Introduction
Aerial migration plays an important part in the life cycle of many insect pests (Dingle, Reference Dingle2014), and it has profound practical consequences for their management (Reynolds et al., Reference Reynolds, Riley, Armes, Cooter, Tucker, Colvin and Dent1997; Chapman et al., Reference Chapman, Reynolds and Wilson2015). Migratory insects are usually too small to directly observe when they are flying hundreds of meters above the ground (Dingle & Drake, Reference Dingle and Drake2007), making entomological radar the only available method for directly observing their migratory behaviors (Chapman et al., Reference Chapman, Drake and Reynolds2011). However, it is often not practicable to use this technique for this reason, flight mills, which constrain tethered insects to fly in circles, have been widely used as a convenient and relatively inexpensive means to assess the relative migration ability of species (Reynolds et al., Reference Reynolds, Riley, Armes, Cooter, Tucker, Colvin and Dent1997). Flight mills have also been used to investigate the effect of migrants’ pre-flight and in-flight environments and physiological state on flight activity (Colvin & Gatehouse, Reference Colvin and Gatehouse1993; Rankin et al., Reference Rankin, Hampoton and Summy1994). Consideration of all these factors is important when assessing the migratory potential of field populations of insects (Cooter & Armes, Reference Cooter and Armes1993). Understand a species’ flight performance facilitates the development of forecasting systems and management strategies (Stefanescu et al., Reference Stefanescu, Páramo, Åkesson, Alarcón, Ávila, Brereton, Carnicer, Cassar, Fox, Heliölä, Hill, Hirneisen, Kjellén, Kühn, Kuussaari, Leskinen, Liechti, Musche, Regan, Reynolds, Roy, Ryrholm, Schmaljohann, Settele, Thomas, Swaay and Chapman2013).
Macdunnoughia crassisigna Warren (Lepidoptera: Noctuidae) is an important insect pest of agricultural crops in the Himalayan-Pacific Region. M. crassisigna larvae are polyphagous, attacking >40 cultivated plants, including several strategic crops such as cotton, maize, beans, etc. Young larvae feed on terminal clusters, seedlings, and stems of host crops, and leaves may be skeletonized and almost completely consumed (Lu et al., Reference Lu, Xu, Chen and Kang1999; Ma et al., Reference Ma, Pati, Chen and Ma2011). In China, this species is widely distributed from northeastern through central China to the Tibetan Plateau, and occasionally its occurrence range can extend into southeastern China (Kumar & Kumar, Reference Kumar and Kumar2013; Hu et al., Reference Hu, Xue and Hua2014). Damage from M. crassisigna has increased on cotton and vegetables in recent years in the Huang-Huai-Hai Rivers Plain in northern China, where it usually undergoes 2–4 generations each year (Wu, Reference Wu1990). M. crassisigna larvae mainly damage cotton and peas in late spring, maize, and soybeans in summer, cruciferous vegetables and asteraceae plants in autumn, and overwinter as diapausing larvae or pupae in the soil (Lu et al., Reference Lu, Xu, Chen and Kang1999; Ma et al., Reference Ma, Pati, Chen and Ma2011). The initial seasonal emergence date of this species becomes progressively earlier moving southward. For example, the first generation normally occurs in mid-July in Jilin province (41°N–46°N), but in late March in Zhejiang province (27°N–31°N), which is ≈1500 km south of Jilin Province (Wu, Reference Wu1990; Lu et al., Reference Lu, Xu, Chen and Kang1999; Wu & Chang, Reference Wu and Chang2004).
M. crassisigna is confirmed to be a long-distance migrant, as large numbers of moths have been caught on an isolated island in the Bohai Strait (Fu et al., Reference Fu, Hu, Feng, Liu and Wu2015). Trans-regional migration is an important reason for the sudden outbreaks of M. crassisigna, which makes it difficult to monitor this species effectively (Fu et al., Reference Fu, Hu, Feng, Liu and Wu2015). Migratory insects usually have strong flight potential and a better understanding of flight behavior is important for studying their migration. To forecast and control M. crassisigna effectively, a thorough appreciation is needed of key biological and ecological facets of this species (e.g., life history, population dynamics, migration, and flight ability). The flight activity of M. crassisigna under different biotic and abiotic conditions is poorly understood by far.
Using a computer-aided flight mill, the age-related flight activity and the relationship between flight and biotic and abiotic factors have been evaluated in a number of Lepidoptera insects, including Helivoverpa armigera (Hübner) (Wu & Guo, Reference Wu and Guo1998), Mythimna separata (Walker) (Jiang et al., Reference Jiang, Luo and Hu2000), and Spodoptera litura Hübner (Tu et al., Reference Tu, Wu, Xue and Guo2008). In this study, the effect of biotic factors (i.e., age and gender) and abiotic factors (i.e., temperature and relative humidity) on flight activity of M. crassisigna were examined. This work will lead to a more comprehensive understanding of the flight and migration behavior of M. crassisigna, and will also help us to develop more effective management strategies against this pest.
Materials and methods
Collection and rearing of M. crassisigna
Macdunoughia crassisigna moths were collected using a searchlight trap from May to June 2014 at the Beihuang Experimental Station of Chinese Academy of Agricultural Sciences (38°24′N, 120°55′E), Shandong Province, China. Beihuang is a small isolated island located in the center of the Bohai Strait, ≈40 km from mainland China to the north and ≈60 km to the south (Fu et al., Reference Fu, Hu, Feng, Liu and Wu2015). A laboratory colony was established and maintained in a growth chamber at 24 ± 1°C, 75 ± 5% relative humidity (RH) and a 16:8 h (L: D) photoperiod. Larvae were reared individually in glass vials (2.5 cm in diameter and 10 cm high) on an artificial diet described by Chen et al. (Reference Chen, Li and Pang2000). After pupation, male and female pupae were separated by reference to Li et al. (Reference Li, Wang and Su1987) and placed in plastic jars (8 cm in diameter and 12 cm high, one individual to a jar) until moth emergence, ensuring a supply of unmated moths of known age for use in the experiments described below. Moths had constant access to a 5% honey solution, which was set in the center of each jar. Moths were used only once.
