs (table S1) were designed to verify the complete open reading frames. All of the sequenced cDNAs were submitted to GenBank (accession numbers: LdBrC Z1, KP340512; LdBrC Z2, KP340513; LdBrC Z3, KP340514; LdBrC Z4, KX881916; LdBrC Z6, KX9881917).
Preparation of dsRNAs
The same method as previously described (Zhou et al., Reference Zhou, Jia, Wan, Kong, Guo, Ahmat and Li2013) was used to express dsJHAMT, dsEcR, dsBrC-1, dsBrC-2 and dsegfp, derived respectively from a 261 bp fragment of LdJHAMT, a 344 bp fragment of LdEcR, a 249 bp and 312 bp fragments targeting two different regions in the common sequence of the five LdBrC isoforms, and a 414 bp fragment of enhanced green fluorescent protein gene from Aequorea victoria. The five dsRNAs were individually transcribed with specific primers in table S1, using Escherichia coli HT115 (DE3) competent cells lacking RNase III. Individual colonies were inoculated, and grown until cultures reached an OD600 value of 1.0. The colonies were then induced to express dsRNA by addition of isopropyl β-D-1-thiogalactopyranoside to a final concentration of 0.1 mM. The expressed dsRNA was extracted and confirmed by electrophoresis on 1% agarose gel. Bacteria cells were centrifuged at 5000g for 10 min, and resuspended in an equal original culture volume of 0.05 M phosphate buffered saline (PBS, pH 7.4). The bacterial solutions (at a dsRNA concentration of about 0.5 µg ml−1) were used for the experiment.
Dietary introduction of dsRNA
The same method as described previously (Fu et al., Reference Fu, Guo, Ahmat and Li2015) was used to dietarily introduce dsRNA into third- and fourth-instar larvae. Ten newly-ecdysed third- or fourth-instar larvae were confined in a Petri dish (9 cm diameter and 1.5 cm height) to feed foliage immersed with a bacterial suspension containing dsJHAMT, dsEcR, dsBrC-1 or dsBrC-2. To test the transcription response of LdBrC to dietary methoprene (a juvenile hormone analog, JHA) or dsJHAMT, six repeats were set. Three replicates were used to collect hemolymph for JH measurement and three were used to extract total RNA, after 3 days’ ingestion of treated foliage (replaced with freshly treated ones each day). To determine the response of LdBrC to dietary 20E or dsEcR, three replicates were performed, which were used to extract total RNA after 3 days’ ingestion of treated foliage. For knockdown of LdBrC, six replicates were set. Three replicates were used to observe the pupation and adults by allowing the larvae to feed on treated leaves for 3 days; and then all the treatments were transferred to untreated foliage until reaching the wandering stage. The other three were collected for RNA extraction after continuously fed on treated foliage for 3 days.
The beetles were weighed 5 days after the initiation of the experiment. The larval mortality and pupation were recorded during a 2-week trial period. The samples on day 3 after the initiation of the experiments were collected. The effects of gene silencing, the levels of LdJHAMT, LdEcR and five LdBrC isoforms, and JH titer were determined.
For the above experiments, three biological replicates were carried out.
Real-time quantitative PCR (qRT-PCR)
For analysis of the tissue expression patterns, RNA templates were derived from the brain-corpora cardiaca-corpora allata complex, prothoracic gland, ventral ganglion, foregut, midgut, hindgut, Malpighian tubules, hemocytes, epidermis and fat body of the day 4 fourth-instar larvae. For analysis of the effects of treatments, total RNA was extracted from treated larvae. Each sample contained 5–10 individuals and repeated three times. The RNA was extracted using SV Total RNA Isolation System Kit (Promega). Purified RNA was subjected to DNase I to remove any residual DNA according to the manufacturer's instructions. Quantitative mRNA measurements were performed by qRT-PCR in technical triplicate, using four internal control genes (LdRP4, LdRP18, LdARF1 and LdARF4, the primers listed in table S1) according to our published results (Shi et al., Reference Shi, Guo, Wan, Zhou, Ren, Tursun, Fu and Li2013). An RT negative control (without reverse transcriptase) and a non-template negative control were included for each primer set to confirm the absence of genomic DNA and to check for primer-dimer or contamination in the reactions, respectively.
