Introduction
The diversity of life keeps fascinating biologists and requires explanation. Diversity is not distributed equally within the tree of life. Some clades comprise many more species than others. Insects show an extremely high number of species compared to other classes of organisms (Stork, Reference Stork1988; Labandeira & Sepkoski, Reference Labandeira and Sepkoski1993; Mayhew, Reference Mayhew2007), and phytophagous insects are particularly diverse (Mitter et al., Reference Mitter, Farrell and Wiegmann1988). This is often explained in terms of their intimate association and strong dependency on their host plants, promoting specific adaptations which in turn may result in genetically based trade-offs in performance on different hosts and ultimately in ecological speciation (Smith, Reference Smith1966; Jaenike 1990; Berlocher & Feder, Reference Berlocher and Feder2002; Nosil Reference Nosil2007). Other groups with unusual species richness are the parasitic wasps from the superfamilies Ichneumonoidea and Chalcidoidea. They comprise at least 45,000 described species (Gaston, Reference Gaston1991), but other estimates go up to one million (Godfray, Reference Godfray1994; Quicke, Reference Quicke1997). This tremendous diversity, just as in phytophagous insects, could be because of ecological speciation (Funk, Reference Funk1998; Schluter, Reference Schluter2001; Stireman et al., Reference Stireman, Nason, Heard and Seehawer2006). Like their herbivorous host insects, parasitoids are often characterized by narrow specialization and/or host-associated genetic differentiation within species using multiple hosts (Pashley, Reference Pashley1986; Stireman et al., Reference Stireman, Nason and Heard2005). Such host races may arise sympatrically and eventually evolve into different species as disruptive selection continues and barriers to gene flow emerge (Bush, Reference Bush1969; Craig et al., Reference Craig, Horner and Itami1997; Drès & Mallet, Reference Drès and Mallet2002). This raises the intriguing possibility that diversification of plant feeding insects can also lead to diversification of their parasitoids, a phenomenon that has been described as cascading host-associated genetic differentiation or sequential sympatric speciation (Stireman et al., Reference Stireman, Nason, Heard and Seehawer2006; Forbes et al., Reference Forbes, Powell, Stelinski, Smith and Feder2009; Feder & Forbes, Reference Feder and Forbes2010).
The genus Lysiphlebus (Hymenoptera: Braconidae) consists of several species of small aphid parasitoids. They use a variety of aphid hosts (Starý, Reference Starý2006). Most common in Europe are species of the Lysiphlebus fabarum group, which is characterized by the frequent occurrence of all-female populations reproducing by thelytokous parthenogenesis (Belshaw et al., Reference Belshaw, Quicke, Völkl and Godfray1999; Starý, Reference Starý1999; Sandrock & Vorburger, Reference Sandrock and Vorburger2011). The group comprises L. fabarum (Marshall), Lysiphlebus cardui (Marshall) and Lysiphlebus confusus (Tremblay & Eady), which are morphologically very similar. L. confusus is distinguished from the other two species by a fringe of long setae on the margin of the forewing, and L. cardui is distinguished from L. fabarum by relatively long and erect setae on the hind femora (Starý, Reference Starý1966). However, a study by Belshaw et al. (Reference Belshaw, Quicke, Völkl and Godfray1999) and more recent work employing mitochondrial DNA sequences as well as nuclear microsatellite markers casts doubt on the validity of this distinction, because morphology does not reliably predict genetic relationships in the L. fabarum group. All three morphotypes are polyphyletic (Sandrock et al., Reference Sandrock, Schirrmeister and Vorburger2011a; Schär, Rouchet & Vorburger, unpublished data). Nevertheless, for simplicity and for the lack of alternative descriptions, the species names will be maintained in this article.