Flight mill and tethered flight
A 24-channel, computer-monitored flight mill system (developed by the Institute of Plant Protection, CAAS, Beijing, China) was used to measure the flight parameters of M. crassisigna moths. Individual moths were anesthetized with ether for 15–20 s and then attached by the tergal side of the mesothorax to the end of the flight mill arm using a 30 cm long copper wire 0.2 cm in diameter, adhered with a drop of cyanoacrylate Super Glue (Yuyao Kexing Adhesive Co., Zhejiang, China). The flight mill system was positioned in a climate controlled room. The temperature could be controlled over a range from 10 to 50°C (±1.5°C) and RH over a range from 30 to 95% (±5%). Both temperature and RH were regulated automatically to set levels.
Flight ability test over a 24 h scotophase
We conducted three different assays in the flight mill tests (table 1). In the first assay, the effect of age on the flight ability of M. crassisigna was examined with 1, 3, 5, 7, 9, and 11-day-old unmated male and female moths tested at 24°C and 75% RH.
Table 1. Combinations of different biotic and abiotic conditions in assays with M. crassisigna.

Subsequently, based on findings from these age-specific flight assays, the effect of temperature on flight ability of M. crassisigna was tested with 3-day-old unmated male and female moths at each of six constant temperatures (12, 16, 24, 28 and 32°C) at 75% RH.
Finally, the effect of RH on flight ability of M. crassisigna was tested with 3-day-old unmated male and female moths at each of six constant RH levels (30, 45, 60, 75, 90 and 100%) at 24°C. Fifty females and fifty males were tested for each treatment, and only those moths still alive at the end of the test were used in analysis. Each test was started at 18:00 (UTC + 8) for a 24 h scotophase.
Computation of flight parameters
Flight data included the time-of-flight initiation, time of cessation, and the number of mill revolutions every 2 s during the assay period recorded by the software system. When flight stopped for ≥1 min, it was considered the end of a flight bout. Based on the number of mill revolutions over a given time period, total flight distance and duration, average flight speed, flight distance and duration in each flight bout were computed for each individual using a custom-made software package. A Gaussian function ![]() $f(X;A,{\rm \mu}, {\rm \delta} ) = (A/{\rm \delta} \sqrt {2{\rm \pi}} )e^{ - (X - {\rm \mu} )^2 /2{\rm \delta} ^2} $ was used to examine changes in flight parameters, where f (X; A, μ, δ) is the flight parameter, A is the area under the curve, μ is the mean of the distribution, and δ is the SD.