According to a previously described method (Bustin et al., Reference Bustin, Benes, Garson, Hellemans, Huggett, Kubista, Mueller, Nolan, Pfaffl, Shipley, Vandesompele and Wittwer2009), the generation of specific PCR products was confirmed by gel electrophoresis. The primer pair for each gene was tested with a tenfold logarithmic dilution of a cDNA mixture to generate a linear standard curve (crossing point plotted vs. log of template concentration), which was used to calculate the primer pair efficiency. All primer pairs amplified a single PCR product with the expected sizes, showed a slope less than −3.0, and exhibited efficiency values ranging from 2.7 to 2.8. Data were analyzed by the 2−ΔΔCT method, using the geometric mean of the four internal control genes for normalization.
Quantitative determination of JH
We quantified JH titers in the beetles according to a previously reported method (Zhou et al., Reference Zhou, Jia, Wan, Kong, Guo, Ahmat and Li2013). Hemolymph in three replicates (a total of 30 larvae) was respectively collected and transferred to a glass vial containing 400 µl of methanol, with 30 ng fenoxycarb (Syngenta Co. Ltd, China) as an internal standard, and then 100 µl of 2% NaCl was added. They were extracted three times with 300 µl of hexane. After adding hexane, the sample was vortexed vigorously incubated for 5 min at room temperature, and centrifuged (965 g) for 5 min. The hexane (upper) phase was collected in a new glass vial. The combined hexane extract (900 µl) was dried completely under vacuum and dissolved in 30 µl of acetonitrile. A liquid chromatography mass spectrometry was used to quantify JH titers (ng per ml hemolymph) (Cornette et al., Reference Cornette, Gotoh, Koshikawa and Miura2008).
Data analysis
The data were given as means ± SE, and were analyzed by one-way ANOVA followed by the Tukey–Kramer test, using SPSS for Windows (SPSS, Chicago, IL, USA). Since no significant difference between dsRNAs targeting two different regions of LdBrC (dsBrC-1 and dsBrC-2) was found, the data of each dsRNA were combined.
Results
Identification of LdBrC
Five full-length LdBrC cDNAs were cloned from L. decemlineata. The LdBrC gene contained 11 exons. The first six exons encoded BTB core region, whereas a specific exon encoded zinc finger in each LdBrC isoform (fig. S1). The homologs of Z1–Z6 were extensively searched in public databases using tblastx with the deduced amino acid sequences of LdBrC isoforms as the query. The Z1–Z5 sequences were respectively obtained from various orders of insects including holometabolan and hemimetabolan. Z6 was only found in a single species, Blattella germanica. The phylogenetic tree of the Z1-Z6 sequences in various insects was constructed based on amino acid sequences of zinc fingers. Based on the phylogenetic analysis, the Z1–Z6 groups were respectively monophyletic. Accordingly, the five LdBrC isoforms in L. decemlineata were named as LdBrC-Z1, LdBrC-Z2, LdBrC-Z3, LdBrC-Z4, and LdBrC-Z6, respectively (fig. 1).

Fig. 1. Phylogenetic analysis of representative insect Broad-Complexes. An unrooted phylogenetic tree is generated by MEGA 6 using the neighbor-joining method based on amino acid sequences of zinc fingers. Bootstrap analyses of 1000 replications are carried out and bootstrap values >50% are shown on the tree. The proteins originate from Blattella germanica (Bge), Tribolium castaneum (Tca), Leptinotarsa decemlineata (Say) (Lde), Drosophila melanogaster (Dme), Apis mellifera (Ame), Bombyx mori (Bmo), and Daphnia magna (Dam).
Tissue expression profiles of LdBrC
To identify tissues at which LdBrC is expressed and potentially required, cDNA samples were prepared from the brain-corpora cardiaca-corpora allata complex, prothoracic gland, ventral ganglion, foregut, midgut, hindgut, Malpighian tubules, hemocytes, epidermis, and fat body of the day 4 fourth-instar larvae analyzed. The five isoforms could be detected at all examined tissues. The highest level of LdBrC-Z1 was found in fat body; the most abundant mRNA levels of LdBrC-Z2, LdBrC-Z3, LdBrC-Z4, and LdBrC-Z6 were noted in the brain-corpora cardiaca-corpora allata complex. The lowest levels were observed in midgut (for LdBrC-Z1, LdBrC-Z3 and LdBrC-Z6), ventral ganglion (for LdBrC-Z2) or hemocytes (for LdBrC-Z4) (fig. 2).

