Introduction
Psyllids (Hemiptera: Sternorrhyncha: Psylloidea) are phloem sap-feeding insects that have intimate associations with symbiotic bacteria. All of the psyllid species examined to date harbor the primary symbiont, Carsonella ruddii (γ-Proteobacteria), within the cytoplasm of specialized cells called bacteriocytes (Buchner, Reference Buchner1965; Fukatsu & Nikoh, Reference Fukatsu and Nikoh1998; Subandiyah et al., Reference Subandiyah, Nikoh, Tsuyumu, Somowiyarjo and Fukatsu2000; Thao et al., Reference Thao, Moran, Abbot, Brennan, Burckhardt and Baumann2000; Spaulding & von Dohlen, Reference Spaulding and von Dohlen2001). Carsonella appears to be an obligate mutualist, which provides the host psyllids with essential amino acids (tryptophan, lysine, methionine, phenylalanine, threonine, valine, leucine, isoleucine, arginine and histidine) that psyllids cannot synthesize and which are scarce in their diet of phloem sap (Buchner, Reference Buchner1965; Clark et al., Reference Clark, Baumann, Thao, Moran and Baumann2001). Since the initial infection more than 100 Myr ago, as in the case of organelles, Carsonella has been subjected to strict vertical transmission and cospeciated with the host psyllids (Spaulding & von Dohlen, Reference Spaulding and von Dohlen1998; Thao et al., Reference Thao, Moran, Abbot, Brennan, Burckhardt and Baumann2000).
During the course of coevolution with the host, Carsonella has reduced its genome size. The genome of Carsonella from the hackberry petiole gall psyllid, Pachypsylla venusta, is only 160 kb in size, representing the smallest cellular genome (Nakabachi et al., Reference Nakabachi, Yamashita, Toh, Ishikawa, Dunbar, Moran and Hattori2006; Nakabachi, Reference Nakabachi, Bourtzis and Miller2008). This size is approximately equivalent to that of chloroplast genomes (generally 120–200 kb; NCBI Entrez Genome/Eukaryota/Organelles, http://www.ncbi.nlm.nih.gov/genomes/static/euk_o.html). Many genes for the syntheses of essential amino acids are retained in the genome, which is consistent with the hypothesis that Carsonella is a nutrition provider. However, the genome lacks numerous genes that appear to be essential for bacterial life (e.g. genes for cell envelope synthesis, cell division, nucleotide metabolism, etc.). This raises the question as to how Carsonella survives within the host bacteriocyte. One of several possible explanations for the absence of these genes is that certain genes were transferred from the genome of Carsonella ancestor to the genome of the psyllid ancestor and are now expressed under the control of the host nucleus. Such lateral gene transfer (LGT) would parallel that known to have occurred from bacterial endosymbionts to host nuclei during the course of the evolution of mitochondria and chloroplasts in eukaryotic hosts (Dyall et al., Reference Dyall, Brown and Johnson2004; Poole & Penny, Reference Poole and Penny2007). Our expressed sequence tag (EST) screening followed by Southern blot and molecular phylogenetic analyses has indicated that some of the genes that are encoded in the psyllid genome and are highly expressed in the bacteriocyte are indeed of bacterial origin (Nakabachi et al., in preparation). This implies that psyllids acquired genes from bacteria by lateral gene transfer (LGT) and make use of these genes to maintain the primary symbiont, Carsonella.
To reveal the complete picture of LGT from symbiotic bacteria to the genome of psyllids, whole genome analysis of psyllids is essential. In the present study, in order to assess the feasibility of the complete genome sequencing of P. venusta, we estimated its genome size by using Feulgen image analysis densitometry and flow cytometry.
Various insect lineages have bacteriocytes, cells that are differentiated so as to harbor obligate mutualistic intracellular bacteria (Buchner, Reference Buchner1965; Moran et al., Reference Moran, McCutcheon and Nakabachi2008). Although bacteriocytes of insects have widely been reported to be polyploid (Koch, Reference Koch1960; Buchner, Reference Buchner1965; Douglas, Reference Douglas1989), only a few reliable estimates based on precise analyses were available. Using Feulgen image analysis densitometry, we further estimated the ploidy of the bacteriocyte of P. venusta that harbors Carsonella.