$f(X;A,{\rm \mu}, {\rm \delta} ) = (A/{\rm \delta} \sqrt {2{\rm \pi}} )e^{ - (X - {\rm \mu} )^2 /2{\rm \delta} ^2} $ was used to examine changes in flight parameters, where f (X; A, μ, δ) is the flight parameter, A is the area under the curve, μ is the mean of the distribution, and δ is the SD.
Data analysis
Flight parameters were first analyzed using a two-way analysis of variance (ANOVA), with age and temperature, or RH and sex as factors. If there were no significant interactions for a certain flight parameter, then the data of each sex were analyzed using a one-way ANOVA followed by a Tukey's HSD (honestly significant difference). Flight data were log-transformed before analyses to meet assumptions of normality and homogeneity for parametric analysis. All statistical analyses were performed with Data Processing System (DPS) V9.5 software (Tang, Reference Tang2010; Tang & Zhang, Reference Tang and Zhang2013).
Results
Effect of age on flight ability
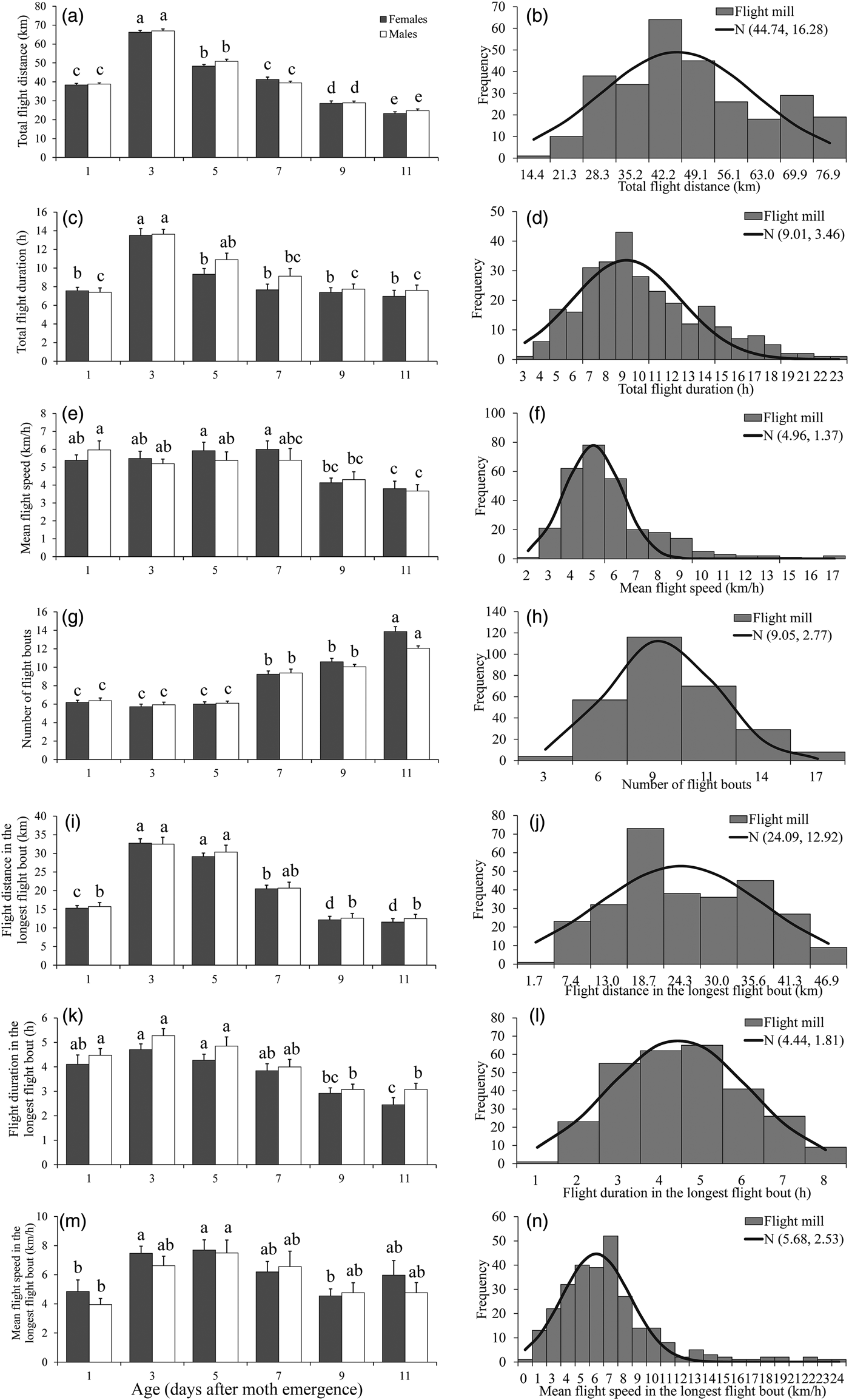
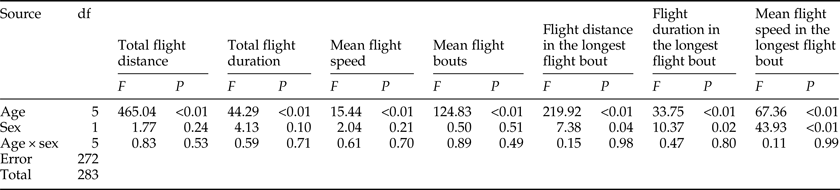
The results of the ANOVA associated with different flight parameters were presented in table 2, which showed that all of the flight parameters significantly differed between various ages. For both sexes, the total flight distance and total flight duration were highest in 3-day-old individuals both throughout the 24 h scotophase (fig. 1a, c). Furthermore, the number of flight bouts increased as a function of age for both sexes (fig. 1g).
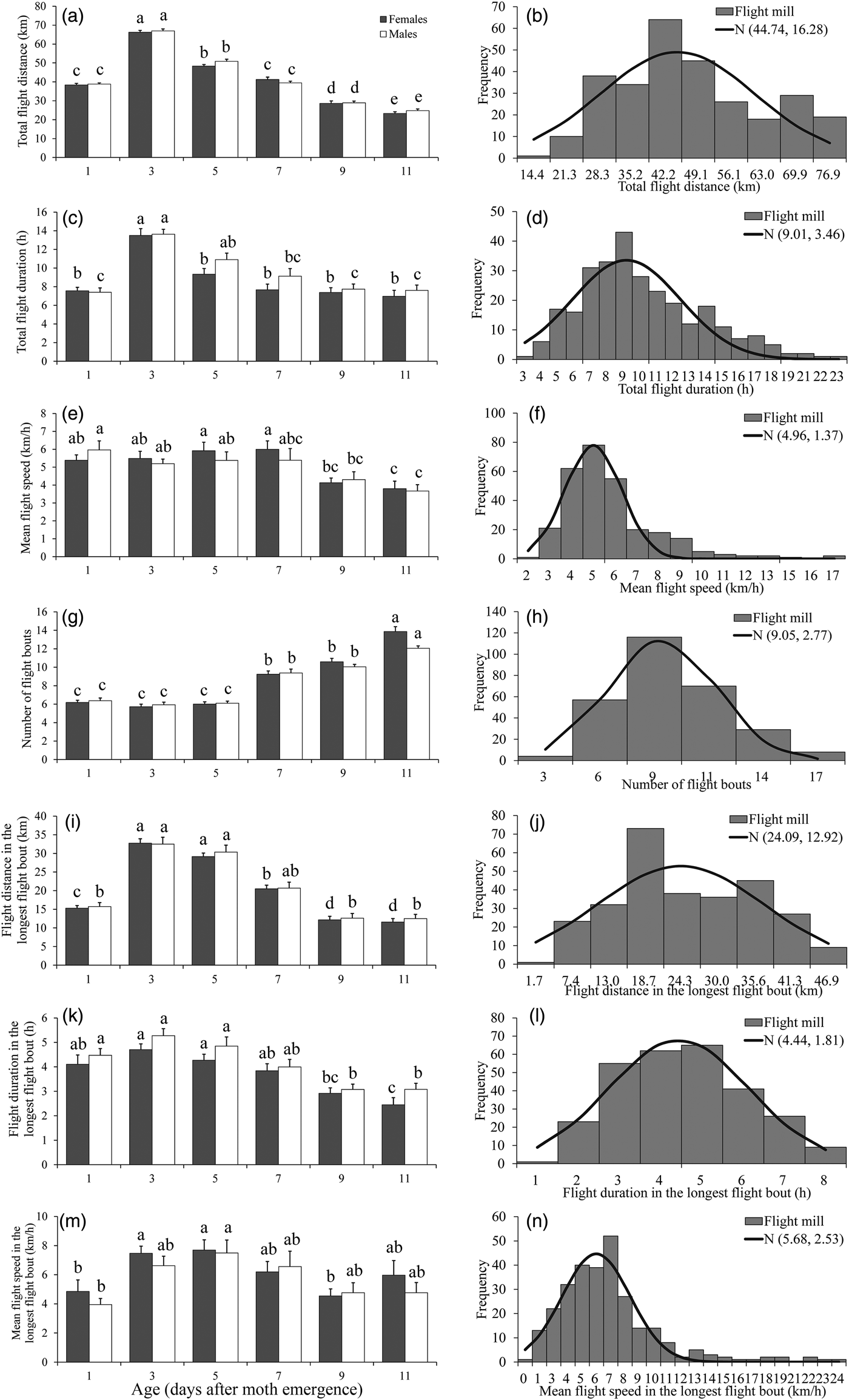
Fig. 1. Flight mill performance as a function with age (left), and the fitted normal distribution (right, n = 284) for M. crassisigna moths at 24°C and 75% RH during a 24 h scotophase. Each bar is the mean and vertical line represents the Standard Error of Mean in the left pictures. For a given sex associated with each age, bars with different letters are significantly different based on Tukey's HSD test (P < 0.05). The same are in figs 2 and 3 below.