Fig. 2. Tissue expression profiles of LdBrC variants in L. decemlineata. The cDNA templates were derived from the brain-corpora cardiaca-corpora allata complex (BCC), prothoracic gland (PG), ventral ganglion (VG), foregut (FG), midgut (MG), hindgut (HG), Malpighian tubules (MT), hemocytes (HC), epidermis (EP) and fat body (FB) of the day 4 fourth-instar larvae. The lowest expression levels in MG for LdBrC-Z1, LdBrC-Z3, and LdBrC-Z6, in VG for LdBrC-Z2 and in HC for LdBrC-Z4 are set as 1. For each sample, three independent pools of 5–10 individuals are measured in technical triplicate using qRT-PCR. The bars represent 2−ΔCt method (±SE) normalized to the geometrical mean of housekeeping gene expression.
JH downregulates the expression of LdBrC isoforms
In L. decemlineata, JHAMT expression levels are highly correlated to circulating JH titers. Therefore, it seems that JHAMT is responsible for JH biosynthesis (Fu et al., Reference Fu, Li, Zhou, Meng, Lü, Guo and Li2016). We tested in vivo effects of methoprene (a JH analog) and dsJHAMT ingestion (fig. S2) on the transcription of LdBrC isoforms in the newly-ecdysed fourth-instar larvae. We found that methoprene ingestion significantly reduced the expression of these transcripts (P < 0.05, ANOVA followed by the Tukey–Kramer test), whereas dsJHAMT feeding significantly increased the transcription of LdBrC isoforms (P < 0.05, ANOVA followed by the Tukey–Kramer test) in the treated beetles, compared with those in the control larvae (fig. 3).

Fig. 3. Ingestion of a juvenile hormone analog (JHA) methoprene and knockdown of LdJHAMT on the expression of LdBrC isoforms in L. decemlineata. The newly-ecdysed fourth-instar larvae are allowed to ingest potato foliage treated with water (blank control, CK), dsegfp (negative control), 100 ng ml−1 JH analog (JHA) methoprene, or dsJHAMT for three days. For each sample, three independent pools of 5–10 individuals are measured in technical triplicate using qRT-PCR. The bars (2−ΔΔCt method values ± SE) marked by different letters indicate significant difference at P value <0.05.
20-Hydroxyecdysone activates LdBrC
LdEcR has been identified as the functional 20E receptor in L. decemlineata (Ogura et al., Reference Ogura, Minakuchi, Nakagawa, Smagghe and Miyagawa2005). Here we determined in vivo effects of 20E and dsEcR ingestion (fig. S3) on the transcription of LdBrC isoforms in the newly-ecdysed fourth-instar larvae. As expected, 20E ingestion significantly upregulated the expression of these transcripts (P < 0.05, ANOVA followed by the Tukey–Kramer test), whereas dsEcR feeding significantly diminished the transcription of LdBrC isoforms in the treated larvae (P < 0.05, ANOVA followed by the Tukey–Kramer test), compared with those in the controls (fig. 4).

Fig. 4. Influence of 20-hydroxyecdysone (20E) signaling on the expression of LdBrC isoforms in L. decemlineata. The newly-ecdysed fourth-instar larvae are allowed to ingest potato foliage treated with water (blank control, CK), dsegfp (negative control), 100 ng ml−1 20E, or dsEcR for three days. For each sample, three independent pools of 5–10 individuals are measured in technical triplicate using qRT-PCR. The bars (2−ΔΔCt method values ± SE) marked by different letters indicate significant difference at P value <0.05.
Ingestion of dsBrC by the final larval instars completely inhibits pupation
We dietarily introduced each of the two dsRNAs of LdBrC directed against the variable common region of all five variants into the newly-molted fourth-instar larvae. Combined data revealed that continuous ingestion of a dsBrC for 3 days significantly downregulated its target gene (fig. 5a).
