Introduction
Insects have evolved a variety of physiological and behavioral responses to various toxins in natural and managed ecosystems (Jallow & Hoy, Reference Jallow and Hoy2005; Li et al., Reference Li, Schuler and Berenbaum2007). These varied responses can reflect the toxin mode of action and the extent to which they influence behavior (Hoy et al., Reference Hoy, Head and Hall1998). Some insects are capable of changing their behavior in response to their sensory perception of insecticides (Gould, Reference Gould1984; Lockwood et al., Reference Lockwood, Sparks and Story1984; Haynes, Reference Haynes1988; Desneux et al., Reference Desneux, Decourtye and Delpuech2007). These behavioral changes may sometimes compromise tests of insecticide efficacy against insect pests; and, consequently, standard assays based on lethality can either overestimate or underestimate the insecticide impact in a population (Moore et al., Reference Moore, Tabashnik and Stark1989; Cox et al., Reference Cox, Fleming, Atkinson, Bannon and Whitefield1997; Bayley, Reference Bayley and Dell'Omo2002). Such limitation is particularly serious for insect pest species with insecticide-resistant populations.
Insecticide studies usually focus on the insecticide lethality or other direct effects of insecticides, whereas relatively little attention is placed on the sublethal behavioral response to insecticidal exposure, particularly in insect pests and in contrast with natural enemies (Kongmee et al., Reference Kongmee, Prabaripai, Akratanakul, Bangsm and Chareonviriyaphap2004; Desneux et al., Reference Desneux, Decourtye and Delpuech2007). One behavioral response of insects to insecticides is the stimulus-dependent avoidance, which refers to the enhanced ability to detect a toxic substance and assumes an irritant or repellent property of the toxicant that elicits avoidance response after detection with or without contact with the insecticide (irritability and repellence, respectively) (Georghiou, Reference Georghiou1972; Lockwood et al., Reference Lockwood, Sparks and Story1984). Another behavioral response of insects to insecticides is the stimulus-independent avoidance, where the insects avoid exposure to a toxicant due to an independent and innate behavioral trait, such as exophily in mosquitos (Anonymous, 1957; Georghiou, Reference Georghiou1972; Lockwood et al., Reference Lockwood, Sparks and Story1984). They are both recognized as behavioral resistance to toxic compounds if such traits are inheritable (Georghiou, Reference Georghiou1972; Lockwood et al., Reference Lockwood, Sparks and Story1984) and will be used here within the same context, although insecticide resistance may also be more restrictively defined as the result of past insecticide selection. If the toxic compound involved is an insecticide and the behavioral trait is enhanced as stimulus-dependent in a population, it is referred to as stimulus-dependent behavioral resistance to the insecticide (Georghiou, Reference Georghiou1972; Gould, Reference Gould1984; Lockwood et al., Reference Lockwood, Sparks and Story1984; Wang et al., Reference Wang, Scharf and Bennett2004). In contrast, if the behavioral trait is not enhanced with increased stimuli in a population, the behavioral response is referred to as stimulus-independent behavioral resistance (Georghiou, Reference Georghiou1972; Lockwood et al., Reference Lockwood, Sparks and Story1984).
Behavioral resistance leads to reduced exposure to insecticides and may minimize selection for physiological insecticide resistance (i.e. insecticide resistance in its strict sense) (Gould, Reference Gould1984; Suiter & Gould, Reference Suiter and Gould1994; Jallow & Hoy, Reference Jallow and Hoy2005). Behavioral resistance to insecticides has been studied in few arthropod species and correlational studies between physiological and behavioral resistance were seldom carried out (Moore, Reference Moore1977; Lockwood et al., Reference Lockwood, Sparks and Story1984; Moore et al., Reference Moore, Tabashnik and Stark1989; Ross, Reference Ross1993; Suiter & Gould, Reference Suiter and Gould1994). The behavioral pattern considered, and its potential dose-dependence relationship, makes such correlations difficult to establish, and the importance of the behavioral resistance remains largely unrecognized.