Materials and methods
Insects
Galls containing 5th instar nymphs of the hackberry petiole gall psyllid, Pachypsylla venusta, were collected from hackberry trees, Celtis reticulata, in Tucson, AZ (a kind gift of Nancy A. Moran, the University of Arizona). Insects were removed from galls and dissected in phosphate-buffered saline (pH 7.4).
Drosophila melanogaster strain Oregon R (a kind gift of Kiyohito Yoshida, Hokkaido University), the haploid C-value for which is 0.18 pg (Rasch et al., Reference Rasch, Barr and Rasch1971; Johnston et al., Reference Johnston, Bennett, Rayburn, Galbraith and Price1999; Bennett et al., Reference Bennett, Leitch, Price and Johnston2003), was used as a standard. In both of the measuring methods described below, the haploid C-values of P. venusta were calculated based on the ratio of signal intensity to that of head cells of D. melanogaster, which were demonstrated to be 2C (Bennett et al., Reference Bennett, Leitch, Price and Johnston2003).
Feulgen image analysis densitometry
Image analysis densitometry was performed as described previously (Koshikawa et al., Reference Koshikawa, Miyazaki, Cornette, Matsumoto and Miura2008). Heads, testes and bacteriocytes were collected from P. venusta, and nuclear DNA was stained using the Feulgen staining method (Hardie et al., Reference Hardie, Gregory and Hebert2002). Heads were collected from five individuals each of both sexes of D. melanogaster strain Oregon R and stained simultaneously. The images of the stained nuclei were captured using a BX-51 microscope and a DP-70 cooled CCD camera (Olympus). The linearity of the camera response was tested by using a stepped density filter (Edmund Optics). The green channel was extracted from images, and the integrated optical density (IOD) of the Feulgen stain in the nuclei was measured using image analysis software, ImageJ (NIH: http://rsb.info.nih.gov/ij/). Background signal intensity was measured in an area adjacent to each nucleus and deduced from the nuclear IOD.
Flow cytometry
Heads were collected from P. venusta, placed in 2 ml of Galbraith buffer (pH 7.2) (Galbraith et al., Reference Galbraith, Harkins, Maddox, Ayres, Sharma and Firoozabady1983), stroked ten times with a pestle in a Dounce tissue homogenizer and filtered through a CellTrics disposable filter (mesh diameter: 30 μm, Partec). Each head from a single individual was processed separately. The nuclear DNA of dissociated cells was stained overnight at 4°C with propidium iodide (final concentration: 50 μg ml−1). Heads were collected from D. melanogaster strain Oregon R and processed in the same manner as the psyllid samples. Subsequently, P. venusta samples were mixed with an aliquot of D. melanogaster samples, and the fluorescence was analyzed using a FACSCanto flow cytometer and FACSDiva software (BD biosciences).
Results
The genome size of P. venusta
Table 1 summarizes the haploid C-values of both sexes of P. venusta that were estimated by using Feulgen image analysis densitometry and flow cytometry.
Table 1. Summary of the haploid C-value of P. venusta.
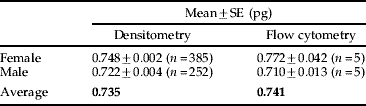
Feulgen image analysis densitometry
Heads were collected from five individuals each of both sexes of P. venusta, and IOD of Feulgen-stained nuclear DNA was analyzed using 385 and 252 cells of females and males, respectively (fig. 1c, d). The IODs of 200 cells (100 cells each from the sexes) of heads of D. melanogaster (fig. 1a) were averaged and used as a standard. Figure 2 shows the histograms indicating the distribution of the estimated C-values of P. venusta based on the IODs of the head cells. Medians for females and males were 0.747 pg and 0.738 pg, respectively. When outliers that appeared to correspond to 4C cells were removed (Smirnov-Grubbs test, P<0.05), the mean C-values for female and male P. venusta were estimated to be 0.748±0.002 pg and 0.722±0.004 pg, respectively. The estimated C-value was slightly larger in females than males (P<0.01, Student's t-test), which is consistent with the karyotype (22+XO/XX) of P. venusta, where the female has two X chromosomes while the male has only one (Riemann, Reference Riemann1966; Maryanska-Nadachowska, Reference Maryanska-Nadachowska2002).

Fig. 1. Feulgen-stained nuclei from different types of cells: (a) cells from the head of a female D. melanogaster strain Oregon R; (b) spermatozoa of P. venusta; (c) cells from the head of a female P. venusta; (d) cells from the head of a male P. venusta; (e) bacteriocytes of a female P. venusta; and (f) bacteriocytes of a male P. venusta. Note that background signals in the cytoplasm are relatively high in the bacteriocytes (e, f) due to the presence of the symbiotic bacterium, Carsonella. Such background signals were subtracted in the same manner used for the other specimens (see experimental procedures). Bar=10 μm.