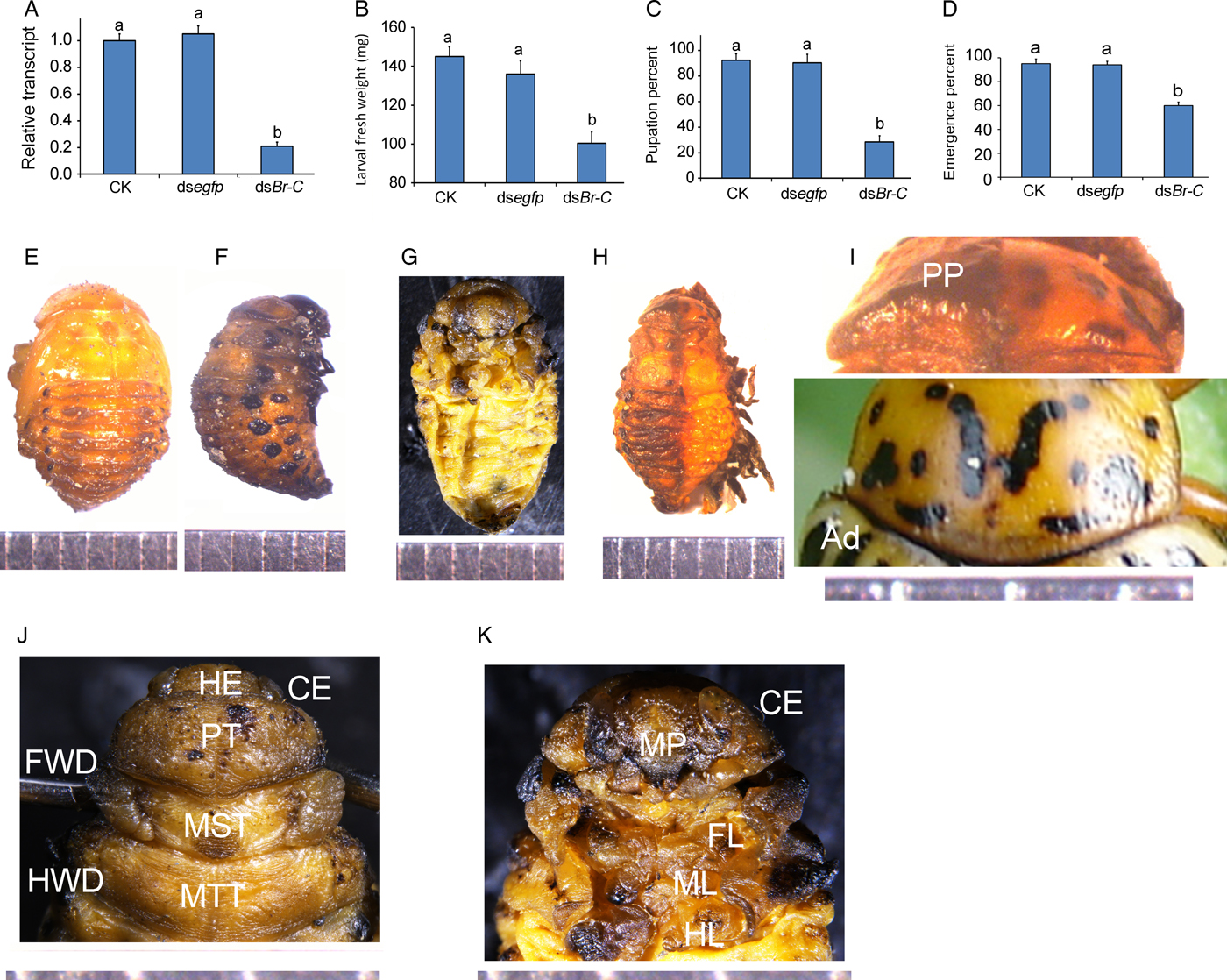
Fig. 5. The negative effects of the LdBrC RNAi on the fourth-instar L. decemlineata larvae. The newly-ecdysed fourth-instar larvae were allowed to ingest PBS (CK)-, dsegfp-, and dsBrC-dipped leaves for 3 days. The larvae were then transferred to untreated foliage if necessary. The relative transcript (a), larval fresh weights (b), pupation (c), and emergence (d) percentages were determined. The bars represent values (±SE). Different letters indicate significant difference at P value <0.05. While the CK larvae pupated 7 days after initiation of bioassay (e), around 70% of the LdBrC RNAi larvae fail to ecdyse. After 9 and 11 days after initiation, these beetles are somewhat withered, dried, and darkened (f). Upon removal of the whole apolysed larval cuticle, a miniature adult is seen (ventral view) (g). Prothorax of the prepupae (PP) shows distinct adult (Ad, the normal adult) cuticle pigmentation (dorsal view) (h, i). The head (HE), compound eyes (CE), prothorax (PT), mesothorax (MST), and metathorax (MTT) are formed. The discs of forewing (FWD) and hindwing (HWD) are attached on the mesothorax and metathorax, respectively (dorsal view) (j). The mouthparts (MT), forelegs (FL), midlegs (ML), and hindlegs (HL) are observed at the miniature adults (ventral view) (k). Scale bar is 1 mm. Approximately 60% of RNAi pupae emerged as adults (d).
