Introduction
The mirid bug Apolygus lucorum (Meyer-Dür) is an important polyphagous pest, broadly distributed in Japan, Russia, Egypt, Algeria, Europe and North America (Miyata, Reference Miyata1994; Watanabe, Reference Watanabe1995; Zhang & Zhao, Reference Zhang and Zhao1996; Watanabe et al., Reference Watanabe, Kikuchi and Tanaka1997; Lee et al., Reference Lee, Lee and Goh2002). It is found in most Chinese provinces except Hainan and Tibet (Lu & Wu, Reference Lu and Wu2008), and is most common in the cotton-planting regions of the Yangtze and Yellow Rivers (Lu et al., Reference Lu, Qiu, Feng, Li, Yang, Wyckhuys and Wu2008a). In recent years, with the wide adoption of Bt (Bacillus thuringiensis) cotton and the increased planting of fruit trees, population levels of A. lucorum and other mirid bugs have greatly increased in China (Lu et al., Reference Lu, Wu, Jiang and Xia2010a), and A. lucorum has become the dominant pest on cotton and many other crops (e.g., alfalfa, Chinese date, grape, apple, pear, peach and tea) (Lu et al., Reference Lu, Wu, Jiang, Xia, Li, Feng, Wyckhuys and Guo2010b; Lu & Wu, Reference Lu and Wu2012).
Both nymphs and adults of A. lucorum prefer feeding on tender plant parts, including leaves, flower buds and fruits (bolls). Damaged feeding sites cease to develop, while the surrounding tissues continued to grow rapidly resulting in abscission of flower buds and fruits, and fruit deformation (Lu & Wu, Reference Lu and Wu2008). The salivary enzymes of A. lucorum play a greater role in causing damage than mechanical injury due to stylet probing (Zhang et al., Reference Zhang, Lu and Liang2013). Polygalacturonase (PG) is an important group of salivary enzymes in A. lucorum, among which two PG genes (PG3-4 and PG3-5) are highly expressed (Zhang et al., Reference Zhang, Xu, Xiao, Lu, Liang, Zhang and Wu2015). Both play key roles in the injury caused by A. lucorum nymphs and adults on host plants (Zhang et al., Reference Zhang, Liu, Lu and Liang2017). These injuries greatly reduce the yield and quality of cotton, causing serious economic loss of 20–30% during years of high infestation (Lu & Wu, Reference Lu and Wu2011), and also greatly reduce the yield and quality of several fruits (Li et al., Reference Li, Men, Ye, Yu, Zhang, Li, Zhang and Zhou2012).
Apolygus lucorum can feed on more than 200 species of host plants (Jiang et al., Reference Jiang, Lu and Zeng2015; Pan et al., Reference Pan, Liu, Lu and Wyckhuys2015). Its adults have a great flight and dispersal capacities (Lu et al., Reference Lu, Wu and Guo2007; Song et al., Reference Song, Feng, Li, Zhang, Qiu and Li2012; Fu et al., Reference Fu, Liu, Li, Lu, Li and Wu2014), and they often switch food plants in the local agro-ecosystem (Pan et al., Reference Pan, Lu, Wyckhuys and Wu2013). One study found that female A. lucorum adults significantly preferred to feed and lay more eggs on flowering individuals of three plant species (Gossypium hirsutum L., Impatiens balsamina L. and Ricinus communis L.), and their offspring performed significantly better on these plants in flower (Dong et al., Reference Dong, Pan, Lu and Yang2013). Moreover, Wang et al. (Reference Wang, Bao, Yang, Xu and Yang2017) developed a molecular gut-content analysis for A. lucorum, which demonstrated the frequent movement of A. lucorum adults among host plants [i.e., cotton and Vigna radiata (L.) Wilczek.]. Host-plant switching has been found to improve the population growth of a number of phytophagous insect pests (Bernays et al., Reference Bernays, Bright, Howard, Raubenheimer and Champagne1992, Reference Bernays, Bright, Gonzalez and Angel1994; Modder & Tamu, Reference Modder and Tamu1996; Hägele & Rowell-Rahier, Reference Hägele and Rowell-Rahier1999). For example, the fecundity and host adaptability of adults of the whitefly Bemisia tabaci (Gennadius) significantly increased after being transferred from the less-preferred host (pepper, Capsicum annuum L.) to the preferred host (tomato, Lycopersicon esculentum Mill.) (Zhou et al., Reference Zhou, Li, Gu, Wang and Ren2011). Furthermore, the aphid Myzus persicae (Sulzer) fed on tobacco showed a higher reproductive capacity when transferred to radish (Zhu et al., Reference Zhu, Zhao, Liu, Li, Yang, Zhang, Yi and Li2012). Lygus lineolaris (Palisot de Beauvois) showed different rates of reproduction on different hosts, suggesting that host switching can considerably increase the population growth and survival of species (Stewart & Gaylor, Reference Stewart and Gaylor1994).