Fig. 2. Histograms showing the distribution of the estimated haploid C-values of P. venusta, (a) female (n=385) and (b) male (n=252). Open columns indicate the outliers, as determined by the Smirnov-Grubbs test (P<0.05).
To confirm the ploidy of head cells, testes were collected from P. venusta males, and the DNA content in spermatozoa was analyzed (fig. 1b). The mean IODs of spermatozoa (n=136) and male head cells (n=252) were in a ratio of 1.00:2.05, clearly demonstrating that the latter are diploid (2C). This implied that spermatozoa can be used to assess the genome size. However, in this paper, we only show estimates based on the head cells, because a comparison between the same cell types with similar DNA compaction levels is essential for the purpose of an accurate estimation (Hardie et al., Reference Hardie, Gregory and Hebert2002).
Flow cytometry
Heads were collected from five individual female and male P. venusta, and the DNA contents of the dissociated cells were analyzed. Each C-value was calculated based on the main peak of 2C cells (G0/G1 phase). The mean estimated C-values for female and male P. venusta were 0.772±0.042 pg and 0.710±0.013 pg, respectively. Once again, the C-value was slightly larger in females than in males (Student's t-test, P<0.01), which is consistent with the karyotype (22+XO/XX) of P. venusta.
Ploidy of the bacteriocytes
Bacteriocytes were collected from five individuals each of both sexes of P. venusta, and the IOD of Feulgen-stained nuclear DNA was analyzed using 173 and 189 bacteriocytes from females and males, respectively. The IOD of each bacteriocyte was divided by the mean IOD of the head cells of the same sex. Figure 3 shows histograms indicating the distribution of the relative IODs of the bacteriocytes of P. venusta. The medians of the relative IODs for females and males were 8.11 and 8.05, respectively. When outliers were removed (Smirnov-Grubbs test, P<0.05), the mean values for the females and males were 8.11±0.05 and 8.07±0.04, respectively. No significant difference was detected between the sexes (Student's t-test, P>0.05). As the head cells are diploid (2C), as mentioned above, it is clearly demonstrated that the vast majority of bacteriocytes are 16C.
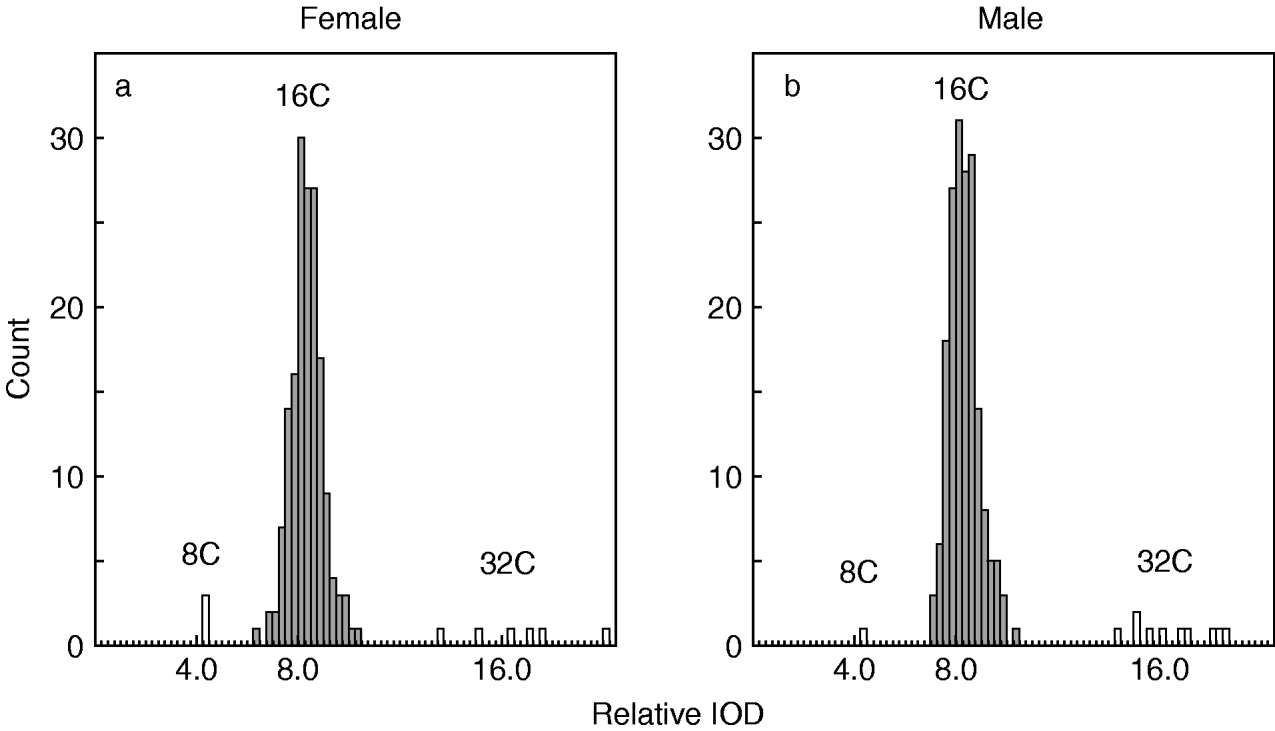
Fig. 3. Histograms showing the distribution of the estimated ploidy of the bacteriocytes of P. venusta, (a) female (n=173) and (b) male (n=189). Open columns indicate the outliers, as determined by the Smirnov-Grubbs test (P<0.05).
Discussion