The dsBrC-fed fully-grown larvae obtained lighter fresh weights than the controls (fig. 5b). While the control larvae pupated 7 days after initiation of bioassay (fig. 5e), around 30% of LdBrC RNAi hypomorphs normally pupated (fig. 5c). The remaining prepupae were completely wrapped in the larval cuticle. After 9 and 11 days after initiation of the experiment, the RNAi beetles were somewhat withered, dried, and darkened (fig. 5f).
A prepupa is a pharate pupa in which larval cuticle has been apolysed and the newly deposited cuticle has attained pupal characters. However, after removal of the apolysed larval cuticle, an arrested LdBrC RNAi hypomorph is a miniature adult (fig. 5g). Distinct adult cuticle pigmentation was seen on the prothorax (fig. 5h, i). The adult head, compound eyes, prothorax, mesothorax, and metathorax were found on the dorsal view. The discs of forewing and hindwing were attached on the mesothorax and metathorax, respectively (fig. 5j). The mouthparts, forelegs, midlegs, and hindlegs could be observed on the ventral view of the miniature adults (fig. 5k).
Approximately 60% of RNAi pupae emerged as adults (fig. 5d). All these LdBrC RNAi adults eventually died within 1 week after molting.
Ingestion of dsBrC by the third larval instars causes lethality
Continuous ingestion of a dsBrC for 2 days significantly reduced its target gene (fig. 6a). Normal control larvae ecdysed 2 days after initiation of bioassay (fig. 6e), around 20% of the LdBrC RNAi moribund beetles remained as prepupae at 13 days after initiation of the experiment (fig. 6f). They were wrapped in the old larval cuticle and finally died. The remaining RNAi larvae grew (fig. 6b) and pupated (fig. 6d) normally.
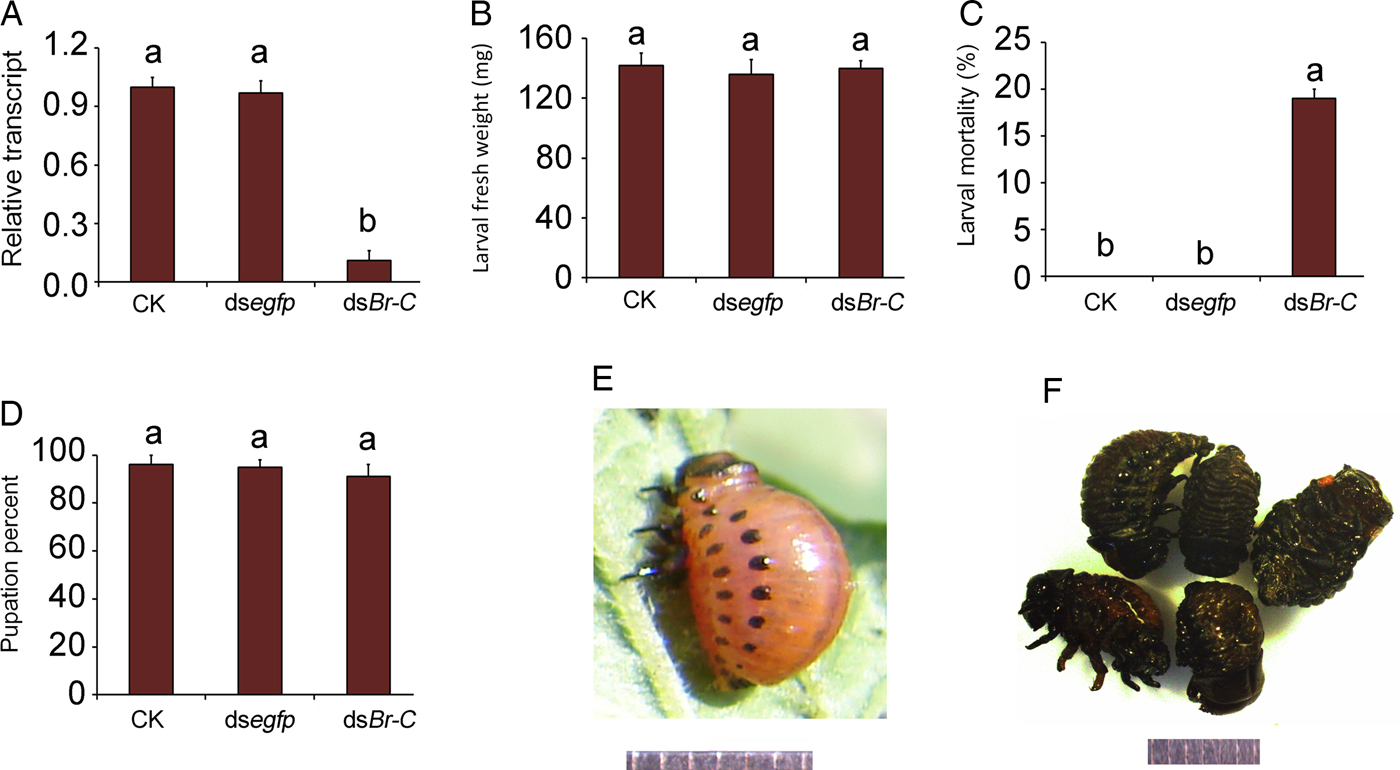
Fig. 6. The negative effects of the LdBrC RNAi on the third-instar L. decemlineata larvae. The newly-ecdysed third-instar larvae are allowed to ingest PBS (CK)-, dsegfp-, and dsBrC-dipped leaves for 3 days. The larvae are then transferred to untreated foliage if necessary. The relative transcript (a), larval fresh weights (b), larval mortalities (c), and pupation percent (d) are determined. The bars represent values (±SE). Different letters indicate significant difference at P value <0.05. While the CK larvae ecdysed to fourth larvae instars 2 days after initiation of bioassay (e), around 20% of the RNAi larvae failed to ecdyse, became moribund and finally died (f). Scale bar is 1 mm.
