Introduction
Insect immune response encompasses all the processes by which insects defend themselves against invading organisms such as bacteria, fungi and parasitoids (Gillespie et al., Reference Gillespie, Kanost and Trenczek1997; Soderhall & Cerenius, Reference Soderhall and Cerenius1998; Lavine & Strand, Reference Lavine and Strand2002; Schmid-Hempel, Reference Schmid-Hempel2005; Zibaee et al., Reference Zibaee, Bandani, Talaei-Hassanlouei and Malagoli2011). The recognition of a foreign particle in the hemocoel induces several cell-mediated responses like changes in hemocyte numbers, clotting, phagocytosis, nodule formation, encapsulation and melanization (Gillespie et al., Reference Gillespie, Kanost and Trenczek1997; Schmid-Hempel, Reference Schmid-Hempel2005). In addition of the cell-mediated immunity, humoral immunity is activated through the synthesis of antimicrobial peptides, lysozymes and activation of phenoloxidase (PO) cascade as well as the production of reactive intermediates of oxygen and nitrogen (Eleftherianos et al., Reference Eleftherianos, Marokhazi, Millichap, Hodgkinson, Sriboonlert, ffrench-Constant and Reynolds2006; Haine et al., Reference Haine, Moret, Siva-Jothy and Rolff2008; Ye et al., Reference Ye, Chenoweth and McGraw2009; Laughton et al., Reference Laughton, Garcia, Altincicek, Strand and Gerardo2011).
Chilo suppressalis Walker (Lepidoptera: Crambidae) is one of the major rice stem borers generating severe economic impact on rice production in Iran, Southern and East areas of Asia and South America (Zibaee et al., Reference Zibaee, Sendi, Alinia, Ghadamyari and Etebari2009). Larval stages feed on inner parts of rice stem leading to dead heart and white head as identified symptoms of damage (Khanjani, Reference Khanjani2006). C. suppressalis produces two to three generations per year generating severe damage to rice plants due to intense feeding, for that reason comprehensive management strategies are needed to decrease constraints on rice production. Although there are some natural biocontrol agents in the field like the wasps Trichogramma spp. and the predatory bug Andrallus spinidens Fabricius (Zibaee et al., Reference Zibaee, Hoda and Fazeli-Dinan2012). However, usually the primary method of control of the pest relies on spraying of chemical pesticides such as Diazinon (more than 95% of fields), Padan and Fenitrothion. Zibaee et al. (Reference Zibaee, Sendi, Alinia, Ghadamyari and Etebari2009) reported increased resistance of C. suppressalis in several regions of Iran, with some populations displaying up to 13.67-fold resistance to diazinon compared to susceptible populations. Esterases, alkaline phosphatases and glutathione transferase seem to be involved in developing resistance.
The combination of the severe damages generated by C. suppressalis and the resistance to Diazinon raises concerns about its indiscriminate use of this insecticide. Diazinon is still used in 200,000 out of 600,000 ha of rice fields with an estimated release of 4000–8000 t diazinon year−1 (Vakili, Reference Vakili1998). In this context, the utilization of biological control agents such as entomopathogenic fungi represents a promising choice. Majidi-Shilsar (Reference Majidi-Shilsar2002) demonstrated pathogenicity of Beauveria. bassiana on all developmental stages of C. suppressalis egg masses, larvae, pupae and adults. Majidi-Shilsar et al. (Reference Majidi-Shilsar, Alinia and Ershad2008) studied pathogenicity of B. bassiana on first- and second generation C. suppressalis larvae in field conditions. Results showed that fungus could infest 60% of larvae inside the rice stems. Pathogenicity of B. bassiana on C. suppressalis has been well documented, but there is no information on the pathogenicity exerted on C. suppressalis by other entomopathogenic fungi. The successful control of an insect population through entomopathogenic fungi relies on well-balanced host–pathogen–environment interactions and requires the understanding of factors responsible for insect susceptibility and resistance to a pathogen. (Hajek & St. Leger, Reference Hajek and St. Leger1994; Chouvenc et al., Reference Chouvenc, Su and Robert2009). For this reason, the objective of this study was to analyze the immune responses of C. suppressalis larvae to different isolated fractions of the entomopathogenic fungi B. bassiana, Metarhizium anisopliae, Isaria fumosoroseus and Lecanicilium lecanii.