Locomotion, either by flight or walking, uniquely determines insecticide exposure by governing the time that animals spend in contact with insecticide. Animals use their freedom of movement to better adapt to their living conditions (Martin & Bateson, Reference Martin and Bateson1993). The locomotory activity is linked to and expresses a synthesis of the animal's physiological processes and its anatomical condition, whilst being central to many aspects of the more complex inter- and intraspecific interactions between animals (Bayley, Reference Bayley and Dell'Omo2002). Most animals express reactive locomotory responses to both abiotic and biotic environmental stimuli, which are certainly important in assessing hazards posed by environmental conditions, such as insecticide-sprayed surfaces.
Physiological and behavioral responses of animals may change under stressful conditions to maximize their chance of survival (Wingfield, Reference Wingfield2003). Stressful conditions resulting from intense and/or prolonged exposure to a given stimuli may lead to a locomotory response impacting the animal fitness (Baatrup & Bayley, Reference Baatrup and Bayley1993). Insecticides may stimulate or depress the general locomotory behavior of arthropods (Haynes, Reference Haynes1988; Desneux et al., Reference Desneux, Decourtye and Delpuech2007). Such behavioral responses may vary in physiologically resistant populations of insect pests, further compromising their control, and should, therefore, be a concern for their management (Georghiou, Reference Georghiou1972; Gould, Reference Gould1984; Lockwood et al., Reference Lockwood, Sparks and Story1984).
Insect pests of stored products frequently show insecticide resistance (e.g. Champ & Dyte, Reference Champ and Dyte1976; Badmin, Reference Badmin, Fleurrat-Lessard and Ducom1990; Subramanyam & Hagstrum, Reference Subramanyam, Hagstrum, Subramanyam and Hagstrum1996). Behavioral avoidance to insecticides has also been detected in these pest species (Watson & Barson, Reference Watson and Barson1996; Cox et al., Reference Cox, Fleming, Atkinson, Bannon and Whitefield1997; Watson et al., Reference Watson, Barson, Pinniger, Roberts and Ludlow1997). Brazilian strains of the maize weevil Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) show problems of pyrethroid resistance (Guedes et al., Reference Guedes, Lima, Santos and Cruz1995; Fragoso et al., Reference Fragoso, Guedes and Rezende2003; Ribeiro et al., Reference Ribeiro, Guedes, Oliveira and Santos2003), but the behavioral correlates associated with such strains and their behavioral responses induced by insecticides have yet to be investigated. These were the objectives of the present investigation, which tested the hypothesis of a potential negative association between physiological and behavioral resistance to pyrethroids. Different dose-dependent locomotory responses were expected among strains, with behavioral resistance less likely to occur in physiologically resistant insects since they are able to withstand higher doses of insecticide. Such expectation is based on the expected relaxation in selection for physiological resistance with the reduced exposure determined by the behavioral resistance, as earlier suggested by Georghiou (Reference Georghiou1972).
Material and methods
Insects and chemicals
Three strains of S. zeamais were used in the present investigation, which were termed here as ‘susceptible’, ‘resistant cost’ and ‘resistant no-cost’. The susceptible strain was collected in Sete Lagoas County and provided by the National Center of Maize and Sorghum of the Brazilian Agricultural Research Corporation (EMBRAPA Milho & Sorgo). This strain has been maintained for nearly 20 years without insecticide exposure and its susceptibility to pyrethroids and organophosphates is well known and periodically checked (Guedes et al., Reference Guedes, Lima, Santos and Cruz1994, Reference Guedes, Lima, Santos and Cruz1995; Fragoso et al., Reference Fragoso, Guedes and Rezende2003; Ribeiro et al., Reference Ribeiro, Guedes, Oliveira and Santos2003; Araújo et al., Reference Araújo, Guedes, Oliveira and Ferreira2008).