The present study demonstrated that the haploid C-value of P. venusta is approximately 0.74 pg, which can be converted (1 pg=978 Mb) (Dolezel et al., Reference Dolezel, Bartos, Voglmayr and Greilhuber2003) into 724 Mb. This is the first report of the genome size of psyllids. Although (i) the genome size of their primary symbiont is extremely small, and (ii) the genome of P. venusta appeared to have acquired bacterial genes via LGT, the value is moderate for Hemiptera; the mean C-value for all of the hemipterans studied to date is 0.91 pg/890 Mb (Brown et al., Reference Brown, Lambert, Ghanim, Czosnek and Galbraith2005; Gregory, Reference Gregory2005; Gomez-Palacio et al., Reference Gomez-Palacio, Jaramillo-Ocampo, Triana-Chavez, Saldana, Calzada, Perez and Panzera2008). Among the hemipterans, the entire genomes of the pea aphid (Acyrthosiphon pisum; Sternorrhyncha: Aphidoidea, 1C=0.54 pg/525 Mb) (http://www.hgsc.bcm.tmc.edu/projects/aphid) and the kissing bug (Rhodnius prolixus; Prosorrhyncha, 1C=0.69 pg/675 Mb) (Panzera et al., Reference Panzera, Ferrandis, Ramsey, Salazar-Schettino, Cabrera, Monroy, Bargues, Mas-Coma, O'Connor, Angulo, Jaramillo and Perez2007) are being sequenced (Tagu et al., Reference Tagu, Klingler, Moya and Simon2008; Megy et al., in press). The genome size of P. venusta is comparable to the sizes of the preceding hemipteran genomes. Moreover, the recent advent of high throughput technologies to produce millions of DNA sequence reads in a single run is dramatically reducing the cost for sequencing, making it feasible to perform whole genome analysis of higher eukaryotes (Hillier et al., Reference Hillier, Marth, Quinlan, Dooling, Fewell, Barnett, Fox, Glasscock, Hickenbotham, Huang, Magrini, Richt, Sander, Stewart, Stromberg, Tsung, Wylie, Schedl, Wilson and Mardis2008; Mardis, Reference Mardis2008; Wheeler et al., Reference Wheeler, Srinivasan, Egholm, Shen, Chen, McGuire, He, Chen, Makhijani, Roth, Gomes, Tartaro, Niazi, Turcotte, Irzyk, Lupski, Chinault, Song, Liu, Yuan, Nazareth, Qin, Muzny, Margulies, Weinstock, Gibbs and Rothberg2008). Complete genome sequencing of P. venusta will reveal the entire picture of LGT from symbiotic bacteria to the host insect genome, which should provide deep insight into the mechanisms involved in the integration of the two organisms. This would parallel the early evolution of mitochondria and chloroplasts, which are known to have transferred essential genes to ancestral eukaryotic hosts and to reimport the products of these genes for their own use (Dyall et al., Reference Dyall, Brown and Johnson2004; Poole & Penny, Reference Poole and Penny2007). As psyllids include important agricultural pests that transmit plant pathogens (Subandiyah et al., Reference Subandiyah, Nikoh, Tsuyumu, Somowiyarjo and Fukatsu2000; Stokstad, Reference Stokstad2006), the genomic data will also be an important resource for studying numerous aspects related to the transmission of such pathogens.