Table 2. Two-way ANOVA analysis on the flight parameters of M. crassisigna as a function of age and sex.
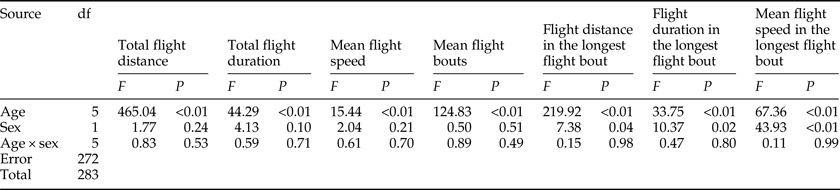
There was no significant difference between sexes in the flight parameters throughout the 24 h scotophase and in the longest flight bout (table 2). Furthermore, no significant age × sex interaction was found in all flight parameters both throughout the 24 h scotophase and in the longest flight bout (table 2).
The fitted normal distributions of flight parameters at six ages showed that the total flight distance, the total flight duration, the mean flight speed, and the mean number of flight bouts throughout the 24 h scotophase was 44.74 ± 16.28 km (fig. 1b), 9.01 ± 3.46 h (fig. 1d), 4.96 ± 1.37 km h−1 (fig. 1f), and 9.05 ± 2.77 (fig. 1h), respectively. While, the mean flight distance, mean flight duration, and mean flight speed in the longest flight bout was 24.09 ± 12.92 km (fig. 1j), 4.44 ± 1.81 h (fig. 1l), and 5.68 ± 2.53 km h−1 (fig. 1n), respectively.
Effect of temperature on flight ability
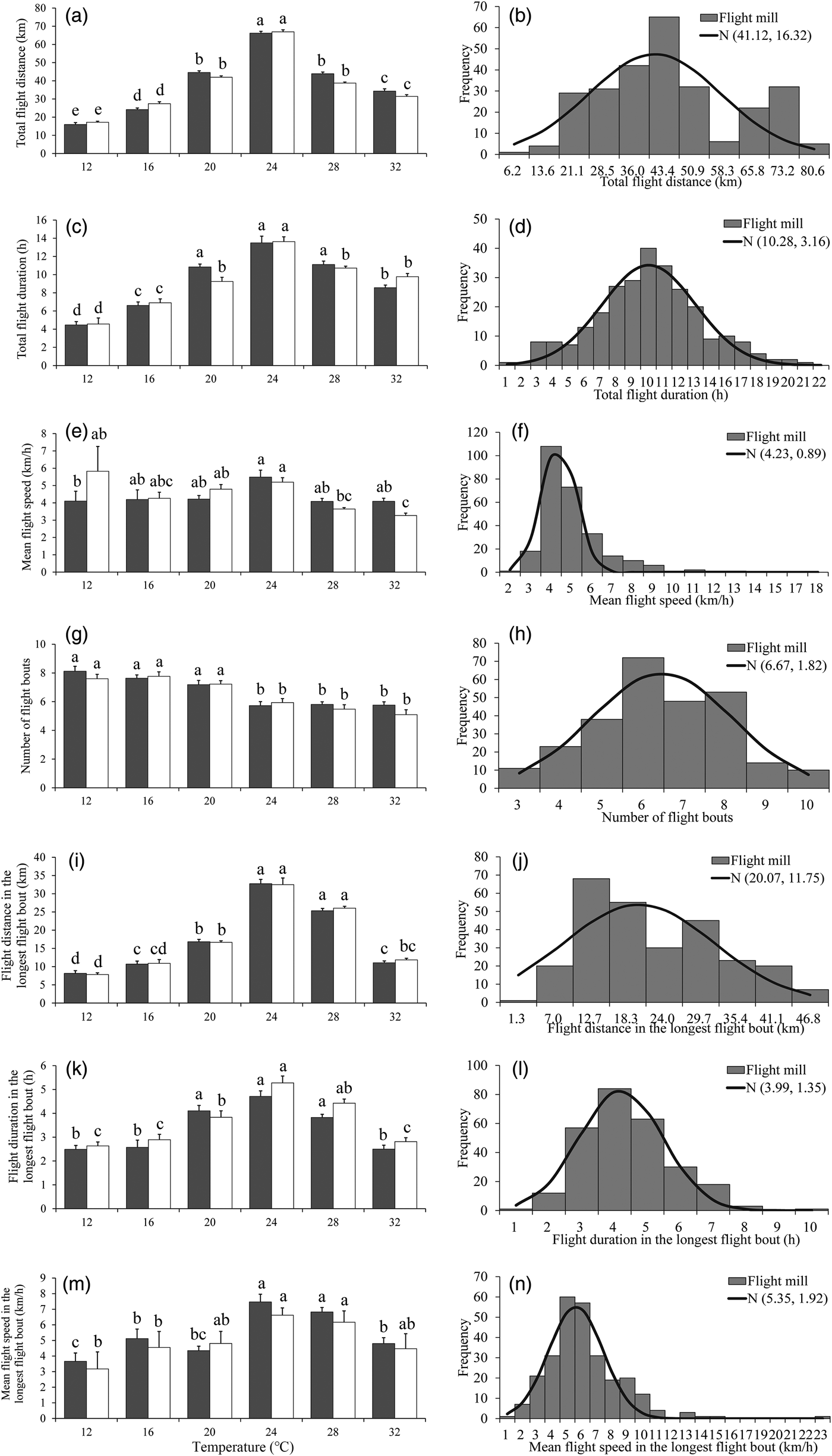
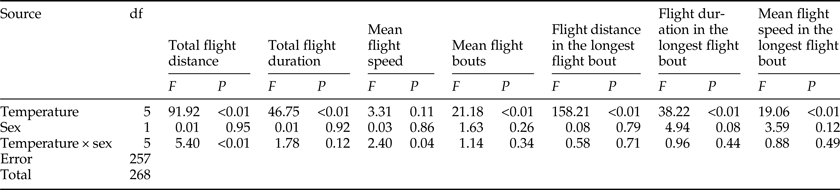
There was a significant effect of temperature on all flight parameters, except for the mean flight speeds throughout the 24 h scotophase (table 3). For both sexes, the flight activity was relatively higher at temperatures of 24–28°C than other temperatures throughout the 24 h scotophase (fig. 2a, c, e) and in the longest flight bout (fig. 2i, k, m). Furthermore, the number of flight bouts decreased as a function of temperature for both sexes (fig. 2g).