Both insecticide-resistant strains are pyrethroid-resistant (≈100-fold resistant and subjected to periodical checks before and during the present study) (Guedes et al., Reference Guedes, Lima, Santos and Cruz1994, Reference Guedes, Lima, Santos and Cruz1995; Ribeiro et al., Reference Ribeiro, Guedes, Oliveira and Santos2003; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007; Araújo et al., Reference Araújo, Guedes, Oliveira and Ferreira2008). The resistant no-cost strain was collected in Jacarezinho County (state of Paraná, Brazil) by the late 1980s, and the resistant cost strain was collected in Juiz de Fora County (state of Minas Gerais, Brazil) by 1999 (Guedes et al., Reference Guedes, Lima, Santos and Cruz1995; Fragoso et al., Reference Fragoso, Guedes and Rezende2003). The resistant cost strain shows fitness disadvantage due to its slower development and reduced progeny production in the absence of pyrethroids, unlike the resistant no-cost strain from Jacarezinho (Fragoso et al., Reference Fragoso, Guedes and Peternelli2005; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007). In addition, the insects from the resistant no-cost strain are heavier and show higher energy reserves than the susceptible and the resistant cost strains (Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007). Both resistant populations share the same major pyrethroid resistance mechanism, altered target-site sensitivity, with secondary involvement of enhanced detoxification by glutathione-S-transferases (Guedes et al., Reference Guedes, Lima, Santos and Cruz1995; Fragoso et al., Reference Fragoso, Guedes and Rezende2003, Reference Fragoso, Guedes and Oliveira2007; Ribeiro et al., Reference Ribeiro, Guedes, Oliveira and Santos2003).
All three insect strains were maintained in whole maize grains free of insecticides under controlled temperature (25±2°C), relative humidity (70±5%) and photoperiod (LD 12:12). Acetone was purchased from Cromato Prod. Quím. (Diadema, SP, Brazil) and technical grade deltamethrin (99% pure) was purchased from ChemService (West Chester, PA, USA).
Flight take-off bioassays
Transparent plastic jars (15 cm diameter×12 cm high) with glue-coated walls were used as experimental units for the flight take-off bioassays, adapting methods from Perez-Mendoza et al. (Reference Perez-Mendoza, Dover, Hagstrum and Hopkins1999). A filter paper (Whatman no. 1) with dried deltamethrin residue (applied as a 3 ml solution using acetone as solvent and left to dry for 30 min) was placed at the bottom of the jar. The bottom of the jar walls received a 2-cm-high layer of Teflon® PTFE (DuPont, Paulínia, SP, Brazil) to prevent the insects from climbing them, the remaining of which was coated with sticky glue (Bio Controle, São Paulo, Brazil). Two hundred non-sexed adult insects (one to two weeks old) were released in the center of each jar, which were maintained at 28±2°C for one hour for subsequent recording of the number of insects glued to the walls while taking-off for flight. Five replicates were used for each combination of strain and insecticide concentration (0.0, 0.004, 0.008, 0.04, 0.17, 0.42 and 0.85 mg a.i. cm−2). These deltamethrin concentrations are in the sublethal range for the strains, and they were selected based on preliminary bioassays and the lowest observed effect concentration (LOEC) established for the susceptible strain (1.0 mg a.i. cm−2).
Walking behavior bioassays
Two behavioral bioassays were carried out in arenas either fully-treated or half-treated with deltamethrin dissolved in acetone (control treatments were treated with acetone only). Filter papers (Whatman no. 1) with dried deltamethrin residue (applied as 1 ml solution and left to dry for 30 min and concentrations of 0, 0.04, 0.16, 0.39, 0.79 mg a.i. cm−2) were placed on Petri dishes (9.0 cm diameter). The range of deltamethrin concentrations used was established as described above. The inner walls of each Petri dish were coated with Teflon to prevent the insects from escaping. Twenty arenas with individual insects were used for each combination of sex, strain and insecticide concentration in each behavioral bioassay (fully- and half-treated arenas), and no mortality was observed within the exposure time used for the behavioral bioassays.