Lu et al. (Reference Lu, Wu, Cai and Liu2008b) developed a method to rear the nymphs and adults of A. lucorum using only fresh green bean pods. Maize is one of the preferred host plants of A. lucorum, and maize cobs have generally low pesticide residues and are annually available in the market. Hence, we examined the population fitness of A. lucorum nymphs and adults on one or two kinds of plant foods [fresh maize kernels (Zea mays L.) and green bean pods (Phaseolus vulgaris L.)] by bugs reared either individually or in groups. The results of this study would contribute to the development of improved rearing techniques for A. lucorum.
Materials and methods
Insects and plants
A laboratory colony of A. lucorum was established with 500–800 nymphs and adults collected from cotton fields at the Langfang Experimental Station (39.53°N, 116.70°E) of the Chinese Academy of Agricultural Sciences in Hebei Province, China. The colony was reared on green bean pods and supplied with a cotton ball soaked with a 10% sucrose solution, as described by Lu et al. (Reference Lu, Wu, Cai and Liu2008b). Insects were held at 25–28°C, 60–70% RH and a 14:10 h L:D photoperiod.
Fresh pods of green bean purchased from a local market were soaked for 10 min in 0.5% NaClO, wiped down to remove any residual pesticides from their surface, and then rinsed several more times in water before being dried with a towel (Lu et al., Reference Lu, Wu, Cai and Liu2008b). We then used a pesticide residue detector (RP-420, Sykam Scientific instrument co., LTD, Beijing, China) to detect any residual pesticides, and only used residue-free pods (each divided into 3 cm length) for the trials. Sweet maize cobs were also purchased from a local supermarket, but because of the limited use of pesticides on maize during its later growth stages, we just removed the bract and filament from them. The previous detection showed that maize kernels were residue-free. Then, we used either maize kernels or slices (groups of kernels from slices along the cob, 2 cm thickness) in the experiments.
Individual rearing
To compare the performance of A. lucorum nymphs between maize and green bean, newly emerged (i.e., first instar) nymphs from the laboratory colony were individually placed into glass vials (3 cm high, 3 cm diam.) covered with a nylon screen (120 mesh). Each glass vial contained one sweet maize kernel or one 3-cm green bean pod for food and a curled strip of paper (5 cm × 1 cm) to increase the activity space of nymphs (Lu et al., Reference Lu, Wu, Cai and Liu2008b). Experiments were conducted at 25 °C in environmental growth chambers (RXZ-500C, Ningbo Jiangnan Instrument Factory, Ningbo, China) with a photoperiod of 14:10 h (L:D) and 65% RH. Plant foods were changed daily. Nymphal development and mortality were recorded daily until nymphs molted to adults or died. The female ratio and weight of emerging adults were also determined. The trial was replicated four times for each plant food, and 28–30 nymphs were assayed for each replicate. Moreover, we reared 800 newly emerged nymphs individually on maize cobs or green beans according to the same procedure as above for the following adult trials.
To measure the effects of maize or green bean on the longevity and fecundity of adult bugs, we tested four plant food combinations: (1) MM, in which both nymphs and adults fed on maize; (2) MG, in which nymphs fed on maize and adults switched to feed on green bean; (3) GM, in which nymphs fed on green bean and adults switched to feed on maize; and (4) GG in which both nymphs and adults fed on green bean. Newly emerged adults that had fed on either maize or green beans were separately paired, and each pair was placed in a glass vial (3 cm high, 3 cm diam.) covered with a nylon screen (120 mesh). Either one sweet maize kernel or one 3-cm green bean pod was provided as both food and oviposition substrate, and a 5 cm × 1 cm paper strip was added to increase the activity space of adults. Experiments were conducted in environmental growth chambers at 25 °C with a photoperiod of 14:10 h (L:D) and 65% RH. Plant foods were changed daily and then inspected for eggs. Adult mortality and fecundity (i.e., the number of eggs laid by a female) were recorded daily. The trial was replicated four times for each plant food combination, and a total of 20 mated pairs were assayed for each replicate.