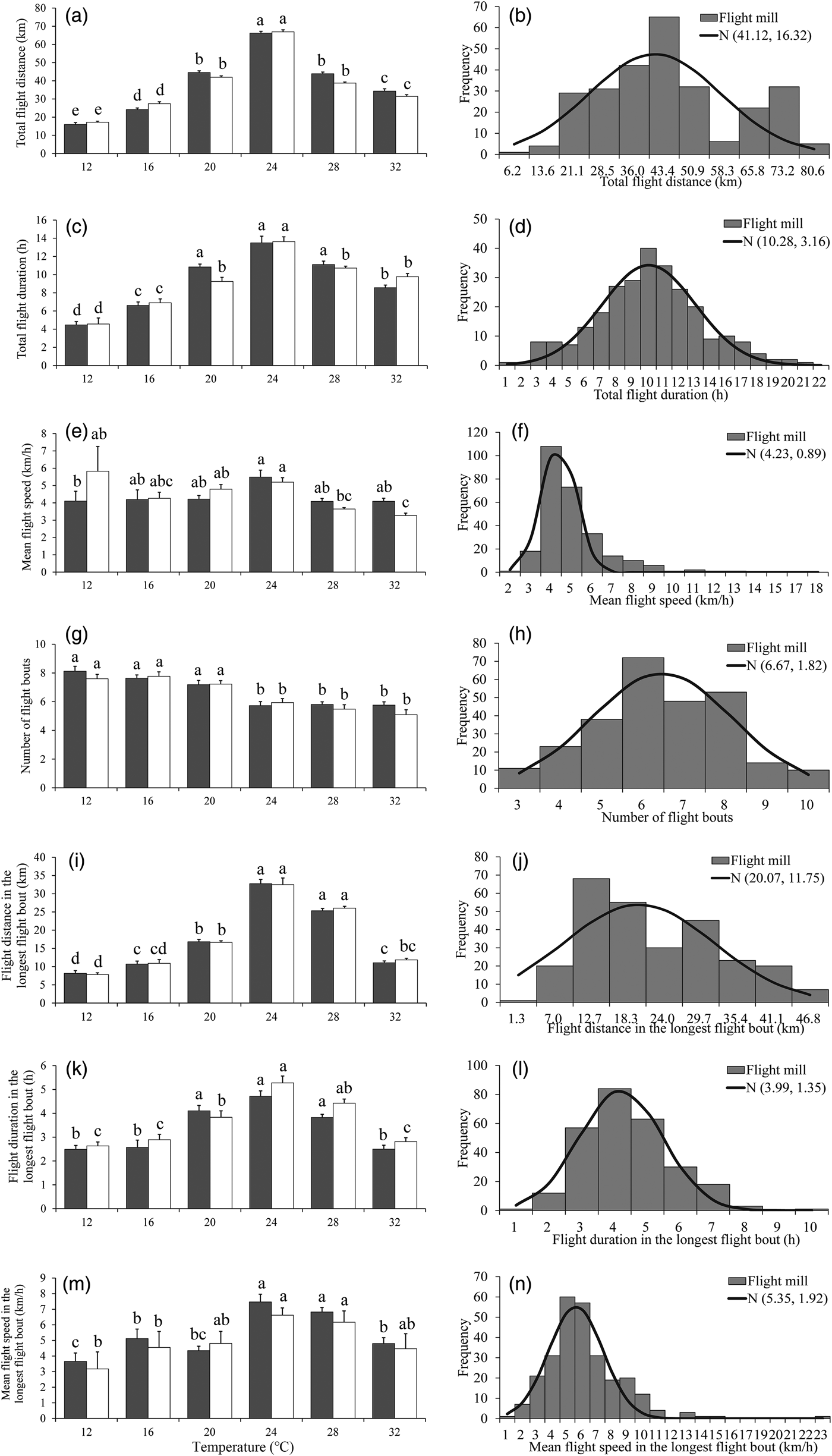
Fig. 2. Flight mill performance as a function with temperature (left), and the fitted normal distribution (right, n = 269) for M. crassisigna moths at 75% RH during a 24 h scotophase.
Table 3. Two-way ANOVA analysis on the flight parameters of M. crassisigna as a function of temperature and sex.