Many insect lineages, especially members of Sternorrhyncha (aphids, psyllids, whiteflies and scale insects), Auchenorrhyncha (cicadas, leafhoppers, treehoppers, spittlebugs and planthoppers), Blattaria (cockroaches) and Coleoptera (beetles), have bacteriocytes, cells that are differentiated so as to harbor obligate mutualistic intracellular bacteria (Buchner, Reference Buchner1965; Moran et al., Reference Moran, McCutcheon and Nakabachi2008). Although the evolutionary and developmental origins of bacteriocytes appear to differ among insect lineages (Buchner, Reference Buchner1965; Douglas, Reference Douglas1989), they almost invariably contain a large nucleus that is widely reported to be polyploid (Koch, Reference Koch1960; Buchner, Reference Buchner1965; Douglas, Reference Douglas1989). The level of polyploidy varies among lineages. The most extreme case is found in adult cockroaches (Periplaneta americana), where the bacteriocytes were inferred to be 256-512-ploid based on the nuclear volume (Baudisch, Reference Baudisch1956). The case of P. venusta demonstrated in this study (16C) is similar to that of leafhoppers (Euscelis plebejus, Hemiptera: Auchenorrhyncha), in which the bacteriocytes are 8C–16C (Körner, Reference Körner1969). The polyploidy, which characterizes insect bacteriocytes, could partly be due to the fact that high ploidy allows cells to increase metabolic output without the need to devote energy to all aspects of cell division (Ravid et al., Reference Ravid, Lu, Zimmet and Jones2002). Indeed, polyploidy is often found in highly metabolically active cells in various animal lineages (Edgar & Orr-Weaver, Reference Edgar and Orr-Weaver2001). This has been clearly demonstrated in the bacteriocyte of the pea aphid (Nakabachi et al., Reference Nakabachi, Shigenobu, Sakazume, Shiraki, Hayashizaki, Carninci, Ishikawa, Kudo and Fukatsu2005). The aphid bacteriocytes are also polyploid and harbor the primary symbiont Buchnera aphidicola (γ-Proteobacteria), which is a lineage distinct from Carsonella (Munson et al., Reference Munson, Baumann and Kinsey1991). Buchnera provides host aphids with essential amino acids that aphids cannot synthesize and are scarce in their diet of phloem sap (Douglas, Reference Douglas1998). Buchnera has a relatively small genome (640 kb) (Shigenobu et al., Reference Shigenobu, Watanabe, Hattori, Sakaki and Ishikawa2000) although the case is not as extreme as the case of Carsonella. The genome of Buchnera retains genes for the synthesis of essential amino acids but lacks many genes, including those for the synthesis of non-essential amino acids (amino acids that metazoa can synthesize; alanine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine and tyrosine) and the genes for amino acid transporters. Complementary to this, genes selectively and highly upregulated in the aphid bacteriocyte included those for (i) synthesis of non-essential amino acids, (ii) utilization of essential amino acids that are supplied by Buchnera and (iii) amino acid transporters (Nakabachi et al., Reference Nakabachi, Shigenobu, Sakazume, Shiraki, Hayashizaki, Carninci, Ishikawa, Kudo and Fukatsu2005). This indicates that the aphid bacteriocyte is dedicated to the maintenance and utilization of Buchnera, which provides the aphid with essential nutrients. It is highly plausible that the psyllid bacteriocytes have adopted similar functional specialization. As the genome streamlining is much more extreme in Carsonella, the psyllid bacteriocytes may have acquired more advanced and elaborate mechanisms to support the survival of the symbiotic bacterium. We are pursuing further analyses of ESTs, which would not only answer this question, but also facilitate gene discovery and annotation in genome analysis.
Now that the present study has verified the feasibility of the complete genome sequencing of P. venusta, efforts have begun to take shape to realize an entire genome project for this psyllid species.
Acknowledgements
We thank Nancy A. Moran and Kiyohito Yoshida for providing insects. This study was partly supported by Grants-in-Aid for Scientific Research (Nos 18370007 and 2065700408) of the Ministry of Education, Culture, Sports, Science and Technology of Japan.