In addition, we also evaluated the influence of nymphal and adult food sources on the hatching rate of A. lucorum eggs, which was done by using filter paper as oviposition substrate to eliminate the influence of plant materials on the hatching rate (Chen et al., Reference Chen, Feng, Li, Guo, Fu and Qiu2012). For each of four plant food combinations (MM, MG, GM and GG), newly-emerged adults that had fed on maize or green beans were separately paired in a glass vial, with a total of 20 pairs for each plant food combination, and the rearing methods and environmental conditions were the same as above mentioned. During peak oviposition stage (10–15 day-old adults) (Dong et al., Reference Dong, Lu and Yang2012), we replaced all plant foods in the vials with four layers of wrinkled moist filter paper as an oviposition substrate at 20:00 pm, because >90% eggs were laid at night (Dong et al., Reference Dong, Lu and Yang2012). After 12 h, the eggs laid in filter paper were counted under microscope, and about 100 eggs were transferred with the filter paper into a petri dish and held at 25 °C, 65% RH and a 14:10 h L:D photoperiod to hatch, as described by Chen et al. (Reference Chen, Feng, Li, Guo, Fu and Qiu2012). Four batches of eggs were assayed for each plant food combination. Egg hatch was recorded daily and each newly emerged nymph was removed.
Group rearing
We repeated the above experiment using a group rearing process, in which 100 newly emerged nymphs were placed into rearing container and held at 25–28 °C, 60–70% RH and a 14:10 h L:D photoperiod. The lid of the container was covered with a nylon screen (120 mesh) for ventilation, and some curled paper strips (5 cm × 1 cm) were added to increase the activity space of nymphs. Either four sweet maize slices or four green bean pods were provided as food, and plant foods were changed daily. We used four containers for each plant food to examine nymphal survival and development, female ratio and adult weight. Moreover, we reared 1200 newly emerged nymphs in groups on maize cobs or green beans according to the same procedure as above for the next adult trials.
From these containers, newly emerged adults fed on maize or green bean were separately paired (30 pairs per rearing container) and were provided with either four maize slices or four green bean pods as food and oviposition substrate, and some 5 cm × 1 cm paper strips were added to increase the activity space of adults. Four plant food combinations (MM, MG, GM and GG) were tested, and plant foods were changed daily and inspected for eggs. Adult mortality and fecundity were recorded daily, and the trial was replicated four times for each plant food combination.
At the same time, the eggs were also collected from each plant food combination (MM, MG, GM and GG) using wrinkled moist filter paper, and egg hatch was recorded daily using the same method as described above in the experiment of individual rearing.
Statistical analysis
Nymphal survival rates and female adult ratio [Female/(Female + Male)] for A. lucorum offspring were compared between maize and green bean using a χ2 goodness-of-fit test (PROC FREQ), and percentage data were arcsine transformed before analysis. Paired t tests (PROC TTEST) were employed to compare the developmental duration of nymphs, adult weight, female and male longevity, female fecundity [log10(n + 1)-transformed] and hatch rate of A. lucorum eggs between maize and green bean reared either individually or in a group. Among the four plant food diets (MM, MG, GM and GG), differences in female and male longevity, female fecundity [log10(n + 1)-transformed], hatch rate of eggs, and population growth rate (i.e., nymphal survival rate×female adult ratio×female fecundity×egg hatching rate) of A. lucorum were further analyzed using one-way ANOVA (PROC ANOVA) followed by Tukey's HSD. All statistical analyses were performed using SAS 9.13 software (SAS Institute, 2005).
Results
Individual rearing
Compared with mirid bugs reared on green bean, the survival rate of A. lucorum nymphs reared on maize was significantly higher (χ2 = 6.65, df = 1, P = 0.0099), the developmental duration was shorter (t = 4.31, df = 6, P = 0.0050), and adult weight was greater (t = 9.35, df = 6, P < 0.0001) (table 1). In contrast, female ratio [Female/(Female + Male)] was not significantly different when bugs reared on maize vs. green bean (t = 1.06, df = 6, P = 0.3311) (table 1).
Table 1. Survival rate and developmental duration of Apolygus lucorum nymphs reared either individually or in groups on maize and green bean.

Note: There were 115 nymphs and 112 nymphs reared individually on maize and green bean respectively, and 400 nymphs reared in groups on both maize and green bean. Data are presented as means ± SE. Asterisk (*) in the same column indicates that there is a significant difference between maize and green bean reared either individually or in groups (P < 0.05), and ns means no significant difference between them (P > 0.05).