Parasitoids of the L. fabarum group occur on a wide range of aphid-plant communities and exhibit a substantial degree of host-associated differentiation (HAD) at presumably neutral molecular markers (Belshaw et al., Reference Belshaw, Quicke, Völkl and Godfray1999; Sandrock et al., Reference Sandrock, Frauenfelder, von Burg and Vorburger2007, Reference Sandrock, Schirrmeister and Vorburger2011a), indicating host specialization and limited gene flow between wasps exploiting different aphids. However, specialization may be facilitated by the fact that different aphid species typically feed on different plant species, generating local geographic separation of their parasitoid populations (e.g., Kavallieratos et al., Reference Kavallieratos, Tomanović, Starý and Bogdanović2008; Tomanović et al., Reference Tomanović, Kavallieratos, Starý, Stanisavljević, Ćetković, Stamenković, Jovanović and Athanassiou2009). An exception is Lysiphlebus on the thistle Cirsium vulgare (Savi) (Asteraceae) on which they attack two aphid species, Brachycaudus cardui (Linné) (Hemiptera: Aphididae) and Aphis fabae cirsiiacanthoides (Scolpoli) (Hemiptera: Aphididae). These aphids can be considered as syntopic because they often feed in mixed colonies on stems and leaves of the same individual plants and during the same time of the year (e.g., Klinkhamer & De Jong, Reference Klinkhamer and de Jong1993; Blackman & Eastop, Reference Blackman and Eastop2000; see the Results section). Observations by Starý (Reference Starý2006) suggest that B. cardui is parasitized by arrhenotokous (sexual) Lysiphlebus and A. f. cirsiiacanthoides is usually parasitized by thelytokous wasps and that the wasps attacking these two hosts also show some morphological differentiation. In consequence, Starý (Reference Starý2006) proposed raising L. fabarum-like parasitoids attacking B. cardui to the level of a separate, host-specific taxon (=Lysiphlebus brachycaudi Starý), but this species has not yet been formally described. Cuticular hydrocarbon profiles (Liepert, Reference Liepert1996) and nuclear genomic DNA confirm their separate status within the L. fabarum group, but mitochondrial DNA sequence divergence does not clearly support the distinction of ‘L. brachycaudi’ as a separate species (Belshaw et al., Reference Belshaw, Quicke, Völkl and Godfray1999, Sandrock et al., Reference Sandrock, Schirrmeister and Vorburger2011a). Here, we address this issue with a systematic field study on the host use and the genetic population structure of Lysiphlebus parasitoids attacking aphids on the host plant C. vulgare. We also aim to shed some light on the poorly known patterns of gene flow and dispersal in this group on the scale of landscape-metapopulations. In addition, we investigated host use at the next trophic level. Parasitoids feeding on phytophagous insect, here aphids, are so-called primary parasitoids. Primary parasitoids may themselves be consumed by hyperparasitoids. The parasitoid community of aphids feeding on C. vulgare gets exploited by a number of such hyperparasitoids. Host associations of hyperparasitoids under syntopic conditions are poorly known. To document and compare those was therefore another objective of our research.
Methods
Sampling
Samples were collected from 22 sites in northern Switzerland (fig. 1a), either in May/June 2007 or between June and August 2009. Sites consisted of patches of C. vulgare harbouring colonies of one or both focal aphid species, and they were separated by 0.5 and 101 km (fig. 1a). Plant parts containing aphid mummies were cut and sealed in cellophane bags. A total of 977 aphid mummies were collected: 460 A. f. cirsiiacanthoides mummies and 517 B. cardui mummies.

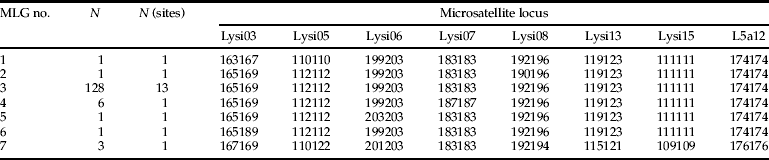
Fig. 1. Map (a) UPGMA tree (b) and individual cluster assignments (c) of Lysiphlebus using two different host aphids on C. vulgare in northern Switzerland. Lysiphlebus populations hatching from B. cardui mummies are shown as white circles, those hatching from A. f. cirsiiacanthoides mummies as black circles and mixed populations are indicated by white circles with bold black frames. The small black points mark sites where only hyperparasitoids were found. The tree in (b) is based on Cavalli–Sforza Chord distance (Dc), UPGMA algorithm and 5000 bootstraps on loci (values are rounded percentages) and includes haploid male and diploid female genotypes (scale bar: Cavalli-Sforza Chord=0.1). (c) Shows the STRUCTURE output with the most probable number of K=3 for the diploid Lysiphlebus females. Each vertical line shows one individual and black lines separate populations. The shaded segments of the lines represent the estimated probability of an individual being member of one of the three inferred clusters. The analyses in (b) and (c) are based on seven presumably neutral microsatellite markers.