Materials and methods
Insect rearing
Rice striped stem borer pupae were collected from rice fields and reared on the same variety of seedling (Hashemi). Insect were reared using Zibaee et al. (Reference Zibaee, Sendi, Alinia, Ghadamyari and Etebari2009) methodology with slight modifications, laboratory conditions were kept at 28±1 °C, 80% relative humidity (RH) and 16 h light : 8 h dark (LD 16:8). Hatched larvae fed on rice seedlings until they reached fourth instars. Laboratory conditions were checked daily, containers were cleaned and fresh stems were provided for larvae.
Entomopathogenic fungi culture
B. bassiana (Isolates: B1, B2 and B3 from Fashand, Iran), M. anisopliae (one isolate), I. fumosoroseus (one isolate) and L. lecanii (Isolate: LiR) were cultured at 25±1 °C on Sabouraud Dextrose Agar (pH=5.6) amended with 1% yeast extract. After 14 days, conidia were washed off with a 0.01% solution of Tween 80 (Sigma Aldrich, USA) and different concentrations of spores were prepared.
Determination of hemocyte types by light microscopy
Hemolymph from ten larvae of C. suppressalis (fourth instar) was carefully collected from one of the wounded prolegs with a 50 μl sterile glass capillary tube (Sigma Aldrich). The collected hemolymph was immediately diluted in an anticoagulant solution (0.01 M ethylenediamine tetraacetic acid, 0.1 M glucose, 0.062 M NaCl, 0.026 M citric acid and pH=4.6) as described by Azambuja et al. (Reference Azambuja, Garcia and Ratcliffe1991) in 1:8 (anticoagulant/hemolymph) proportion. Hemolymph samples were directly put onto a glass slide and allowed them to dry at dark in natural air conditions for 20–30 min. Six replicates were performed to obtain images from hemocytes. Cells were then fixed in methanol for 10 min at room temperature. Fixed hemocytes were stained with Giemsa (diluted 1:9 in distilled water) for 15–35 min and slides were rapidly rinsed with distilled water (Brayner et al., Reference Brayner, Araujo, Cavalcanti, Alves and Peixoto2005). After staining the slides were dehydrated in ethanol and mounted in xylene. Hemocytes were monitored under light microscope (Olympus company).
Bioassay
Spores of 14-day-old SDA culture were removed by a scalpel and the serial concentrations of each Entomopathogenic fungi (EF) were prepared from 102 to 108 spores ml−1. Larvae were topically exposed by adding 2 μl of the solution with a sampler device (Brand Co., Germany). Control larvae were treated with 2 μl of Tween 80 (0.05%) solution. Mortality was recorded to death of individuals in the highest concentration of EFs. Lethal concentration values were calculated using POLO-PC software.
Effect of fungal spore isolates on circulating hemocyte number
To determine the effects of enthomopathogenic fungi on hemocyte number, fourth instar larvae were injected laterally into the third thoracic segment with 1 μl of solution containing 105 spores ml−1 of the fungal isolates in Tween 80 (0.05%). Latex beadsFootnote 1 and Tween 80 (0.05%) solution were used as positive and negative controls, respectively. Hemolymph was collected at intervals of 1, 3, 6, 12, 24, 48 and 72 h after injection from the control, latex bead- and spore-injected groups, separately. Samples of hemolymph from five larvae were blend in a 1.5 ml plastic tube with 1 ml of ice-cold anticoagulant buffer. The tubes were gently inverted five to seven times to facilitate mixing, and both total and specific hemocyte numbers were counted using an improved Neubauer hemocytometer (Chemkind Co.). For each treatment (Time interval), six larvae were used and the experiment had five replicates (N=30 for each isolate, n=5).
