Introduction
Early studies on host-parasitoid interactions indicated that these systems are inherently unstable, mainly due to a decelerating functional response in foraging parasitoids (Nicholson & Bailey, Reference Nicholson and Bailey1935; Hassell, Reference Hassell1978). This suggestion spurred a flurry of studies suggesting that mechanisms, such as area-restricted search, metapopulation dynamics, parasitoid interference, host feeding and other types of density feedbacks, may reduce temporal fluctuations and enhance stability (Hassell, Reference Hassell2000a,Reference Hassellb). While these studies mainly focused on monophagous parasitoids, the same general reasoning can be extended to polyphagous species (Holt & Lawton, Reference Holt and Lawton1993; Bonsall & Hassell, Reference Bonsall and Hassell1999, Reference Bonsall and Hassell2000; Hassell, Reference Hassell2000b). The theory for polyphagous species suggests that, in the absence of other stabilising factors, parasitoid-mediated interactions will generally lead to exclusion of host species that have a relatively low growth rate and experience a relatively high attack rate (Holt & Lawton, Reference Holt and Lawton1993; Hambäck & Björkman, Reference Hambäck and Björkman2002).
When trying to understand the stability of multihost-parasitoid interactions, the main focus has been on the host selection behaviour by parasitoids (Teder & Tammaru, Reference Teder and Tammaru2003) and how trade-offs between selective parasitism and host growth rates may affect coexistence (Luhring et al., Reference Luhring, Millar, Paine, Reed and Christiansen2004; Hambäck et al., Reference Hambäck, Stenberg and Ericson2006). Less effort has been devoted to differences among hosts as resources for parasitoids, despite the fact that studies on koinobiont parasitoid systems suggest that resource competition within hosts and host immunity processes may affect female body size (Godfray, Reference Godfray1994; Vet et al., Reference Vet, Datema, Janssen and Snellen1994; Bernal et al., Reference Bernal, Luck and Morse1998; Zaviezo & Mills, Reference Zaviezo and Mills2000; Mackauer & Chau, Reference Mackauer and Chau2001), offspring sex ratio (Godfray, Reference Godfray1994; Vet et al., Reference Vet, Datema, Janssen and Snellen1994; Bernal et al., Reference Bernal, Luck and Morse1998; Pandey & Singh, Reference Pandey and Singh1999; Bertschy et al., Reference Bertschy, Turlings, Bellotti and Dorn2000; Harvey, Reference Harvey2000) and larval survival (Hoogendoorn & Heimpel, Reference Hoogendoorn and Heimpel2002; Heimpel et al., Reference Heimpel, Neuhauser and Hoogendoorn2003), with implications for population growth. If sex allocation and offspring fitness of oligophagous parasitoids differ between host species, this may have profound effects on parasitization rates, and ultimately provide mechanisms for asymmetry or coexistence in systems with multiple host species (Heimpel et al., Reference Heimpel, Neuhauser and Hoogendoorn2003).
In this paper, we investigate the relationships between host size, brood size, progeny size and sex ratio in a koinobiont endoparasitoid, Asecodes mento (Hymenoptera: Eulophidae), which parasitizes larvae of two closely related and co-occurring species, Galerucella tenella (hereafter Gt) and G. calmariensis (hereafter Gc) (Coleoptera: Chrysomelidae). Earlier studies suggest that parasitoid selectivity for Gt favours Gc; but, despite this strong asymmetric interaction, mixed populations frequently occur (Hambäck et al., Reference Hambäck, Stenberg and Ericson2006: see fig. 1 for an illustration of the food web). We were, therefore, interested in the possibility that density-feedbacks on parasitoid fitness may differ between the two hosts, providing an alternative stabilising factor in the system. The two chrysomelid hosts have similar life histories but utilize different, though coexisting, host plants, and differ in size. Thus, they provide an optimal system for studying how host size and species characteristics contribute to parasitoid fitness. In this system, we investigated the distribution of brood sizes among the two hosts by collecting parasitized hosts from the field. This approach has the advantage, relative to laboratory conditions, that parasitoids probably experience lower rates of host encounter and life expectancy under field conditions than under optimal conditions in laboratory studies. Here, we hypothesise that Gc mummies return less fit and female-biased parasitoid broods than Gt mummies. If such differences exist between the two hosts, this could counterbalance the effect of parasitoid selectivity for Gt and explain why coexisting host populations exist in nature (Hambäck et al., Reference Hambäck, Stenberg and Ericson2006; Stenberg et al., Reference Stenberg, Heijari, Holopainen and Ericson2007).

Fig. 1. Schematic illustration of the food web, which consists of three trophic levels. The parasitoid Asecodes mento is at the highest trophic level, parasitizing two leaf beetles, Galerucella tenella (left) and G. calmariensis (right), which in turn feed on one host-plant each, Filipendula ulmaria and Lythrum salicaria, respectively. The dashed lines illustrate that parasitoids hatching from one of the leaf beetles can migrate to the other, thus producing an indirect interaction between the two prey species.