Parasitoid eclosion and classification
After collection the still uneclosed mummies were carefully removed from the plants and placed individually in gelatin capsules. This treatment allowed unambiguous identification of the host aphid of each individual wasp. After the parasitoids had eclosed from mummies they were killed with vaporized ethyl acetate and then classified by use of the available taxonomic literature (Genera Lysiphlebus and Binodoxys: Starý (Reference Starý1966), genera Asaphes and Pachyneuron: de Vere Graham (Reference de Vere Graham1969), genus Syrphophagus: Erdös (Reference Erdös1964), genus Dendrocerus: Fergusson (Reference Fergusson1980), genus Alloxysta: Andrews (Reference Andrews1978)). The final set of ecological data contained information on the date of collection, the site of collection, the individual host plant, the host aphid species as well as the species and sex of each eclosed parasitoid wasp. Aphid parasitoids of the genus Lysiphlebus all belonged to the L. fabarum group and were further distinguished based on the presence or absence of long semi-erect setae on the femora (absent=L. fabarum, present=L. cardui) and then stored in 96% ethanol at −20 °C until molecular investigation. We did not find any individuals belonging to L. confusus (females with a fringe of long setae along the margin of the forewing) in our samples.
DNA extraction and microsatellite analysis
The DNA was prepared using the Chelex® method: a single wasp was placed in a 1.5-ml Eppendorf tube and squashed in 100 μl of a 5% Chelex solution (BioRad). After that, 5 μl Proteinase K were added, and the mixture was incubated overnight at 56 °C. The next day, the content of the tubes was mixed again, heated at 95 °C for 15 min and centrifuged at 7000 g for 5 min. Finally, 50 μl of the clear supernatant were separated in a fresh Eppendorf tube and used as DNA template in PCRs.
All wasps belonging to the L. fabarum group were genotyped at eight microsatellite markers developed for L. fabarum (Lysi03, Lysi05, Lysi06, Lysi07, Lysi08, Lysi13, Lysi15, and Lysi16; Sandrock et al., Reference Sandrock, Frauenfelder, von Burg and Vorburger2007) and one for L ysiphlebus testaceipes (L5a12; Fauvergue et al., Reference Fauvergue, Tentelier, Genson, Audiot, Guillemaud and Streiff2005), following a published PCR protocol (Sandrock et al., Reference Sandrock, Frauenfelder, von Burg and Vorburger2007). The fragment analysis was carried out on an ABI 3730 automated sequencer, using an internal size standard (GeneScan 500 LIZ). Electropherograms were analyzed with the program GENEMAPPER version 3.7 (Applied Biosystems).
Comparing host associations
To test for biases in host use of the different parasitoids species, we used generalized linear-mixed models including all 977 collected aphid mummies. The analysis was carried out using PROC GLIMMIX (SAS 9.2; SAS Institute, Cary, NC, USA). Hatching events of each parasitoid species were coded as a binary response variable with hatching coded as 1. The host aphid species was treated as a fixed effect and the site and the interaction between site and aphid species were included as random effects in the model to correct for variation in host use between sites, non-independence of replicates within sites and local host-abundance. We assumed a binary distribution of the response variable and chose the logit-link function. This type of analysis caused some problems when testing for host associations of aphid parasitoids because their perfect host specialization led to non-convergence of the models. In those cases, we introduced one artificial hatching-event data point at a randomly chosen site of the parasitoid species using the non-associated aphid species. This allowed us to calculate the conservative upper limit of the P-value for host associations.
Genetic data analysis
Owing to the haplodiploid sex-determination system in the investigated Lysiphlebus parasitoids, only diploid female genotypes were considered in all analyses except for the population tree based on allele frequencies, which were estimated including the haploid male genotypes (fig. 1b).