The movement of each insect within the arena during the 30 min trial was recorded and digitally transferred to a computer using an automated video tracking system equipped with a CCD camera (Videomex-One, Columbus Instruments, Columbus, OH, USA). The video images of the arenas were maintained either undivided, for the behavioral bioassay with fully-treated arenas, or divided into two symmetrical zones: one untreated and the other treated with deltamethrin, for the behavioral bioassay with half-treated arenas. The parameters calculated for the fully-treated arenas were walking distance (cm), velocity (cm s−1), time spent walking (s), time spent immobile (i.e. resting time (s)), and time spent stationary while participating in non-forward motion (i.e. stationary time (s)). Stationary time was distinguished based on the presence of insect movement, but lack of horizontal body displacement, unlike when either walking (i.e. with horizontal body displacement) or resting (i.e. without any movement). For the half-treated arenas, these same parameters were calculated for each arena zone (i.e. untreated and deltamethrin-treated zones) in addition to the time spent on the deltamethrin-treated zone (i.e. visiting time).
Statistical analyses
The results of the flight take-off bioassays were subjected to analysis of covariance with deltamethrin concentration as covariate (PROC GLM: SAS Institute, 2002). The overall results for fully-treated arenas were subjected to a two-way (sex×strain) multivariate analysis of covariance with deltamethrin concentration as the covariate (PROC GLM with MANOVA statement: SAS Institute, 2002). Individual two-way analyses of covariance for each parameter were eventually carried out followed by Fisher's LSD test, if appropriate (P<0.05) (PROC GLM: SAS Institute, 2002).
The results for half-treated arenas were subjected to two distinct sets of analyses. First, the results of untreated×deltamethrin-treated zones of each sex and strain were contrasted using multivariate analyses of covariance with deltamethrin concentration as covariate (PROC GLM with MANOVA statement: SAS Institute, 2002). Second, the results of the deltamethrin-treated half of the arenas were subjected to a two-way (sex×strain) multivariate analysis of covariance, again with deltamethrin concentration as covariate (PROC GLM with MANOVA statement: SAS Institute, 2002). As with the results for fully-treated arenas, individual analyses of covariance for each parameter assessed in the half-treated arenas were also carried out and followed by Fisher's LSD test, if appropriate (P<0.05) (PROC GLM: SAS Institute, 2002).
Results
Flight take-off from deltamethrin-treated surface
The flight take-off from deltamethrin-treated surfaces differed among strains of the maize weevil (F2,66=51.36, P<0.0001), but the differences among deltamethrin concentrations and the interaction strain-deltamethrin concentration were not significant (F6,66=0.49, P=0.81 and F8,66=0.88, P=0.54, respectively). The rate of flight take-off was over threefold higher for the resistant cost strain compared with the resistant no-cost strain, while the rate of flight take-off for the susceptible strain was intermediate between that of both pyrethroid-resistant strains, regardless of the deltamethrin concentration to which they were exposed (fig. 1).

Fig. 1. Proportion of adult insects (±SE) from three strains of the maize weevil Sitophilus zeamais taking-off for flight from deltamethrin-treated surfaces. Histogram bars with the same letter do not significantly differ by Fisher's LSD test (P<0.05).