Effect of fungal spores on nodulation
After spore-injections the numbers of nodules were calculated after 1, 3, 6, 12, 24, 48 and 72 h. Injected larvae were chilled on ice, hemolymph was collected in a capillary tube and 200 μl were placed onto a hemocytometer for nodule counting (Franssens et al., Reference Franssens, Smagghe, Simonet, Claeys, Breugelmans, DeLoof and Vanden Broeck2006). For each treatment (time interval), six larvae were used and the experiment had five replicates (N=30 for each isolate, n=5).
Effects of fungal spores on PO activity
After injecting the larvae with fungal spores, the hemolymph was collected after 1, 3, 6, 12, 24, 48 and 72 h as mentioned earlier. The hemocyte lysate was prepared based on the protocol described by Leonard et al. (Reference Leonard, Kenneth and Ratcliffe1985). Collected hemolymph was mixed with anticoagulant buffer and centrifuged at 13,000 rpm for 5 min; the supernatant was discarded and the pellet washed gently twice with a phosphate buffer (0.02 M, pH=7.1). Cells were homogenized in 200 μl of phosphate buffer centrifuged at 13,000 rpm for 15 min, and the supernatant was used in PO assays. Samples (10 μl) were pre-incubated with phosphate buffer solution at 30 °C for 3 min followed by adding of 20 μl of a 10 mM solution of dihydroxyphenylalanin. The mixture was incubated for an additional 5 min at 30 °C and PO activity was measured in the spectrophotometer at 495 mm. One unit of PO activity represents the amount of enzyme required to produce an increase in absorbance of 0.01 units of absorbance per min (Dularay & Lackie, Reference Dularay and Lackie1985). Activity of injected larvae was compared with that of Tween 80-injected controls (n=3).
Statistical analysis
Data were analyzed using one-way analysis of variance using (add procedure) (SAS reference). Tukey's studentized test was performed on those comparisons displaying significant differences (P≤0.05). To determine the differences among fungi in each time point a factorial test was performed (SAS, 1997).
Results
Determination of hemocyte types by light microscopy
Light microscopy observations after Giemsa staining of larval hemolymph revealed the presence of four morphological distinct types of hemocyte (fig. 1A–D) (Lavine & Strand, Reference Lavine and Strand2002). Prohemocytes are a small, oval cells with a large and central nucleus and thin cytoplasm (fig. 1A). Granulocytes display a regularly ellipsoidal shape, a large nucleus and a highly granular cytoplasm (fig. 1B). Plasmatocytes are large spindle-shaped cells, with central nucleus and very few granules in the cytoplasm (fig. 1C). Oenocytoids are a circular cell with a large unconventional nucleus and a granular cytoplasm (fig. 1D).

Fig. 1. Light microscopy images of C. suppressalis hemocytes: (A) prohemocyte with a large nucleus and a thin peripheral cytoplasm; (B) granulocyte filled with numerous granules in the cytoplasm and large nucleus; (C) plasmatocyte exhibiting a spindle shape, small nucleus and a few granules. (D) oenocytoid with a large nucleus and agranular cytoplasm Magnification 40× with the exception of (b) (60×). Bar=5 μm.
Effects of fungi on mortality of larvae
All entomopathogenic fungi used had different effects on mortality of C. suppressalis larvae in a dose-dependent manner (fig. 2). The highest mortality (90%) was observed at 108 spore ml−1 up to 90% (fig. 3). Specifically, BB1, BB3 and M. anisopliae lead to the highest mortality on larvae and L. lecanii caused the lowest mortality on larvae (fig. 3).

Fig. 2. Mortality of entomopathogenic fungi on larvae of C. suppressalis. Bars with different letters are statistically different (Tukey's test, P≤0.05).

Fig. 3. Nodule formation in C. suppressalis 6 h after injection by different entomopathogenic fungi.