Owing to the high fraction of thelytokous L. cardui samples, mostly belonging to the same clonal lineage, deviations from linkage and Hardy–Weinberg equilibria were only calculated for the subset of sexually reproducing L. fabarum. The analysis of linkage disequilibrium between pairs of loci was done using exact probability tests (Guo & Thompson, Reference Guo and Thompson1992) with the program GENEPOP 4.1.0 (Raymond & Rousset, Reference Raymond and Rousset1995). For the diploid L. fabarum females, F IS and global as well as pairwise F ST values (Weir & Cockerham, Reference Weir and Cockerham1984) were calculated using the software FSTAT (Goudet, Reference Goudet2005). Isolation by distance was assessed by testing for a correlation between genetic distance (F ST/(1−F ST)) (Slatkin, Reference Slatkin1995) and log-transformed straight line geographic distance as per Rousset (Reference Rousset1997). The geographic distance between sample sites was measured using the software ’Geographic Distance Matrix Generator 1.2.3’ (Ersts, Reference Ersts2007) from WSG84 coordinates. Matrix correlation was analyzed using a Mantel-test with 10,000 permutations in Arlequin v 3.1 (Excoffier et al., 2005).
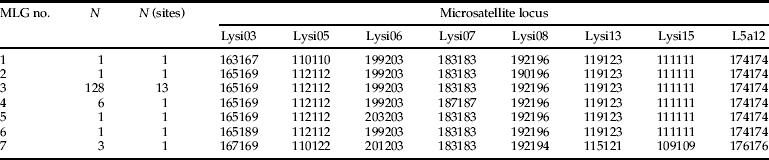
Genetic diversity, frequencies of multilocus genotypes (MLGs) of Lysiphlebus as well as the probability of multiple copies of the same MLG being produced independently by sexual recombination (P sex) were analyzed by use of the program GenClone v 2.0 (Arnaud-Haond & Belkhir, Reference Arnaud-Haond and Belkhir2007) according to the ‘round robin fashion’ mode (Parks & Werth, Reference Parks and Werth1993). A matrix of allelic distances was plotted for the MLGs of L. cardui to assess the allelic distances between them.
A microsatellite-based Unweighted Pair Group Method with Arithmetic Mean (UPGMA) tree of all Lysiphlebus populations defined as individuals from the same site and host aphid was created using the software packages Populations v 1.2.32 (Langella, Reference Langella1999) and TreeView v 1.6.6 (Page, Reference Page1996) (fig. 1b). The tree was calculated based on the Cavalli–Sforza Chord distance method (Cavalli-Sforza & Edwards, Reference Cavalli-Sforza and Edwards1967) and using the UPGMA (Sneath & Sokal, Reference Sneath and Sokal1973). Haploid male genotypes were included for the calculation of genetic distances between populations based on allele frequencies within populations. A total of 5000 bootstraps on locus were performed to estimate the support of the nodes of the tree.
In addition, we investigated genetic structuring using Bayesian clustering as implemented in STRUCTURE v 2.3.3 (Pritchard et al., Reference Pritchard, Stephens and Donelly2000; Falush et al., Reference Falush, Stephens and Pritchard2003). We chose the admixture model, which assumes that each individual potentially received a part of its genome from each of the K ancestor populations and assumed correlated allele frequencies among populations. The following parameters were chosen: burnin length of 100,000, followed by 1 million MCMC iterations. Ten independent runs for each value of K were generated to test for consistency between runs. The values for K varied between two and seven between independent runs of the program. The most accurate number of populations (K) was visually examined when plotting K against ΔK and using the Evanno method in STRUCTURE HARVESTER (Earl & vonHolt, Reference Earl and vonHolt2012). The program Distruct v 1.1 (Rosenberg, Reference Rosenberg2004) was used to visualize the results of the structure output (fig. 1c).