Study species
Asecodes mento (Hymenoptera: Eulophidae) is known to attack young larval stages of all species in the Galerucella genus, as well as species in related chrysomelid genera (Askew & Viggiani, Reference Askew and Viggiani1978; Dolgin, Reference Dolgin1979; Hippa & Koponen, Reference Hippa and Koponen1984; Hansson, Reference Hansson1996). In our study area, the Skeppsvik Archipelago (63°44–49′N, 20°34–40′E), its only hosts are Galerucella tenella L (Gt) and G. calmariensis L (Gc). The parasitoid overwinters as pupae inside mummified hosts, and the adults emerge in July when new hosts are available. Emerging females may parasitize new host larvae immediately, with no foraging needed. Superparasitism may occur, at least under laboratory conditions.
In the Skeppsvik Archipelago, Gt and Gc are the main enemies of their respective host plant (Filipendula ulmaria, Rosaceae, and Lythrum salicaria, Lythraceae) and may at high densities completely devastate their host plants (Katovich et al., Reference Katovich, Becker and Ragsdale1999; Hambäck et al., Reference Hambäck, Ågren and Ericson2000; Stenberg et al., Reference Stenberg, Witzell and Ericson2006, Reference Stenberg, Heijari, Holopainen and Ericson2007). Gt and Gc have very similar life cycles, are univoltine and over-winter as adults in the soil but show slight differences in phenology (Hambäck et al., Reference Hambäck, Stenberg and Ericson2006). Oviposition usually starts in mid-June, and the first larvae can be seen by early July.
Materials and methods
Naturally occurring 2nd and 3rd instar Gt and Gc larvae were sampled in July 2005 on islands in the Skeppsvik Archipelago (63°44–49′N, 20°34–40′E) from floriferous Filipendula and Lythrum, respectively. The larvae were fed with fresh leaves from their respective host plant until they were either mummified or pupated. Mummies were placed individually in 1.5-ml plastic tubes, and stored outdoors at a shaded location at the Umeå University campus until the adult Asecodes emerged. In total, 856 Gt and 782 Gc mummies were obtained of which 25% and 18%, respectively, hatched during the same year and 75% and 82% hatched during the following summer. Seven days after the first individual had emerged from each mummy, its plastic tube with the mummy and the emerged parasitoids were placed in a freezer at −40°C, where they were stored until counting, measurements and sex determinations were undertaken. The number of parasitoids which emerged from each mummy was counted, and the resulting distributions were compiled separately for each host species and year of emergence.
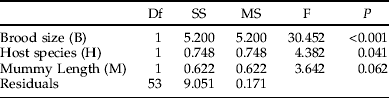
To determine whether (secondary) sex ratio and size were dependent upon host species, host length and brood size, we randomly sampled 30 mummies each of Gt and Gc. We then counted, sex determined and estimated sizes of all emerging parasitoid individuals. As a size estimate we used hind tibia length, as it has been used frequently in previous studies on parasitoids, and because preliminary studies suggested that this measure was less prone to measurement errors. We then performed a generalised linear model with Poisson errors and log link to evaluate whether brood size was significantly influenced by host species and mummy length, and an ancova to evaluate whether the size of emerged adults was significantly influenced by brood size, host species and mummy length (males and females were tested separately). We further performed a generalized ancova, with binomial error, to determine whether the sex ratio (proportion females) was significantly influenced by brood size, host species and mummy length. Interaction terms which were highly unsignificant were removed from the models.
Results
Between 1 and 14 adult parasitoids emerged from each mummy. The natural distributions of brood sizes were almost identical for Gt and Gc (fig. 2). However, a large difference can be seen between frequency distributions of individuals that overwintered and those that emerged the same year. The peak of the frequency curve for overwintered individuals was strongly pushed towards smaller brood sizes compared with those that emerged during the first year.

Fig. 2. Natural distributions of brood sizes in a cohort of Asecodes mento in the Skeppsvik Archipelago. Grey bars denote broods emerging from Galerucella tenella mummies, and black bars denote brood emerging from G. calmariensis mummies. (a) Distribution for individuals that emerged the same year. (b) Distribution for individuals that overwintered and emerged the following summer.