For adults, male longevity of A. lucorum on the plant food combination GM was significantly higher than on MM (F = 3.95, df = 3, 12, P = 0.0358) (table 2), while female longevity on plant food combinations MG and GG was significantly greater than on MM or GM (F = 43.87, df = 3, 12, P < 0.0001) (table 2). Meanwhile, female fecundity on plant food combination GM and GG was higher than on MM and MG (F = 17.49, df = 3, 12, P < 0.0001) (table 2). In addition, the hatch rate of A. lucorum eggs on MG was significantly higher than on the other three plant food combinations (F = 33.90, df = 3, 12, P < 0.0001) (table 2). The population growth rate of A. lucorum on plant food combination MG was significantly higher than on GG, but was similar to that on MM or GM (F = 3.90, df = 3, 12, P = 0.0371) (fig. 1); meanwhile a significant difference in the population growth rate was found between GM and GG (t = 3.03, df = 6, P = 0.0232), but no significant difference was found between MM and MG (P > 0.05) (fig. 1).
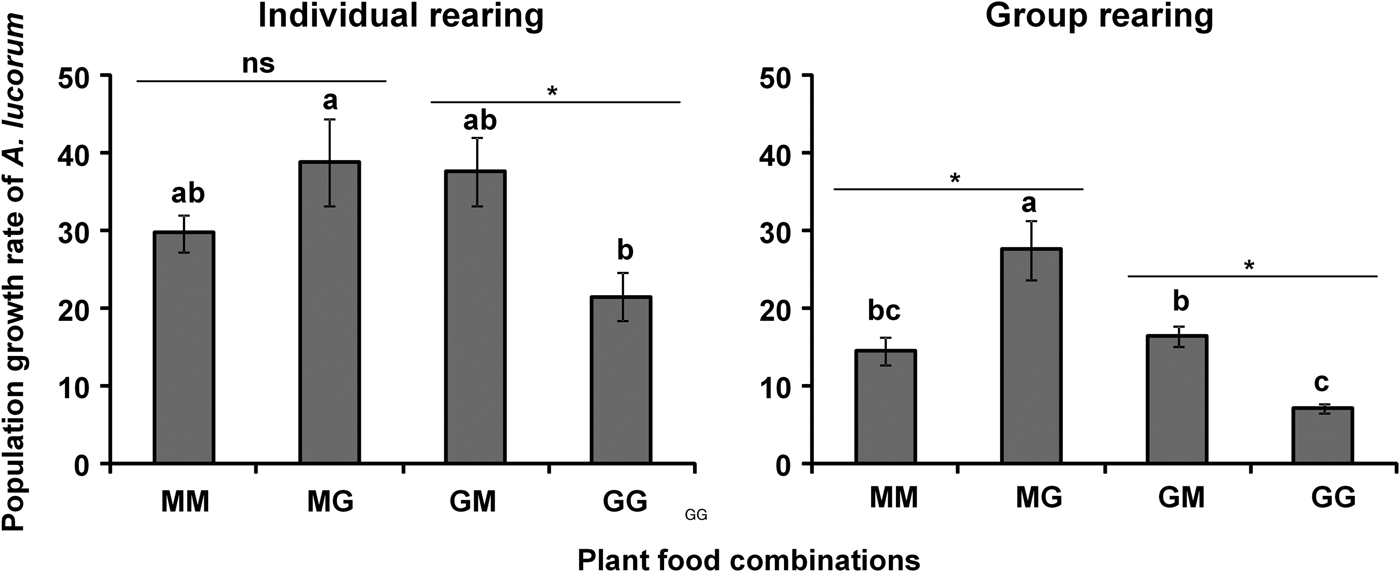
Fig. 1. Population growth rate of Apolygus lucorum both individually and group reared on maize and green bean. MM means that both nymphs and adults fed on maize; MG means that nymphs fed on maize and adults switched to feed on green bean; GM indicates that nymphs fed on green bean and adults switched to feed on maize; GG means that both nymphs and adults fed on green bean. Means (± SE) followed by different letters denotes significant differences among four plant food combinations (Tukey's HSD, P < 0.05). Asterisk (*) indicates that there is a significant difference between maize and green bean when nymphs fed on the same plant food (Paired t tests, P < 0.05), and ns means no significant difference between them (Paired t tests, P > 0.05).
Table 2. Longevity and female fecundity of Apolygus lucorum adults reared either individually or in groups on maize and green bean.