Effect of fungal spore on hemocyte numbers
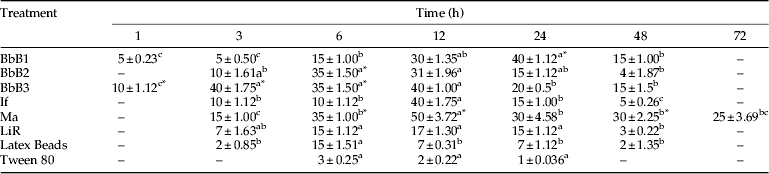
Table 1 summarizes the total hemocyte counts over time in C. supressalis larvae injected with spores of different entomopathogenic fungi. The highest number of total hemocytes was obtained 3 h after injection by spores of the three isolates of B. bassiana (table 1, F=20.18, P>F: 0.001). The highest number of total hemocytes after the injection of I. fumosoroseus or M. anisopliae spores was found 6 h post-injection, even though of the effects of M. anisopliae isolates after 6 h were comparable to those registered 12 h post-injection (table 1, F=0.024, P>F: 2.92; F=0.0115, P>F: 3.43). LiR isolates from L. lecanii and latex beads caused the highest total hemocyte numbers between 3 and 6 h post-injection (table 1, F=3.43, P>F: 0.0115).
Table 1. Changes of total hemocyte numbers in C. suppressalis larvae injected by 105 spore ml−1 of different entomopathogenic fungal isolates.

1 Mean (n=5) values must be considered as ×104 cells ml−1.
2 Different letters indicate significant differences among times in each treatment (P≤0.05). Also, asterisks showed significant differences in each time intervals for all treatments (column).
In terms of differential count, the highest number of granulocytes was found 3 h post-injection of BbB1 and BbB3 isolates and 6 h after injection of BbB2 spores (table 2). I. fumosoroseus and M. anisopliae isolates exerted their maximal effects after 6 h, when the highest number of granulocytes was observed (table 2, F=2.92, P>F: 0.0245, F=3.43, P>F: 0.0115). Similarly, the highest number of granulocytes after L. lecanii spore injection and latex beads was observed 6 h after injection (table 2). For plasmatocytes, the highest number was observed after 3 h for BbB1 isolate and 6 h for BbB2 and BbB3 isolates of B. bassiana (table 3, F=6.59, P>F: 0.0002; F=20.18, P>F: 0.0001). For granulocytes, the number of circulating plasmatocytes reached its maximum after 6 h of spore injection (table 3, F=2.92, P>F: 0.0245; F=3.43, P>F: 0.115). After injection of LiR and latex beads, the highest number of circulating plasmatocytes was registered 6 h post-injection (table 3, F=3.43, P>F: 0.0115).
Table 2. Changes in plasmatocyte number in C. suppressalis larvae injected by 105 spore ml−1 of different entomopathogenic fungal isolates.

1 Mean (n=5) values must be considered as ×104 cells ml−1.
2 Different letters indicate significant differences among times in each treatment (P≤0.05). Also, asterisks showed significant differences in each time intervals for all treatments (column).
Table 3. Changes in granulocyte number in C. suppressalis larvae injected by 105 spore ml−1 of different entomopathogenic fungal isolates.

1 Mean (n=5) values must be considered as ×104 cells ml−1.
2 Different letters indicate significant differences among times in each treatment (P≤0.05). Also, asterisks showed significant differences in each time intervals for all treatments (column).
Effect of fungal spores on nodulation
Injection of C. suppressalis larvae with spores of BbB1, BbB2 and BbB3 isolates of B. bassiana exerted its maximal effects on nodule formation (fig. 3) after either 12 or 24 h (table 4, F=6.59, P>F: 0.0002; F=20.18, P>F: 0.0001) (fig. 3). I. fumosoroseus, M. anisopliae and L. lecanii generated maximum nodule formation 12 h after the injection, but in the case of LiR, there was no significant differences between time intervals of 12 and 24 h (table 4, F=2.91, P>F: 0.0245; F=3.43, P>F=0.0115). Latex beads injection of larvae resulted in the highest number of nodules after 6 h of the injection (table 4, F=2.941, P>F: 0.0245).