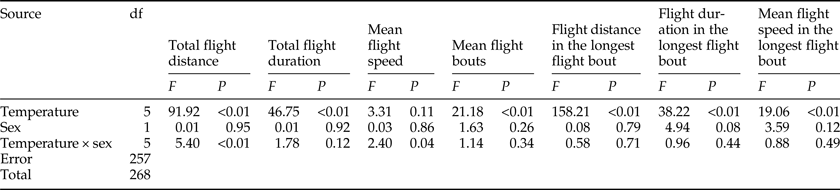
For 3-day-old M. crassisigna moths at six continuous temperatures, there was no significant difference between sexes in all flight parameters both throughout the 24 h scotophase and in the longest flight bout (table 3). Furthermore, no significant temperature × sex interaction was found in all flight parameters, except for the total flight distance and the mean flight speed throughout the 24 h scotophase (table 3).
The fitted normal distributions of flight parameters of 3-day-old moths at six temperatures showed that the total flight distance, total flight duration, mean flight speed, and mean number of flight bouts was 41.12 ± 16.32 km (fig. 2b), 10.28 ± 3.16 h (fig. 2d), 4.23 ± 0.89 km h−1 (fig. 2f), and 6.67 ± 1.82 (fig. 2h), respectively. Meanwhile, the mean flight distance, mean flight duration, and mean flight speed in the longest flight bout was 20.07 ± 11.75 km (fig. 2j), 3.99 ± 1.35 h (fig. 2l), and 5.35 ± 1.92 km h−1 (fig. 2n).
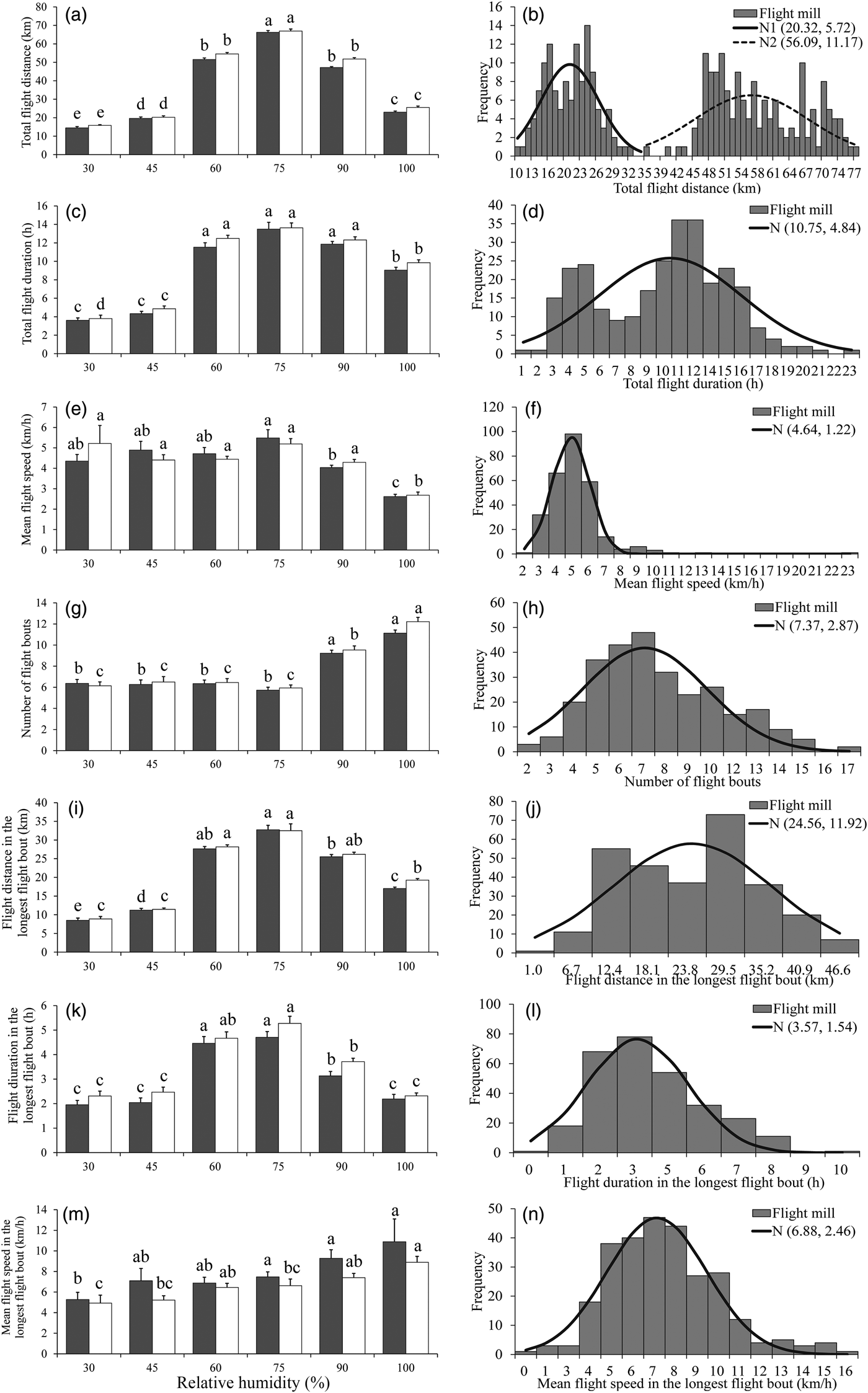
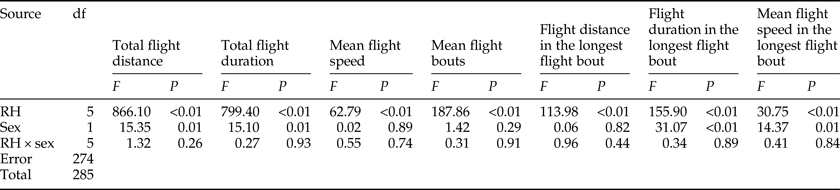
Effect of RH on flight ability
There was a significant effect of RH on all flight parameters (table 4). For both sexes, the flight activity was relatively higher at RH of 60–75% than other RH levels throughout the 24 h scotophase (fig. 3a, c, e) and in the longest flight bout (fig. 3i, k, m). Furthermore, the number of flight bouts increased as a function of temperature for both sexes (fig. 3g).