Results
Wasp diversity
A total of 589 parasitoid wasps hatched from the 977 aphid mummies collected. They were classified into five families, seven genera and at least nine different species (Lysiphlebus fabarum and L. cardui, Binodoxys angelicae (Haliday) (Hymenoptera: Braconidae), Alloxysta sp. (Foerster) (Hymenoptera: Figitidae), Asaphes vulgaris (Walker), A saphes suspensus (Nees) and Pachyneuron aphidis (Bouché) (Hymenoptera: Pteromalidae), Dendrocerus carpenteri (Curtis) and D. laevis (Ratzeburg) (Hymenoptera: Megaspilidae) and Syrphophagus aphidivorus (Mayr) (Hymenoptera: Encyrtidae) (table 1). The species belonging to the genera Lysiphlebus and Binodoxys are aphid parasitoids, whereas the other genera are all hyperparasitoids (Müller et al., Reference Müller, Adriaanse, Belshaw and Godfray1999). The taxonomically poorly resolved Alloxysta species were not classified to species level because comprehensive taxonomic literature is lacking for that genus in the palaearctic region (Andrews, Reference Andrews1978; Evenhuis & Kiriak, Reference Evenhuis and Kiriak1985).
Table 1. Sample sizes and host use of all collected parasitoid species. Significant associations between parasitoids and aphids are indicated with bold letters and by asterisks (generalized linear mixed-model, *P<0.05, **P<0.01, ***P<0.001).

A total of 251 Lysiphlebus wasps (97.3% of all primary parasitoids) hatched from mummies collected on 38 different plants in 17 different sampling localities. Both species were collected at eight sites, five sites yielded only L. fabarum and four sites only L. cardui (table 1, fig. 1a). In five cases, both parasitoid species occurred together on the same plant, on 14 plants only L. fabarum was found and on 19 plants only L. cardui.
Sex ratios
All 154 individuals of L. cardui were females, consistent with thelytokous reproduction in this lineage. The sex ratio in L. fabarum was 31 male and 66 female individuals, that is approximately 1/3 males. Similarly female-biased sex ratios were also observed in hyperparasitoids. The difference in sex ratio between L. cardui and L. fabarum was highly significant (Fisher's exact test, P<0.001).
Host associations
The host associations of the two aphid parasitoid taxa belonging to the genus Lysiphlebus were clearly distinct and non-overlapping. All wasps determined as L. cardui enclosed from mummies of A. f. cirsiiacanthoides, whereas all wasps determined as L. fabarum enclosed from mummies of B. cardui (host association of L. fabarum with B. cardui: F 1,14=15.43, P<0.002, L. cardui with A. f. cirsiiacanthoides: F 1,14=22.06, P<0.001, table 1). B. angelicae eclosed exclusively from A. f. cirsiiacanthoides mummies, but only seven individuals from a single site were found overall, precluding a firm statement on its host association. Hyperparasitoids showed less pronounced host associations and all species represented by more than one individual hatched from both aphid species. Nevertheless, the hyperparasitoid species P. aphidis emerged significantly more often from B. cardui/L. fabarum mummies than expected by chance (F 1,14=6.20, P=0.026, table 1). The overall relative proportion of hyperparasitoids was much higher in B. cardui mummies compared to A. f. cirsiiacanthoides mummies (Fisher's exact test, P<0.001, table 1), suggesting higher vulnerability to hyperparasitism of L. fabarum compared with L. cardui. We did not observe any phenological differences between L. fabarum and L. cardui which could bias our findings.
Microsatellite variation
Eight polymorphic microsatellite markers amplified consistently in all sampled populations. Marker Lysi16 amplified only in Lysiphlebus parasitoids hatching from A. f. cirsiiacanthoides mummies (L. cardui) but not in those hatching from B. cardui mummies (L. fabarum), providing a first indication of genetic differentiation between these two host-associated lineages. This locus was therefore excluded from all further analyses. The marker Lysi07 is not neutral because it is usually linked with the reproductive mode in the L. fabarum group (Sandrock & Vorburger, Reference Sandrock and Vorburger2011) and was excluded from all analyses of the genetic population structure. These were carried out with the seven remaining microsatellite loci.