Table 4. Number of nodules formation counted in C. suppressalis larval hemolymph after injection with 105 spore ml−1 from different entomopathogenic fungal isolates.
1 Different letters show statistical differences among times in each treatment (P≤0.05). Also, asterisks showed significant differences in each time intervals for all treatments (column).
Effects of fungal spores on PO activity
The highest activities of PO after spore injection of BbB1, BbB2 and BbB3 were obtained after 12, 6 and 12 h, respectively (table 5, F=3.43, P>F: 0.0115), with the BbB3 extract showing the highest effect. After spore injections of I. fumosoroseus, M. anisopliae, LiR and latex beads, PO activity was observed to reach its maximum between 6 and 12 h post-injection (table 5).
Table 5. PO specific activity (U mg−1 protein) measured in C. suppressalis larval hemolymph after injection with 105 spore ml−1 from different entomopathogenic fungal isolates.

1 Different letters show statistical differences among times in each treatment (P≤0.05). Also, asterisks showed significant differences in each time intervals for all treatments (column).
Discussion
The environmental concerns raised by the continued use of chemical pesticides, the rising of resistant strains of insect pests and the suitable conditions for the utilization of entomopathogenic fungi in rice fields, represent promising premises for their use as biological control agent of C. suppressalis in Iran. The identification of hemocyte types of an insect represents the first step of a comprehensive immune characterization and it is fundamental for understanding how the immune system may react against a specific pathogen. Through light microscopy observations, four basic types of hemocytes were identified in larvae of C. suppressalis prohemocytes, granulocytes, plasmatocytes and oenocytoids. These types of hemocytes have been well documented in other lepidopteran larvae (Lavine & Strand, Reference Lavine and Strand2002; Nakahara et al., Reference Nakahara, Shimura, Ueno, Kanamori, Mita, Kiuchi and Kamimura2009). In Bombyx mori L. (Lepidoptera: Bombycidae) frequently referred to as the main model for lepidopteran immunity, it has been pointed out that prohemocyte is a multi-potent stem cell (Yamashita & Iwabuchi, 2001), plasmatocytes and granulocytes are responsible of cell-mediated immune reactions (Wago, 1991) and oenocytoids are involved in melanization (Iwama & Ashida, 1986). Nakahara et al. (Reference Nakahara, Shimura, Ueno, Kanamori, Mita, Kiuchi and Kamimura2009) used various arrays of flow-cytometry to characterize silkworm hemocytes. Also, Nakahara et al. (Reference Nakahara, Shimura, Ueno, Kanamori, Mita, Kiuchi and Kamimura2009) synthesized cDNA from these hemocytes and subset-specific gene expression was examined by RT-PCR (Nakahara et al., Reference Nakahara, Shimura, Ueno, Kanamori, Mita, Kiuchi and Kamimura2009). Results revealed that granulocytes, plasmatocytes and oenocytoids expressed different classes of immune genes and showed their multiple roles in silkworm immunity. Studies have been shown that spherulocytes has not genetically based immune functions because they failed to express most of the immune-involved genes. So, it has been suggested to play a distinct role from the other three cell types (Nakahara et al., Reference Nakahara, Shimura, Ueno, Kanamori, Mita, Kiuchi and Kamimura2009). Due to the correspondence between C. suppressalis and B. mori cell morphology, we used the results collected by Nakahara et al. (Reference Nakahara, Shimura, Ueno, Kanamori, Mita, Kiuchi and Kamimura2009) to interpret the changes of granulocytes and plasmatocytes number as a marker of the immune challenge represented by the injection of spores from different entomopathogenic fungi.