Walking behavior in fully-treated arenas
The overall mobility parameters of the maize weevil strains on the surface treated with increasing deltamethrin concentrations differed for strains (df=10, 610, Wilks' lambda=0.8385, F=5.61, P<0.0001), sex (df=5, 305, Wilks' lambda=0.7289, F=22.69, P<0.0001) and the interaction strain×sex (df=10, 610, Wilks' lambda=0.7126, F=11.26, P<0.0001). Deltamethrin concentration (df=20, 1012.5, Wilks' lambda=0.9236, F=1.23, P=0.22) and its interactions with strain (df=40, 1332.3, Wilks' lambda=0.8824, F=0.95, P=0.53), sex (df=20, 1012.5, Wilks' lambda=0.9003, F=1.60, P=0.06) and both (df=40, 1332.3, Wilks' lambda=0.8923, F=0.88, P=0.68) were not significant, indicating that the walking behavior is characteristic of the sex and strain but independent from the insecticide stimuli for the range of sublethal insecticide concentrations tested. Univariate analyses of covariance were, therefore, carried out for each parameter measured. There were significant differences for all of the parameters, but there was no significant effect of deltamethrin concentration and its interactions for any of them. The interaction between sex and strain was significant for all parameters (F2,309>9.94, P<0.0002) except resting time (F2,309=1.57, P=0.21), where the effects of sex and strain alone were significant, regardless of each other (F1,309=69.93, P<0.0001 and F2,309=12.05, P<0.0001, respectively). Resting time was significantly higher for the resistant no-cost strain (fig. 2a). Resting time was significantly higher for females rather than males regardless of the strain (fig. 2b). The remaining mobility parameters showed little variation among females from different strains, which were significantly lower only for velocity and stationary time of the resistant cost strain. In contrast, the overall male mobility parameters were higher than the female parameters, with the males from the resistant cost strain exhibiting higher mobility than the resistant no-cost males (table 1). The susceptible males showed intermediate mobility between both resistant strains (table 1), following a pattern similar to that observed for flight take-off.

Fig. 2. Resting time (i.e. time spent immobile) (±SE) of (A) three strains and (B) both sexes of the maize weevil Sitophilus zeamais exposed to arenas fully treated with either solvent (acetone) or deltamethrin. Data from acetone-treated and deltamethrin-treated arenas were pooled together since they did not differ by Fisher's F-test from the respective analyses of covariance (P<0.05). Histogram bars with the same letter do not significantly differ by either (a) Fisher's LSD test (P<0.05) or (b) Fisher's F-test (P<0.05).
Table 1. Walking distance, velocity, walking time and stationary time (i.e. time spent on non-forward motion) (±SE) of both sexes from three strains of the maize weevil Sitophilus zemais, one insecticide-susceptible (susceptible) and two insecticide-resistant strains (resistant cost and resistance no-cost), exposed to fully-treated arenas (data from acetone-treated and deltamethrin-treated arenas were pooled together since they did not differ by Fisher's F-test from the respective analyses of variance at P<0.05).
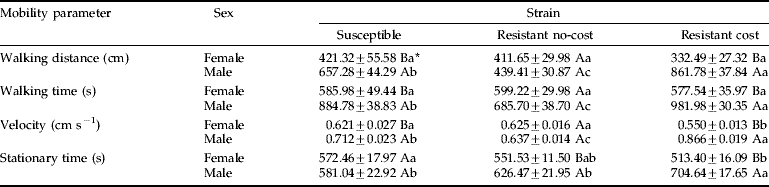
* Means followed by the same lower case letter in a row or the same capital letter in a column, for each mobility parameter, are not significantly different by Fisher's LSD test (P<0.05).
Walking behavior in half-treated arenas
Untreated×deltamethrin-treated halves
The results obtained on both untreated and deltamethrin-treated zones of each arena were contrasted for each strain and sex using deltamethrin concentration as covariate. There was no significant overall difference between untreated and deltamethrin-treated zones of the arena, except for the males of the susceptible strain (df=5, 68, Wilks' lambda=0.8395, F=2.60, P=0.03). The univariate analysis of covariance carried out for each mobility parameter of males of the susceptible strain indicated significant differences only in resting time (F1,72=9.85, P=0.002) and visiting time (F1,72=9.21, P=0.003) between untreated and deltamethrin-treated zones of the arenas. Susceptible males exhibited higher resting and visiting time on the deltamethrin-treated surfaces (fig. 3), spending therefore more time on the deltamethrin-treated surface and moving less on such a surface than on its untreated portion.