Note: There were 80 pairs and 120 pairs of adults reared either individually or in groups on maize and green bean, respectively. The data in the brackets ‘()’ indicate the number of tested eggs for each plant food combination. Data are presented as means ± SE. Means in the same column followed by different letters are significantly different among four plant food combinations reared either individually or in groups (Tukey's HSD, P < 0.05).
Group rearing
Under conditions of group rearing, the survival rate of A. lucorum nymphs was also significantly higher when they fed on maize than on green bean (χ2 = 3.47, df = 1, P = 0.0134) (table 1), and the weight of adults fed on maize was likewise higher than on green bean (t = 8.23, df = 6, P = 0.0002), whereas no significant difference was found in developmental duration and female ratio when insects reared on maize vs. green beans (all P > 0.05) (table 1).
Male longevity of A. lucorum adults on plant food combination MM was significantly shorter than on MG, GM or GG (F = 37.05, df = 3, 12, P < 0.0001), and there were no significant differences among the last three mentioned plant food combinations (P > 0.05) (table 2). Female longevity on plant food combination MG was significantly greater compared with other plant food combinations (F = 24.27, df = 3, 12, P < 0.0001) (table 2), while female fecundity on plant food combinations MG and GM was higher than on MM or GG (F = 47.13, df = 3, 12, P < 0.0001), and there was no significant difference between MG and GM (P > 0.05) (table 2). Moreover, the hatching rates of A. lucorum eggs on three plant food combinations (MM, MG, and GM) were significantly higher than on GG (F = 73.13, df = 3, 12, P < 0.0001), and there were no significant differences among MM, MG, and GM (table 2). Meanwhile, the population growth rate of A. lucorum on plant food combination MG was higher than on the other three plant food combinations, and those of GM were significantly higher than GG (F = 14.77, df = 3, 12, P = 0.0002) (fig. 1). In addition, there were significant differences between MM and MG (t = 3.14, df = 6, P = 0.0201), and between GM and GG (t = 6.26, df = 6, P = 0.0008) (fig. 1).
Discussion
Lygus bugs have usually been reared on green bean pods in the laboratory (Beards & Leigh, Reference Beards and Leigh1960; Wilson, Reference Wilson1973), and in China, mirid bugs (A. lucorum and Adelphocoris spp.) have been successfully reared on this plant food for the last 10 years (Lu et al., Reference Lu, Wu, Cai and Liu2008b, Reference Lu, Wu, Wyckhuys and Guo2009). In this study, we found that fresh kernels of sweet maize were better for the rearing of A. lucorum nymphs and that the survival rate of A. lucorum nymphs was significantly higher when they fed on maize than on green beans, both individually and in groups. Maize cobs possess the advantage of having low pesticide residues, being enclosed in the bract. In our present study, both maize kernels and green bean pods were changed by the same time interval (i.e., daily) to avoid the potential effect of rearing practice. But in fact of mass laboratory rearing, green bean pods must be changed every 2 days (Lu et al., Reference Lu, Wu, Cai and Liu2008b), and maize cobs need only be changed every 7 days (Personal observation). This greatly reduces the workload, as well as potential injury and disturbance to mirid bugs. Female A. lucorum adults have been found to lay more eggs on flowering plants, and their offspring performed significantly better on them (Dong et al., Reference Dong, Pan, Lu and Yang2013), likely because the sugar content (e.g., sucrose) was significantly higher in flowers than in new foliage of host plants. We found sweet maize can enhance survival and development of A. lucorum nymphs over green bean, possibly because of the high content of sugars contained in sweet maize kernels.
In our study, female longevity of A. lucorum adults and egg hatch rate significantly increased if adults switched to feed on green bean after being reared on maize as nymphs, and such food switching was also associated with that prolonged female and male longevity, improved female fecundity and population growth rate compared with individuals where both nymphs and adults fed on maize. Likewise, bugs, where green bean was the nymphal food and maize, was the adult food showed significantly increased female fecundity, egg hatching rate and population growth rate compared with those of where both nymphs and adults fed on a green bean. These findings suggested that switching food plants from nymphs to adults can improve the survival and population growth of A. lucorum, a pattern common for other mirid bugs (Womack & Schuster, Reference Womack and Schuster1987; Panizzi, Reference Panizzi1997; Esquivel & Mowery, Reference Esquivel and Mowery2007; Esquivel & Esquivel, Reference Esquivel and Esquivel2009). For example, rearing nymphs on green bean pods and then switching the newly-emerged adults to pods of Crotalaria lanceolata, Desmodium tortuosum or Sesbania vesicaria, or mature seeds of Glycine max or Arachis hypogaea, improved the longevity and reproductive performance of Nezara viridula (L.) adults (Panizzi & Slansky, Reference Panizzi and Slansky1991), while the reproductive performance and percentage gain in adult weight of N. viridula were better for those whose food was switched from radish to soybean from nymph to adulthood, as compared with those solely fed on radish (Panizzi & Saraiva, Reference Panizzi and Saraiva1993). Under field conditions, host-plant switching of mirid bug adults is common as bugs search for the most suitable plant foods for themselves and their offspring (Wheeler, Reference Wheeler2001; Geng et al., Reference Geng, Pan, Lu and Yang2012; Dong et al., Reference Dong, Pan, Lu and Yang2013).