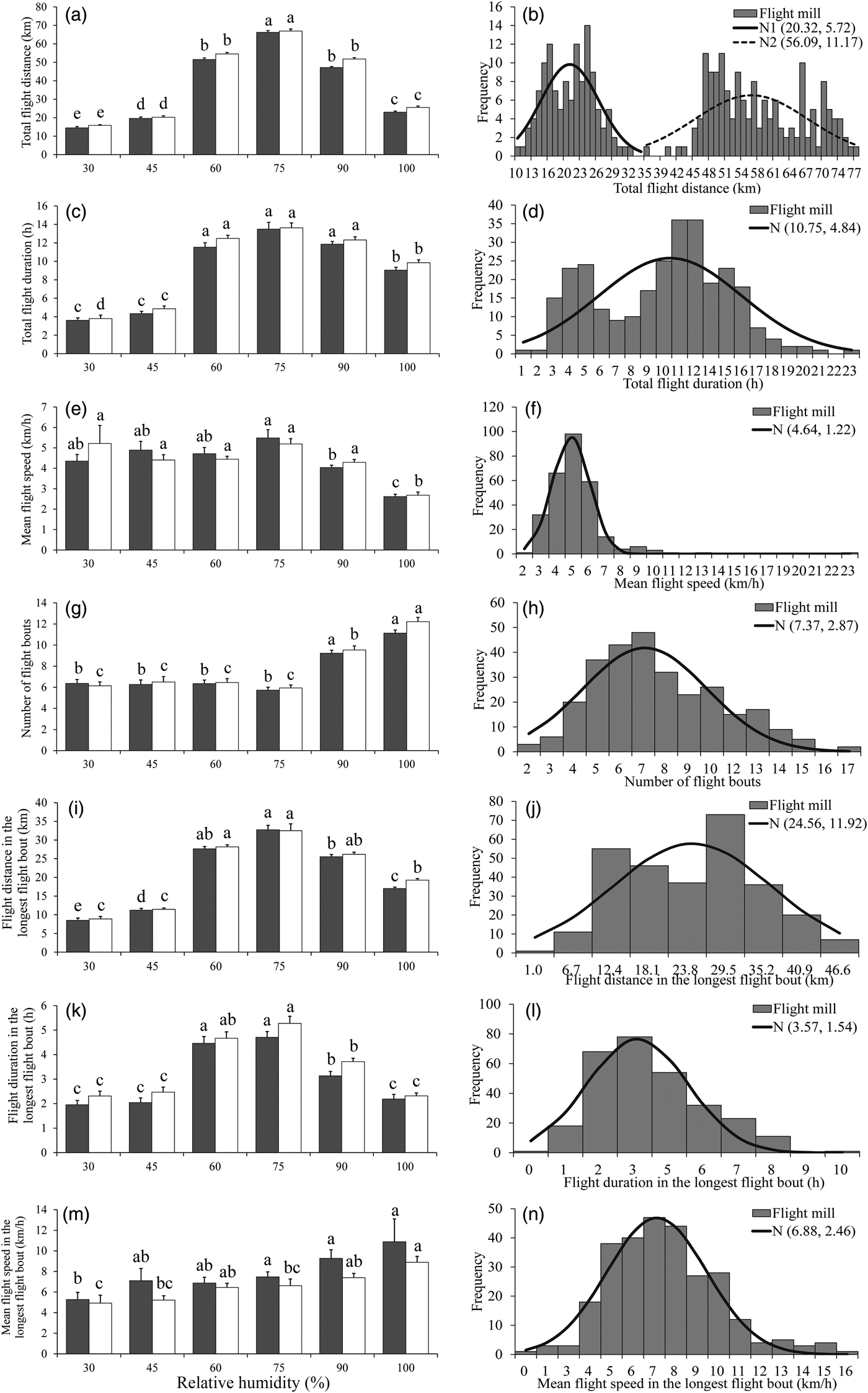
Fig. 3. Flight mill performance as a function with RH (left), and the fitted normal distribution (right, n = 286) for M. crassisigna moths at 24°C during a 24 h scotophase.
Table 4. Two-way ANOVA analysis on the flight parameters of M. crassisigna as a function of RH and sex.
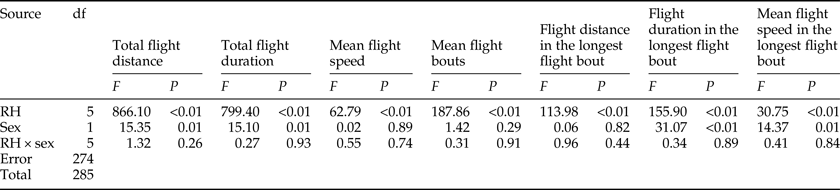
For 3-day-old M. crassisigna moths at six RH levels, there was a significant difference between sexes in the total flight distance and total flight duration throughout the 24 h scotophase, as well as the mean flight duration and speeds in the longest flight bout, while other flight parameters were not significantly differed between sexes (table 4). Furthermore, no significant relative humidity × sex interaction was found in all flight parameters both throughout the 24 h scotophase and in the longest flight bout (table 4).
The fitted normal distributions of flight parameter of 3-day-old moths at six RH levels showed the total flight distance (double unimodal) was 20.32 ± 5.72 and 56.09 ± 11.17 km, respectively (fig. 3b), the total flight duration was 10.75 ± 4.84 h (fig. 3d), the mean flight speed was 4.64 ± 1.22 km h−1 (fig. 3f) and the mean number of flight bouts throughout 24 h scotophase was 7.37 ± 2.87 (fig. 3h). In the longest flight bout, the mean flight distance, the mean flight duration, and the mean flight speed was 24.56 ± 11.92 km (fig. 3j), 3.57 ± 1.54 h (fig. 3l), and 6.88 ± 2.46 km h−1 (fig. 3n), respectively.