Isolates from entomopathogenic fungi modified both total and specific hemocyte number in C. suppressalis larvae. Although, isolates from diverse fungi exerted effects of different amplitude, in general the highest increase in hemocyte number was observed from 3 to 6 h post-injection. The increase observed within the first 24 h was followed by a decrease 72 h post-injection. This type of fluctuation in hemocyte, number of insects during pathogenic challenges have been previously recorded in Melanoplus sanguinipes Fabricius (Orthoptera: Acrididae) (Bidochka & Khachatourians, Reference Bidochka and Khachatourians1987), Schistocerca gregaria L. (Orthoptera: Acrididae) (Gunnarsson & Lackie, Reference Gunnarsson and Lackie1985), Periplaneta americana L. (Blattaria: Blattidae) Spodoptera exigua Hubner (Lepidoptera: Noctuidae) (Hung & Boucias, Reference Hung and Boucias1992), Galleria mellonella L. (Lepidoptera, Pyralidae) (Sewify & Hashem, Reference Sewify and Hashem2001), Reticulitermes flavipes Kollar (Isoptera: Rhinotermitidae) (Chouvenc et al., Reference Chouvenc, Su and Robert2009), Oxya japonica Thunberg (Orthoptera: Acrididae) and Eurygaster integriceps Puton (Hemiptera: Scutelleridae) (Zibaee et al., Reference Zibaee, Bandani, Talaei-Hassanlouei and Malagoli2011). The observed increase in total hemocyte count relies on the increment of both granulocytes and plasmatocytes, suggesting a general triggering of cell-mediated immunity against the fungal extracts. Observed decrease in hemocyte numbers at the end of the experiments is due to reduction of both granulocytes and plasmatocytes populations. Different explanations have been proposed in other insects for the trend we have observed in C. suppressalis: (i) a possible involvement of hemocytes in nodule formation, (ii) the cytotoxic effect of fungal secondary metabolites on hemocytes and (iii) a membrane damage due to the composition of spore surface, mainly represented by hydrophobic proteins. Mazet et al. (Reference Mazet, Hung and Boucias1994) reported that B. bassiana produced toxic metabolites while infecting the larvae of the moth S. exigua, thus reducing the activity of larval hemocytes. For example, Destruxins is a compound produced by M. anisopliae that is toxic against the hemocytes of Manduca sexta L. (Lepidoptera: Sphingidae) (Samuels et al., Reference Samuels, Charnley and Reynolds1988; Huxham et al., Reference Huxham, Lackie and McCorkindale1989). Bandani (Reference Bandani2005) observed that the total hemocyte number of G. mellonella decreased in comparison with control in a dose-dependent fashion subsequent to the infection with entomopathogenic fungus, Tolypocladium cylindrosporum. Zibaee et al., (Reference Zibaee, Bandani, Talaei-Hassanlouei and Malagoli2011) showed that fungal secondary metabolites inhibited phagocytic activity of E. integriceps hemocytes and hampered nodule formation. In case of latex bead and Lle, although an increase in hemocyte numbers was observed but it was so lower than B. bassiana isolates. This increase could be attributed to surface of spores or lower production of secondary metabolites. Meanwhile, pathogenicity of L. lecanii was so lower than other entomopathogenic fungi.
To explore the possible cause of hemocyte decrease, we also quantified the effects of fungal extracts on nodule formation. Nodule formation is one of the major cellular responses of insects to pathogens, especially when the invader is too big for being engulfed through phagocytosis (Chouvenc et al., Reference Chouvenc, Su and Robert2009). Our fungal extracts stimulated nodulation in C. suppressalis larvae the highest number of nodules was found at 6 and 12 h of post-injection, in concomitance with the increase of circulating hemocytes. In these respects, the hemocyte decrease we have registered might be due to a toxic effect of fungal isolates rather than the recruitment of hemocytes in nodule formation. Solter et al. (Reference Solter, Maddox and McManus1997) and Solter & Maddox (Reference Solter and Maddox1998) proposed that infections in lepidopteran larvae were accompanied by nodule formation and melanin deposition. Gillespie et al. (Reference Gillespie, Burnett and Charnley2000) demonstrated an inverse correlation between hemocyte counts and number of nodules after a fungal infection. In our study this is not the case, and the number of nodules varied on the basis of injected entomopathogenic fungi in parallel with hemocyte number. The positive control latex beads caused the lowest nodule formation in comparison with entomopathogenic fungi. Cellular encapsulation or nodulation is induced by adhesion of hemocytes to the surface of non-self targets (Ling & Yu, Reference Ling and Yu2006). Pech & Strand (Reference Pech and Strand1996) showed that proteins containing RGD tripeptidic motif have a critical role in encapsulation. The authors suggested that cell adhesion molecules such as integrins recognize RGD sequence and participate in cellular encapsulation (Pech & Strand, 1995; Ruoslahti, Reference Ruoslahti1996). In addition to RGD and integrins, surface characteristics of non-self targets may also be important in eliciting of encapsulation (Gorman et al., Reference Gorman, Schwartz and Paskewitz1998; Lavine & Strand, Reference Lavine and Strand2001). Lavine & Strand (Reference Lavine and Strand2001) found no encapsulation of latex bead incubated by Pseudoplusia includes Walker (Lepidoptera: Noctuidae) hemocyte in vitro. Conversely, encapsulation occurred after pre-incubation of beads by plasma or injection in vivo and Lavine & Strand (Reference Lavine and Strand2001) suggested the involvement of humoral recognition molecules in encapsulation.