Fig. 3. (A) Resting time and (B) visiting time (±SE) of males from an insecticide-susceptible strain of the maize weevil Sitophilus zeamais on each half of arenas half-treated with deltamethrin. Histogram bars with the same letter do not significantly differ by Fisher's F-test (P<0.05).
Walking behavior on the deltamethrin-treated half of the arenas
The overall mobility parameters of the maize weevil strains on the deltamethrin-treated zone of half-treated arenas were significantly different for the sex-strain interaction. However, subsequent univariate analyses of covariance for each mobility parameter indicated significant differences only for velocity, where the interaction sex-strain was significant (F2,249=3.64, P=0.03). As in the determination for fully-treated arenas, female velocity was indistinguishable among strains and smaller than male velocity (except for the susceptible strain) (table 2). Males from the resistant cost strain exhibited significantly higher velocity than males of the other two strains, which were undistinguishable (table 2).
Table 2. Velocity (±SE) of insects from both sexes from three strains of the maize weevil Sitophilus zeamais, an insecticide-susceptible (susceptible) and two insecticide-resistant strains (resistant cost and resistance no-cost), on the treated half of deltamethrin half-treated arenas (data from different insecticide concentrations were pooled together since they did not differ by Fisher's F-test from the analysis of variance at P<0.05).

* Means followed by the same lower case letter in a row, or the same capital letter in a column, are not significantly different by Fisher's LSD test (P<0.05).
Discussion
An insect's chance of survival may be greatly increased if its behavior is modified to avoid insecticide-treated surfaces (Watson & Barson, Reference Watson and Barson1996; Cox et al., Reference Cox, Fleming, Atkinson, Bannon and Whitefield1997). Earlier studies on behavioral resistance to insecticides led to Georghiou's theoretical hypothesis of negative correlation between behavioral and physiological resistance to insecticides (Georghiou, Reference Georghiou1972). Such divergent evolution would take place because the most insecticide-susceptible individuals within a population are more likely to show higher avoidance to the insecticide. Insecticide application for successive generations would, therefore, favor divergent selection with the more susceptible individuals being selected for higher avoidance.
The negative correlation predicted between behavioral and physiological resistance to insecticides was observed in several insect species, but the number of exceptions to this expectation is far from negligible (e.g. Lockwood et al., Reference Lockwood, Sparks and Story1984; Suiter & Gould, Reference Suiter and Gould1994; Renou et al., Reference Renou, Henninot-Rodes, Delorme, Augé and Touton1997; Kongmee et al., Reference Kongmee, Prabaripai, Akratanakul, Bangsm and Chareonviriyaphap2004; Wang et al., Reference Wang, Scharf and Bennett2004). The present results with maize weevil strains also challenge this hypothesis. The results of flight take-off and walking behavior on deltemethrin-treated surfaces do not seem related to physiological resistance to insecticides since the susceptible strain showed intermediate rates of flight take-off and walking behavior between the physiologically resistant strains, in contrast with the initial expectation of an inverse relationship between behavioral and physiological resistance to insecticides.
Stimulus-independent behavioral resistance to the pyrethroid deltamethrin does exist in strains of the maize weevil since these strains differ in their exposure to this insecticide regardless of the presence of insecticide. The trait is apparently inheritable and maintained within each strain generation after generation. However, such behavioral resistance is not negatively correlated with physiological resistance to insecticides as earlier suggested by Georghiou (Reference Georghiou1972), nor is it positively correlated with physiological resistance to insecticides as alternatively suggested by Lockwood et al. (Reference Lockwood, Sparks and Story1984). Rather, the origin of the (locomotory) behavioral resistance in strains of maize weevil is independent from physiological resistance to this compound, a possibility earlier recognized by Chareonviriyaphap et al. (Reference Chareonviriyaphap, Roberts, Andre, Harlan and Bangs1997) while studying pesticide avoidance in mosquitoes.