Discussions
BrC controls pupal commitment and pupal morphogenesis, and inhibits adult differentiation (Kiss et al., Reference Kiss, Bencze, Fodor, Szabad and Fristrom1976; Kiss et al., Reference Kiss, Beaton, Tardiff, Fristrom and Fristrom1988; Zhou et al., Reference Zhou, Hiruma, Shinoda and Riddiford1998; Zhou & Riddiford, Reference Zhou and Riddiford2002; Zhou et al., Reference Zhou, Zhou, Truman and Riddiford2004; Konopova & Jindra, Reference Konopova and Jindra2008; Parthasarathy et al., Reference Parthasarathy, Tan, Bai and Palli2008; Suzuki et al., Reference Suzuki, Truman and Riddiford2008; Daimon et al., Reference Daimon, Uchibori, Nakao, Sezutsu and Shinoda2015). Therefore, the feasibility of using an RNAi-based pest-control strategy targeting LdBrC gene in the third- and fourth-instar larvae in L. decemlineata was carefully examined in this study.
LdBrC should be an important regulator to L. decemlineata metamorphosis
In the present paper, we provided three lines of evidence to suggest that LdBrC was an essential regulator of the larval-pupal metamorphosis in L. decemlineata. Firstly, we cloned five full-length LdBrC cDNAs (LdBrC-Z1, LdBrC-Z2, LdBrC-Z3, LdBrC-Z4, and LdBrC-Z6) in L. decemlineata. These BrC isoforms (Z1–Z6) are conserved in both hemimetabolan and holometabolan insects (DiBello et al., Reference DiBello, Withers, Bayer, Fristrom and Guild1991; Bayer et al., Reference Bayer, Holley and Fristrom1996; Nishita & Takiya, Reference Nishita and Takiya2004; Spokony & Restifo, Reference Spokony and Restifo2007; Piulachs et al., Reference Piulachs, Pagone and Bellés2010; Nagamine et al., Reference Nagamine, Kayukawa, Hoshizaki, Matsuo, Shinoda and Ishikawa2014). In Psacothea hilari, although seven PhBrC isoforms (referred to as Z1, Z2, Z3, Z2/Z3, Z4, Z5/Z6, and Z6, respectively) are cloned, the Z5/Z6 isoform is aberrant in that it contained a premature stop codon (Nagamine et al., Reference Nagamine, Kayukawa, Hoshizaki, Matsuo, Shinoda and Ishikawa2014).
The second line of evidence was that all isoform-specific mRNAs were easily detected in all tested tissues. The wide distribution of LdBrC variants among various tissues indicates its requirement for pupal differentiation in L. decemlineata. Similar tissue expression patterns of BrC isoforms have also been reported in other insect species (Emery et al., Reference Emery, Bedian and Guild1994).