Effects of host size
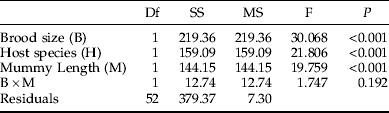
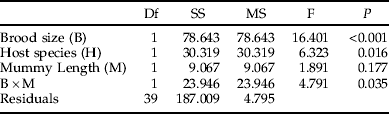
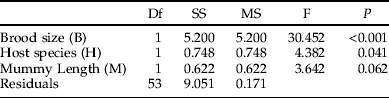
As this investigation concerns secondary parasitism, we have no data on host sizes at the time of encounter. Our measure of mummy length is, however, likely to be correlated with the larval size faced by the ovipositing female at the time of parasitization. Gc is a larger species than Gt (Hambäck et al., Reference Hambäck, Stenberg and Ericson2006) and this was reflected in mummy sizes (fig. 3), although they overlapped to a large extent. Both brood size (fig. 3, table 1) and the size of emerging female parasitoids (fig. 3, table 2) were positively and significantly correlated with mummy length for both host species. However, although the emerging males showed the same general pattern as females, this effect was not statistically significant (table 3), probably due to the lower sample size for males (as shown below, while females were always present, very often no males were present at small brood sizes, making their sample size smaller). The effect of mummy length on sex ratio was not significant (table 4).

Fig. 3. (a) Brood size of Asecodes mento as a log-linear function of mummy size of Galerucella tenella (Gt, upper curve) and G. calmariensis (Gc, lower curve). (b) Female size of emerging A. mento as a linear function mummy size of Gt (lower line) and Gc (upper line) of mummy size. Open symbols denote Gt and filled symbols denote Gc.
Table 1. Effects of host species (Galerucella tenella and G. calmariensis) and mummy length (mm) on brood size in Asecodes mento, using a generalised linear model with Poisson errors and log link.
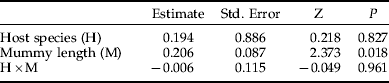
Table 2. ANCOVA table showing the effects of brood size, host species (Galerucella tenella and G. calmariensis) and mummy length (mm) on female hind tibia length (mm) in Asecodes mento.
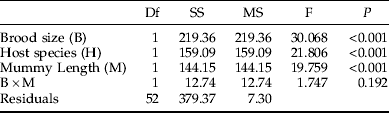
Table 3. ANCOVA table showing the effects of brood size, host species (Galerucella tenella and G. calmariensis) and mummy length (mm) on male hind tibia length (mm) in Asecodes mento.
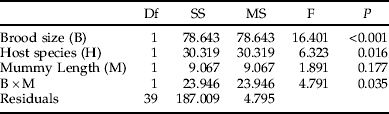
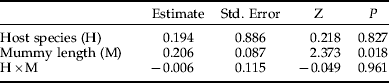
Table 4. Logistic ANCOVA table showing the effects of brood size, host species (Galerucella tenella and G. calmariensis) and mummy length (mm) on the sex ratio (proportion females) in Asecodes mento.
Effects of brood size
The proportion of females was, for both host species, reduced in parallel with an increasing brood size (fig. 4, table 4). At small brood sizes (⩽2), the proportion of females was close to 1, while very large brood sizes (>11) produced more males than females (fig. 4). Likewise, the size of emerging parasitoids was, for both sexes and both host species, negatively related to brood size (fig. 4, tables 2 and 3).

Fig. 4. (a) Sex distribution (logistic) and (b) size (linear) of emerging Asecodes mento as a function of brood size. Data for parasitoids emerging from Galerucella tenella (Gt) mummies and G. calmariensis (Gc) mummies are separated in both charts, and sexes are additionally shown in the lower chart (♀=females, ♂=males). Note that some data points overlap (large filled symbols arise when Gt symbols overlap entirely with Gc symbols).
Effects of host species
In accordance with data on the natural distribution of brood sizes (fig. 2), host species had no effect on brood size, but significant effects were shown on both the size of emerging parasitoids (figs 3 and 4, tables 2 and 3) and on the sex ratio (fig. 4, table 4). When the effects of mummy size and brood size had been accounted for, both females and males were generally smaller when emerging from Gt than from Gc (figs 3 and 4). In contrast, the proportion of females emerging was higher for Gt than for Gc (fig. 4).
Discussion
Previous studies suggest that host growth and parasitoid selectivity create scope for an asymmetry in the parasitoid-mediated apparent competition between Gt and Gc, making the inferior host (Gt) suffer substantially higher parasitism in mixed vs. monospecific populations (Hambäck et al., Reference Hambäck, Stenberg and Ericson2006; Stenberg et al., Reference Stenberg, Heijari, Holopainen and Ericson2007). The results from this study show that Gc, as a consequence of its larger size, also delivers larger-sized parasitoids (understood as more fit: Godfray, Reference Godfray1994; Bernal et al., Reference Bernal, Luck and Morse1998; Zaviezo & Mills, Reference Zaviezo and Mills2000) to the shared parasitoid pool (fig. 1). This difference in host quality may, along with the parasitoid preference for Gt and the superior growth of Gc, further expose Gt to a higher risk of exclusion through apparent competition (Holt & Lawton, Reference Holt and Lawton1993). Our study, however, also suggests one possible stabilising mechanism to this strong assymetric apparent competition, differential parasitoid sex allocation. The results imply that the sex ratio of the parasitoid population should be more female biased in monospecific Gt populations and that the presence of Gc in a mixed population should act to reduce the overall proportion of females in the shared parasitoid pool. This shifting sex ratio may cause the inferior host (Gt) to experience a lower proportion of parasitizing females in mixed populations, and indirectly promote species coexistence.