Discussion
We examined the effects of various biotic and abiotic factors on the flight performance of M. crassisigna moths of different ages, under different temperatures and RH levels by a flight-mill system. The results showed that the flight activity of this species is greatly affected by age, sex, temperature, and relative humidity.
This study confirms that the strong fliers of M. crassisigna have the potential for long-distance migration from a very early age, with 3-day-old males and females flying an average distance of 66.93 ± 1.11 and 66.19 ± 1.01 km throughout the 24 h successive assay. Although flights of this distance did not occur often, they may be frequent enough to contribute to the trans-regional migration of M. crassisigna, especially considering the tremendous numbers of this species that build up in local populations. These findings are similar to the results of flight mill tests with other Lepidoptera, including the black cutworm, Agrotis ypsilon (Rottemberg) (Jia & Cao, Reference Jia and Cao1992), the cotton bollworm, H. armigera (Armes & Cooter, Reference Armes and Cooter1991; Wu & Guo, Reference Wu and Guo1996), the oriental armyworm, M. separata (Luo et al., Reference Luo, Jiang, Li and Hu1999), and the beet armyworm, S. exigua (Jiang et al., Reference Jiang, Luo and Hu1999, Reference Jiang, Luo, Li, Cao, Hu and Liu2002a, Reference Jiang, Lei and Zhangb). Our laboratory captured thousands of M. crassisigna moths annually using a searchlight trap placed on an isolated small island in the center of the Bohai Strait from 2003 to 2014, which indicated that this species migrates at least 40–60 km (and probably much greater distances) across the sea (Fu et al., Reference Fu, Hu, Feng, Liu and Wu2015). This study provides additional evidence that M. crassisigna possesses strong potential for long-distance migration.
Our results demonstrate that both sexes of M. crassisigna have the capacity for sustained flight and the flight ability was highest in 3 days after emergence, at a time when they are still sexually immature. Then, their flight ability decreased after this point as the moths aged 7–11 days old. These findings may helpful explaining why the vast majority of female M. crassisigna moths, which took cross-sea migration described above, were virgins with little or no ovarian development (i.e., younger individuals) (Fu et al., Reference Fu, Hu, Feng, Liu and Wu2015). The particular relationship between age and flight capacity has been attributed to species-specific temporal changes in physiological status (Saito, Reference Saito2000). An insect's flight ability likely decreases with age due to the gradual autolysis of flight muscles (Johnson, Reference Johnson1954) or structural changes within flight muscles (Blackmer et al., Reference Blackmer, Lindley and Byrne2005). Furthermore, previous studies have reported that moths could produce aggregation pheromones attract both sexes, and unmated individuals may maintain high flight activity until they find conspecific aggregations that contain potential mates (Jiang et al., Reference Jiang, Luo, Li, Cao, Hu and Liu2002a, Reference Jiang, Lei and Zhangb). However, this needs to be tested by comparing the flight activity of mated and unmated individuals, which merits further studies of M. crassisigna moths.
Environmental conditions, especially temperature and relative humidity, have a significant influence on the flight ability of insects (Jiang et al., Reference Jiang, Luo and Hu2000, Reference Jiang, Luo, Li, Cao, Hu and Liu2002a, Reference Jiang, Lei and Zhangb; Kroder et al., Reference Kroder, Samietz and Dorn2006; Lu et al., Reference Lu, Wu and Guo2007). Our research confirms that temperature and RH significantly affect the flight ability of M. crassisigna, with the strongest flight activity always occurred in 24–28°C, and 60–75% RH. These results were in agreement with the recorded optimum temperature and RH for other Lepidoptera, such as A. ypsilon (Jia & Cao, Reference Jia and Cao1992), H. armigera (Armes & Cooter, Reference Armes and Cooter1991; Wu & Guo, Reference Wu and Guo1996), M. separata (Luo et al., Reference Luo, Jiang, Li and Hu1999), and S. exigua (Jiang et al., Reference Jiang, Luo and Hu1999, Reference Jiang, Luo, Li, Cao, Hu and Liu2002a, Reference Jiang, Lei and Zhangb). Weak flight ability was found at higher temperatures (32°C) or lower RH levels (30 and 45%), which was likely due to water loss during tethered flight.
The flight potential of various moths has been determined using similar techniques to those employed in this study. The flight activity of anesthetized insects tethered on a flight mill is not expected to accurately reflect the natural flight activity of individuals under normal environmental conditions, and the limitations of such studies have been discussed previously (Armes & Cooter, Reference Armes and Cooter1991; Beerwinkle et al., Reference Beerwinkle, Lopez, Cheng, Lingren and Meola1995; Riley et al., Reference Riley, Downham and Cooter1997). However, the flight mill does allow for quantitative estimates of the relative flight capacity of species (Taylor et al., Reference Taylor, Nault, Styer and Cheng1992). For example, it has been used to examine insect flight activity as a function of age, sex, size, mating status, and physiological state (Colvin & Gatehouse, Reference Colvin and Gatehouse1993; Rankin et al., Reference Rankin, Hampoton and Summy1994; Lu et al., Reference Lu, Wu and Guo2007; Sarvary et al., Reference Sarvary, Bloem, Bloem, Carpenter, Hight and Dorn2008), as well as lipid utilization in flight (Williams & Robertson, Reference Williams and Robertson2008). Despite the potential shortcomings, this study offers some insights into the flight performance of M. crassisigna, which is helpful for understanding the flight behavior of this species.
Acknowledgements
The authors would like to thank Jianglong Guo and Xiao Wu from the Institute of Plant Protection, Chinese Academy of Agricultural Sciences, along with Yatao Zhou, Runfeng Li, Dandan Dong, and Huihui Chen from the College of Plant Protection, Henan Agricultural University, for their contributions in moth collecting and larval rearing. The authors would like to thank Dr Roy Van Driesche, University of Massachusetts, for his hard work to polish this paper. This research was supported by the Special Fund for Agro-scientific Research in the Public Interest of China (grant number 201403031).