Thirdly, we found that the expression of the five LdBrC isoforms was suppressed by JH signaling, whereas the transcription was upregulated by 20E signaling. Tight regulation of the expression levels indicates important physiological functions of the five LdBrC isoforms in the larval-pupal metamorphosis in L. decemlineata. Consistent with our results, removal of the corpora allata (the primary organs for JH synthesis) in M. sexta and B. mori induces BrC expression and subsequent precocious pupation, and application of exogenous JH to allatectomized larvae inhibits BrC transcription and pupation in these larvae (Zhou et al., Reference Zhou, Hiruma, Shinoda and Riddiford1998; Reza et al., Reference Reza, Kanamori, Shinoda, Shimura, Mita, Nakahara, Kiuchi and Kamimura2004). Moreover, treatment with methoprene at the onset of the adult molt causes re-expression of BrC in some insect species (Zhou & Riddiford, Reference Zhou and Riddiford2002; Konopova & Jindra, Reference Konopova and Jindra2008; Minakuchi et al., Reference Minakuchi, Tanaka, Miura and Tanaka2011). JH-mediated repression of BrC transcription and activity has also been reported in cultured insect epidermis (Zhou & Riddiford, Reference Zhou and Riddiford2001; Muramatsu et al., Reference Muramatsu, Kinjoh, Shinoda and Hiruma2008).
For 20E signaling, it is known that BrC is one of the early 20E response genes in holometabolous insect species (Karim et al., Reference Karim, Guild and Thummel1993; Bayer et al., Reference Bayer, Holley and Fristrom1996). In B. mori, the expression pattern of BmBrC in the carcass and silk gland roughly coincides with the fluctuation of 20E titers in the hemolymph (Nishita & Takiya, Reference Nishita and Takiya2004). Moreover, an induction of BmBrC by 20E is detected in the Bombyx culture cell line BM-N (Nishita, Reference Nishita2014).
It can accordingly be hypothesized that JH represses LdBrC in young larvae. When JH declines at the final larval instar, LdBrC isoforms are expressed. The resultant proteins control pupal commitment and pupal morphogenesis in L. decemlineata, resembling other holometabolous insect species (Huang et al., Reference Huang, Lozano and Belles2013; Ureña et al., Reference Ureña, Chafino, Manjón, Franch-Marro and Martín2016).
Targeting LdBrC with dsRNA is a potential control strategy
In Drosophila, Bombyx and Tribolium, all BrC isoforms share some functions, or at least have partially overlapping functions during metamorphosis (Bayer et al., Reference Bayer, von Kalm and Fristrom1997; Parthasarathy et al., Reference Parthasarathy, Tan, Bai and Palli2008; Suzuki et al., Reference Suzuki, Truman and Riddiford2008). In T. castaneum, for example, all larvae treated for a single isoform ecdyse into pupae displaying a degree of aberrancies increasing in the order of isoforms: Z5 < Z1 < Z4 < Z3 < Z2. The most visible effect is the shortening of the wings and legs (Parthasarathy et al., Reference Parthasarathy, Tan, Bai and Palli2008; Suzuki et al., Reference Suzuki, Truman and Riddiford2008). Injection of dsBrCZ1 and dsBrCZ2, dsBrCZ1 and dsBrCZ3, dsBrCZ2 and dsBrCZ4, and dsBrCZ3 and dsBrCZ4 results in individuals that resemble the complete BrC knockdown phenotypes, except that the degrees of pigmentation and segmentation are weaker and the urogomphi are more pupal-like in character (Parthasarathy et al., Reference Parthasarathy, Tan, Bai and Palli2008; Suzuki et al., Reference Suzuki, Truman and Riddiford2008).
Therefore, we dietarily introduced each of the two dsRNAs of LdBrC direct against the variable common region of all five variants into the newly-molted third- and fourth-instar larvae in the present paper, to test the lethal effects on the old larvae in L. decemlineata. Our results revealed that the fourth-instar dsBrC-fed fully-grown larvae obtained lighter fresh weights and approximately 30% LdBrC RNAi larvae did not get buried into the soil for pupation and finally died at the larval stage. Moreover, most remaining dsBrC-fed larvae did not normally pupate, or did not emerge as adults. Furthermore, around 20% third-instar LdBrC RNAi larvae remained as moribund beetles and finally died. Similar to our results, 98% of the TcBrC RNAi larvae in T. castaneum eventually die during the prepupal stage (Konopova & Jindra, Reference Konopova and Jindra2008). Moreover, 95% of the dsBrC injected Chrysopa larvae arrest at the prepupal stage, and 41% of them fail to complete or even initiate spinning their cocoons (Konopova & Jindra, Reference Konopova and Jindra2008).