No significant deviations from linkage equilibrium were found among the seven microsatellite markers in the sexual L. fabarum populations, consistent with two detailed reports (Sandrock et al., Reference Sandrock, Frauenfelder, von Burg and Vorburger2007, Reference Sandrock, Schirrmeister and Vorburger2011a). The mean observed heterozygosity in L. fabarum (H obs=0.204) was lower than expected heterozygosity (H exp=0.234). In accordance with this homozygote excess, the mean F IS-value of 0.211±0.096 (SE) was significantly larger than zero (P<0.001). There was also significant genetic differentiation among populations of L. fabarum (global F ST=0.296±0.064, P<0.001), but the degree of pairwise differentiation between populations was unrelated to the geographic distance separating them, as we could not detect any isolation by distance across the study area (r=−0.014, R 2<0.001, P=0.539). The high F IS values combined with strong genetic differentiation unrelated to geographic distance suggests that there may be some family structure in our data (e.g., from collecting multiple offspring of the same female per site), which would not be surprising. Yet because we could only include data coming from just 66 sexual, diploid females spread across 13 collection sites, all population genetic indices should be interpreted cautiously.
Genetic diversity was extremely low in L. cardui. The 141 samples with completely analyzed genotypes comprised only seven distinct MLGs. One of those (MLG 3) was shared by 91% of the individuals (table 2). Another 7% consisted of very closely related genotypes, differing by one and four alleles only from the most abundant MLG 3 and by one and six alleles among each other (table 1). Interestingly, the L. cardui samples also comprised three individuals with an additional, very distinct MLG which differed by 10 and 13 alleles from all other L. cardui genotypes (MLG 7 in table 2). This genotype was just discovered in one sampling site, namely ‘Buchs ZH’ (no. 5 in fig. 1), where it co-occurred with the most abundant MLG 3 (fig. 1c). The probability of being generated independently by sexual recombination (P sex) was below 0.001 for all MLGs of L. cardui represented by more than one individual. A very different pattern was observed in L. fabarum. Among the 57 females with completely analyzed genotypes, 43 distinct MLGs were found. These results are consistent with former reports of asexual reproduction in L. cardui and sexual reproduction in L. fabarum on C. vulgare (Starý, Reference Starý2006).
Table 2. Abundance and microsatellite genotypes of the seven observed L. cardui MLGs.
Genetic relationships among populations
The UPGMA tree of all Lysiphlebus samples showed complete separation between L. fabarum and L. cardui (fig. 1b). Most L. cardui samples from A. f. cirsiiacanthoides clustered closely together because they essentially consisted of the same asexual lineage. Only the population ‘Buchs ZH’ (no. 5 in fig. 1) was clearly differentiated from all other L. cardui populations because it contained individuals belonging to the second, morphologically cryptic asexual lineage (MLG 7 in table 2). STRUCTURE identified the highest probability for K=3 populations, corresponding to L. fabarum and the two cryptic L. cardui lineages (fig. 1c).
Discussion
This study showed that parasitoid wasps of the L. fabarum group exploiting aphids living on C. vulgare belong to two genetically and morphologically distinct lineages with different reproductive modes. Those showed virtually perfect host specialization in the same microhabitat. Mummies of A. f. cirsiiacanthoides exclusively yielded female wasps morphologically belonging to L. cardui, whereas mummies of B. cardui yielded wasps of both sexes with L. fabarum morphology, even when the two aphid species formed mixed colonies on the very same plants. The entire primary parasitoid community using A. f. cirsiiacanthoides as a host was strongly dominated by a single asexual MLG with L. cardui morphology, but we also discovered a second, genetically distinct but morphologically cryptic asexual L. cardui lineage (table 2, fig. 1b, c).