In this study, the population growth rate of A. lucorum reared both individually and as a group, on the plant food combination, MG (maize as nymphal food and green bean as adult food) was significantly increased compared with those on GG (green bean as foods of both nymph and adult). Such food changing from nymphs to adults has been found to have varied effects on a number of phytophagous insects (Simpson & Simpson, Reference Simpson, Simpson and Bernays1990; Panizzi, Reference Panizzi1997; Lee et al., Reference Lee, Behmer and Simpson2006; Bonoan et al., Reference Bonoan, Al-Wathiqui and Lewis2015). For instance, when soybean was provided as adult food, the male longevity of N. viridula was greater when soybean was the nymphal food than those radish as nymphal food (Panizzi & Saraiva, Reference Panizzi and Saraiva1993). Previous studies also demonstrated that adequate nymphal food can partially alleviate the deleterious effects of poor adult food, while adequate adult food will likewise mitigate the impact of unsuitable nymphal food (Panizzi & Slansky, Reference Panizzi and Slansky1991; Panizzi & Saraiva, Reference Panizzi and Saraiva1993; Velasco & Walter, Reference Velasco and Walter1993; Pinto & Panizzi, Reference Pinto and Panizzi1994). Our study suggests that feeding sweet maize to A. lucorum nymphs and then feeding adults with green bean had the best outcome.
Using the rearing method described by Lu et al. (Reference Lu, Wu, Cai and Liu2008b), mirid bugs (inc. A. lucorum) were successfully reared on green bean pods in the laboratory, with an egg hatch rate of 80%. During the course of these experiments, we found that maize kernels will dry out in 3–5 days, which severely reduced the hatch rate of A. lucorum eggs. Since fresh plant tissues easily lose water, the type of oviposition substrate (such as cotton, alfalfa, soybean, cowpea, and kidney bean) significantly affects egg hatching rate of mirid bugs (Fu et al., Reference Fu, Feng, Qiu, Guo and Chen2008; Guo et al., Reference Guo, Fu, Feng, Qiu and Guo2008). To eliminate the impact of plant materials on egg hatching rate, we chose instead to use four layers of wrinkled moist filter paper as an oviposition substrate to evaluate the influence of plant foods of nymphs and adults on the hatching rate of A. lucorum eggs. Chen et al. (Reference Chen, Feng, Li, Guo, Fu and Qiu2012) also used wet filter papers to attract mirid bugs (A. lucorum and Adelphocoris suturalis) for oviposition and had an egg hatching rate above 80%.
Life tables are one of the most useful tools in the study of insect population dynamics and play an important role in biological and ecological research (Gao & Yang, Reference Gao and Yang2015). When establishing these tables, it is important to distinguish the results between the individual and group rearing (Guo et al., Reference Guo, Fu, Feng, Qiu and Guo2008; Feng et al., Reference Feng, Jin, Li and Feng2012). In our study, the population growth rate of group-reared A. lucorum on the plant food combinations MG and GM were significantly higher compared with those on MM and GG, respectively, but there were no significant differences between MG and MM, or GM and GG when individually reared. In general, group rearing is closer to laboratory rearing, and as such provides important information about conditions suitable for mass rearing of A. lucorum in the laboratory.
In conclusion, we demonstrated that host food switching promotes population fitness of A. lucorum, and our study shows the value of a new food combination (i.e., maize as nymphal food and green bean as adult food) for mass rearing of A. lucorum in the laboratory.
Acknowledgements
This study was financially supported by the National Key Research and Development Program of China (2017YFD0201900), the Special Fund for China Agriculture Research System (CARS-15-19), the National Natural Science Funds of China (No. 31501645, 31621064).