The strain and sex differences in locomotion observed in the maize weevil may result from differences in body mass among the strains and sex (Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007), since the heavier strain (i.e. resistant no-cost) and sex (i.e. female) exhibited lower levels of locomotion. However, strain differences in intermediate metabolism are also likely to be important (Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007; Araújo et al., Reference Araújo, Guedes, Oliveira and Ferreira2008). As previously reported for the walking activity of insecticide-resistant and susceptible strains of a related species, the granary weevil Sitophilus granarius (L.) (Surtees, Reference Surtees1966), heavier maize weevils showed lower rate of flight take-off (represented by the resistant no-cost insects) and lower mobility (females and males particularly from the resistant no-cost strain are heavier). Such lower rate of flight take-off and mobility reduces the gene flow among populations increasing the probability of within-strain mating and may be important for the spread of insecticide resistance among populations of maize weevil (Georghiou, Reference Georghiou1972; Gould, Reference Gould1984).
Behavioral resistance to insecticides may be either stimulus-dependent or stimulus-independent (Georghiou, Reference Georghiou1972; Gould, Reference Gould1984; Lockwood et al., Reference Lockwood, Sparks and Story1984). Irritability and repellence are regarded as two types of stimulus-dependent behavioral resistance to insecticides (i.e. require sensory stimulation), which has been relatively frequently reported in mosquitoes and other arthropods (e.g. Lockwood et al., Reference Lockwood, Byford, Story, Sparks and Quisenberry1985; Wang et al., Reference Wang, Scharf and Bennett2004; Muenworn et al., Reference Muenworn, Akaratanakul, Bangs, Parbaripai and Chareonviriyaphap2006). In contrast, stimulus-independent behavioral resistance to insecticides has been the object of little attention and only detected in malaria mosquitoes, but with important consequences for their management (Anonymous, 1958; Akiyama, Reference Akiyama1973; Ribeiro & Janz, Reference Ribeiro and Janz1990).
The behavioral resistance expressed in the locomotory activity in strains of maize weevil was stimulus-independent because no sensory stimulation was required for its expression (as observed in the untreated surfaces), which was characteristic for each strain (and sex within the strain). In addition, the locomotion-based behavioral resistance in maize weevil strains was independent of low (sublethal) insecticide concentrations. Therefore, the behavioral resistance expressed in locomotory activity of maize weevil strains was stimulus-independent and concentration-independent as a result of the different genetic make-up of the strains allowing some of them to minimize insecticide exposure by their high rate of flight take-off and reduced mobility.
The varied behavioral patterns of locomotion leading to stimulus-independent behavioral resistance to insecticides are also important for the spread of physiological resistance to insecticides, which may co-exist with behavioral resistance in some strains. Furthermore, these behavioral patterns potentially lead to reduced insecticide exposure compromising the efficacy of such compounds in at least some insect populations and should, therefore, be considered in insecticide resistance surveys and management programs.
The stimulus-independent behavioral resistance to deltamethrin was expressed at sublethal insecticide concentrations, which are found in storage facilities after insecticide spraying and the start of the insecticide degradation. Such low residue levels are unlikely to interfere with the regular behavior of the exposed insects, but the diverse (and inate) locomotory behavior among strains will affect insect exposure and should be considered in behaviorally-target management strategies (i.e. Push-Pull strategies (Cook et al., Reference Cook, Khan and Pickett2007)). At higher residue levels of deltamethrin, concentration-dependent behavioral resistance may also exist, which deserves further studies and does also have potential impact on the maize weevil management.
Acknowledgements
We thank the Minas Gerais State Foundation for Research Aid (FAPEMIG), the CAPES Foundation (Brazilian Ministry of Education) and the Brazilian National Council of Scientific and Technological Development (CNPq) for the financial support provided for the present study. Appreciation is also expressed to Dr J.P. Santos (EMBRAPA Milho & Sorgo) for providing two of the original stock strains used in the present investigation. Comments and editing by Drs J.E. Throne, J.F. Campbell and K.Y. Zhu on an early draft of the manuscript were greatly appreciated, as were the comments and suggestions provided by two anonymous referees.