In the present paper, we found that an arrested fourth-instar LdBrC RNAi hypomorph was a miniature adult in L. decemlineata, after removal of the apolysed larval cuticle. In agreement with our results, it is well known that BrC is involved in the metamorphic control of pupal commitment, pupal morphogenesis and the inhibition of adult differentiation (Kiss et al., Reference Kiss, Bencze, Fodor, Szabad and Fristrom1976; Kiss et al., Reference Kiss, Beaton, Tardiff, Fristrom and Fristrom1988; Zhou et al., Reference Zhou, Hiruma, Shinoda and Riddiford1998; Zhou & Riddiford, Reference Zhou and Riddiford2002; Zhou et al., Reference Zhou, Zhou, Truman and Riddiford2004; Konopova & Jindra, Reference Konopova and Jindra2008; Parthasarathy et al., Reference Parthasarathy, Tan, Bai and Palli2008; Suzuki et al., Reference Suzuki, Truman and Riddiford2008; Daimon et al., Reference Daimon, Uchibori, Nakao, Sezutsu and Shinoda2015). In the present paper, we found that the LdBrC RNAi larvae bypassed pupal stage, and became a miniature adult, although some structures, such as wing discs, were not fully developed in L. decemlineata. Similarly, BrC RNAi prepupae show a blend of larval, pupal, and partially even adult features in Tribolium and Chrysopa (Konopova & Jindra, Reference Konopova and Jindra2008; Suzuki et al., Reference Suzuki, Truman and Riddiford2008).
In short, exposure the fourth-instar larvae to dsBrCs killed most treated beetles. Therefore, our results demonstrate that targeting LdBrC with dsRNA can be used to control the final instar larvae in L. decemlineata.
However, there are still ample amount concerns and risks before application of dsBrC to control L. decemlineata larvae. Among them we will focus on three issues here. Firstly, expression in plants, chemical synthesis and production in bacteria and other microorganisms are the three major methods that are being developed to produce dsRNA. It is undoubted that the expression of dsRNA in transgenic plants might work well for commercial crops such as cotton. In contrast, dsRNA synthesized in bacteria may be a better choice for pests attacking food crops that is consumed directly by humans, such as potato (Palli, Reference Palli2014). Our results in the present paper raise the possibility to control L. decemlineata larvae by bacterially-produced dsRNA.
Secondly, the first through fourth larval instars often occurred simultaneously in the potato field in Xinjiang Uygur Autonomous Region in China in L. decemlineata (Guo et al., Reference Guo, Tan and Zhang2013). The practical implementation of RNAi to protect potato necessarily requires the development of broad-spectrum larvicidal effects against both young and old larvae. A way is to construct a fused dsRNA including sequences from several genes respectively effective to young and old larvae. In fact, transgenic potato lines (Solanum tuberosum cv. Desiree) expressing fused viral coat protein coding sequences from Potato virus X, Potato virus Y and Potato virus S resulted in nearly 100% resistance against the infection of the three virus, when compared with untransformed controls (Hameed et al., Reference Hameed, Tahir, Asad, Bilal, Van Eck, Jander and Mansoor2017). Moreover, spraying barley (Hordeum vulgare) with a 791 nt long dsRNA (CYP3-dsRNA) targeting three fungal ergosterol biosynthesis genes (CYP51A, CYP51B, CYP51C) successfully knocked down the three genes in Fusarium graminearum, and inhibited fungal growth (Koch et al., Reference Koch, Biedenkopf, Furch, Weber, Rossbach, Abdellatef, Linicus, Johannsmeier, Jelonek, Goesmann, Cardoza, McMillan, Mentzel and Kogel2016). We will conduct further experiments to test this issue.
The third concern is how to develop species-specific dsRNA pesticide given that BrC is conserved among insects. Since RNAi operates in a very sequence-specific manner, dsRNAs specific to the unique 5′- and/or 3′-untranslated region of BrCs could selectively kill only one species without adversely affecting other closely related species. Furthermore, when dsRNAs are harnessed as potential pesticides, it will also be important to assess the likelihood of cross-reactivity with other species (Whyard et al., Reference Whyard, Singh and Wong2009).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485318001050.
Acknowledgments
This research was supported by China Agriculture Research System (CARS-09-P22), the National Key R & D Program of China (2017YFD0200900), the National Natural Science Foundation of China (31760544) and the Fundamental Research Funds for the Central Universities (KYTZ201403).