Considering that host-associated genetic differentiation of various strengths is observed across the entire L. fabarum group (Sandrock et al., Reference Sandrock, Schirrmeister and Vorburger2011a), some degree of host specialization was expected here, especially since Starý (Reference Starý2006) already reported phenotypic differences between wasps from B. cardui and A. f. cirsiiacanthoides. More surprising was the complete lack of overlap in host use, even when the two aphids formed mixed colonies. In general, there is evidence that host-associated populations of L. fabarum group parasitoids are connected by gene flow (Sandrock et al., Reference Sandrock, Schirrmeister and Vorburger2011a), suggesting less than perfect host specialization. This is further supported by the observation that wasps collected from different hosts can often be reared on the same host (A. fabae) in the laboratory (e.g., Sandrock et al., Reference Sandrock, Gouskov and Vorburger2010). Even sexual and asexual populations do not show complete reproductive isolation in the L. fabarum group, because asexual lineages are known to spontaneously (albeit very rarely) produce males that can cross-breed with females of sexual populations (Belshaw et al., Reference Belshaw, Quicke, Völkl and Godfray1999; Sandrock & Vorburger, Reference Sandrock and Vorburger2011). Yet in the present case with two syntopic hosts belonging to different aphid genera, specialization and reproductive isolation of their Lysiphlebus parasitoids appear to be very strong if not complete. This indicates selection for fitness-related traits associated with host use and against hybridization. The present system of Lysiphlebus on C. vulgare is unlikely to represent an example of syntopic divergence, because Lysiphlebus lineages found on other aphid-plant associations genetically fall between the lineages found on C. vulgare (Sandrock et al., Reference Sandrock, Schirrmeister and Vorburger2011a), but it does show that host specialization and reproductive isolation are upheld when recently evolved parasitoid lineages using different host species meet in the same microhabitat. Still unclear is how the strict host specialization is maintained. Does it reflect perfect host choice by ovipositing females or are the two parasitoid lineages even unable to develop in the alternative host? The complete lack of overlap is certainly suggestive of the latter, but this remains to be tested. The role of reproductive mode variation for the evolution of host specialization is unclear because both, sexual and asexual, parasitoids showed the same high degree of host specificity.
Aphid parasitoids on C. vulgare are themselves hosts of generalist as well as host-associated hyperparasitoid wasp species. Especially P. aphidis appears to preferentially exploit the mummies of B. cardui containing the sexual L. fabarum primary parasitoids (F 1,14=6.20, P=0.026, table 1). That host associations cascade upwards to the hyperparasitoid level on the same plant has been reported before (reviewed in Sullivan, Reference Sullivan1987), but here we found it surprising, given that the two Lysiphlebus host-lineages (97.3% of all collected aphid parasitoids) are closely related and presumably not even differentiated at the species level (Sandrock et al., Reference Sandrock, Schirrmeister and Vorburger2011a). Again, the question remains whether this result reflects a preference for the aphid species (mummies of B. cardui tend to be slightly larger), the aphid parasitoid inside the mummy, or a difference in survival between the two environments. The much lower relative proportion of hyperparasitoids in A. f. cirsiiacanthoides mummies compared to B. cardui mummies is interesting with respect to biological control. It would suggest that asexual parasitoids may be better suited for biological control not only because of their faster reproduction, but possibly also because of lower rates of hyperparasitism.
Finally, the strong genetic differentiation among sampling sites is indicative of very limited and local dispersal of L. fabarum, although the lack of isolation by distance at least at the geographic scale of our study does not support this interpretation. However, note that the patterns of genetic differentiation were estimated unreliably because of small sample sizes of sexual females per site, and that they may have been distorted from collecting closely related individuals within sites (see the Results section). Very limited dispersal was previously proposed for L. cardui (Weisser & Völkl, Reference Weisser and Völkl1997), as well as for Lysiphlebus hirticornis, a specialized parasitoid of the tansy aphid, Metopeurum fuscoviride (Nyabuga et al., Reference Nyabuga, Loxdale, Heckel and Weisser2011). Thus, aphid parasitoids of the genus Lysiphlebus may generally be poor dispersers. This is in contrast to the aphid hosts of Lysiphlebus, especially from the genus Aphis, which migrate over large distances and show a very limited spatial population structure. In samples of A. fabae covering large parts of Europe, only about 5% of the molecular variation was explained by differences among sites (Sandrock et al., Reference Sandrock, Razmjou and Vorburger2011b). Different mobilities could be of importance for the study of aphid–parasitoid co-evolution, because local adaptation evolves more readily in the antagonist with the higher dispersal ability (Gandon et al., Reference Gandon, Capowiez, Dubois, Michalakis and Olivieri1996). This would generate the testable prediction that aphids tend to be locally adapted to their Lysiphlebus parasitoids, rather than the other way around.
Acknowledgements
Special thanks go to Christoph Sandrock for his help in the laboratory, for answering many analytical questions and for sharing his impressive knowledge. Furthermore, we would like to thank Uli Reyer for his comments on the manuscript, Sandra Röthlisberger for help during laboratory work and David Nash for statistical advice. This study was funded by the Swiss National Science Foundation and the Institute of Environmental Studies at the University of Zurich.