PO cascade and melanization are intimately connected with encapsulation. POs are stocked pre-enzymes or zymogens in insect hemolymph and they become activated upon wounding or infection as part of the innate immune response (Kanost & Gorman, Reference Kanost, Gorman and Beckage2008). The enzymes have two biochemical function in hydroxylation of tyrosine to form L-dihydroxyphenylalanine, and oxidizing o-diphenols to form quinones (Gorman et al., Reference Gorman, An and Kanost2007). After forthcoming reactions, quinones form melanin, which is deposited on the surface of encapsulated parasites, hemocyte nodules and wound sites (Kanost & Gorman, Reference Kanost, Gorman and Beckage2008). In our experiments, the highest activity of PO was found after 6 and 12 h post-injection which it corresponds to the hemocyte increase and nodule formation. Results may be explained by melanin deposition as a complement of the nodule formation process. The melanin deposition (melanization) of nodules and capsules around non-self targets is one of the important defensive responses in insects (Gillespie et al., Reference Gillespie, Kanost and Trenczek1997; Christensen et al., Reference Christensen, Li, Chen and Nappi2005; Michel & Kafatos, Reference Michel and Kafatos2005; Nappi & Christensen, Reference Nappi and Christensen2005; Chouvenc et al., Reference Chouvenc, Su and Robert2009; Zibaee et al., Reference Zibaee, Bandani, Talaei-Hassanlouei and Malagoli2011). Melanin deposition blocks absorption of nutrients by parasites leading to their killing due to starvation (Chen & Chen, Reference Chen and Chen1995). Also, formation of cytotoxic reactive oxygen and nitrogen intermediates during melanin synthesis causes to kill invading organisms (Nappi & Christensen, Reference Nappi and Christensen2005).
In conclusion, our study demonstrated that C. suppressalis larvae present at least four different types of circulating hemocytes, in agreement with previous observations performed in other lepidopteran models. Our experiments evidenced a different susceptibility of C. suppressalis larvae to entomopathogenic fungi. In all cases the effects of the fungi involved cell-mediated immunity that is initially triggered and then strongly reduced. The immediate response of larvae to fungi infection was a significant increase of total circulating hemocytes. This increase included both granulocytes and plasmatocytes. However, after 3 days from the immune challenge, the number of circulating hemocytes was significantly lower. Consequently, the larvae showed nodulation and increased PO activity in the immediacy of the challenge, but progressively the phenomenon decreased. Further research is required in order to completely understand the effects of entomopathogenic fungi on immune system of C. suppressalis. These results combined with field mortality caused by entomopathogenic fungi will lead to the arsenal of bio-compatible controller for the striped rice stem borer C. suppressalis.
Acknowledgements
This research was supported by a grant of research from university of Guilan. The authors would like to thank Dr M. Fazeli-Dinan, H. Hoda and M. Salimi for their kind assistantship in the experiments.