The mechanism underlying the differential sex ratio is not easily understood. Theoretically, parasitoid mothers are expected to vary the proportion of female offspring (fertilised eggs) according to the available resources (Godfray, Reference Godfray1994; Vet et al., Reference Vet, Datema, Janssen and Snellen1994; Bernal et al., Reference Bernal, Luck and Morse1998; Pandey & Singh, Reference Pandey and Singh1999; Bertschy et al., Reference Bertschy, Turlings, Bellotti and Dorn2000; Harvey, Reference Harvey2000), as female parasitoids often benefit more from a larger body size than males (Godfray, Reference Godfray1994; Bernal et al., Reference Bernal, Luck and Morse1998). Hence, one would expect the parasitoid to allocate more daughters to Gc, which is the larger, and qualitatively superior host, but A. mento does just the opposite. Why this seemingly maladaptive behaviour occurs is unclear but possible mechanisms include both constraints during the valuation of host quality and yet unknown benefits from the observed behaviour. The continued growth of larvae after parasitization for koinobiont parasitoids, such as A. mento, may cause females to underestimate the final size difference between Gc and Gt. This hypothesis is supported by the larger size of parasitoid offspring, emerging from Gc pupae. An alternative hypothesis derives from studies of other parasitoids, where females seem able to control the future growth and development of their hosts by injecting polydnaviruses during oviposition (Harvey et al., Reference Harvey, Jervis, Gols, Jiang and Vet1999). If this is the case also in A. mento, it calls for an adaptive explanation for why more daughters are allocated to the smaller host. We conclude that more controlled laboratory experiments need to be done with A. mento before any mechanistic and adaptive explanations can be drawn.
Similarly to previous studies (Godfray, Reference Godfray1994; Vet et al., Reference Vet, Datema, Janssen and Snellen1994; Bernal et al., Reference Bernal, Luck and Morse1998; Zaviezo & Mills, Reference Zaviezo and Mills2000; Mackauer & Chau, Reference Mackauer and Chau2001), this study also shows that host size has a strong and positive effect on both brood size and progeny size, whereas brood size reduces progeny size. The likely mechanisms for these patterns are (i) that female parasitoids adjust clutch sizes to the amount of available resources, and (ii) that individual progeny grow larger the more resources they have. In A. mento, the density-dependent effect on progeny size is also coupled to a change in sex ratio with a larger proportion of females at small brood sizes. Two mechanisms may account for this observation, depending on the presence of superparasitisms (Godfray, Reference Godfray1994). Because female parasitoids benefit more from a larger size than do male parasitoids, optimality theory suggests an allocation of females to high resource hosts. From this follows that egg-laying females would loose less from aggregating male off-spring compared to aggregating female off-spring. A similar reasoning applies to situations with superparasitism, where sex allocation theory suggests that the second mother should produce a clutch with a higher proportion of males than the first female for the reason that mate competition from the few males from the first clutch will be relatively low (Godfray, Reference Godfray1994). Previous studies have revealed both positive and negative relationships between brood size and sex ratio (e.g. Vet et al., Reference Vet, Datema, Janssen and Snellen1994; Bertschy et al., Reference Bertschy, Turlings, Bellotti and Dorn2000), and this may largely reflect different ovipositing strategies in cases when superparasitism is rare vs. common.
The distribution of brood sizes in the field was virtually the same in both host species, contradicting findings of a previous more restricted study (Hambäck et al., Reference Hambäck, Stenberg and Ericson2006), which suggested that Gc returns larger brood sizes than Gt. However, a marked difference in brood size distribution was observed for parasitoids that emerged the same year vs. those that overwintered. The most likely cause for this difference is differential winter mortality between large and small broods, but only future studies can identify the underlying mechanism.
Concluding remarks
The findings of this study imply that polyphagous parasitoids may experience differential fitness and sex ratios in different host species. As put forward by Ode (Reference Ode2006), these herbivore differences may be bottom-up controlled by host-plant chemistry. The differences imply that polyphagous parasitoids experience differential selection in different areas depending on the relative abundance and composition of local plant and herbivore communities. Ultimately, such divergent selection should create scope for host race formation and speciation in the parasitoid. Therefore, we suggest that future studies should strive to disentangle the geographic mosaic of co-evolution (Thompson, Reference Thompson2005) in polyphagous parasitoids and its implications for food web stability on the larger scale.
Acknowledgements
This study was funded by the Swedish Research Council Formas.