Introduction
Brassicaceae is an economically important botanical family worldwide, with approximately 3.6 million hectares worldwide cultivated in 2011 (FAOSTAT, 2011). This plant family is important in most parts of Brazil, where its cultivation is shared by subsistence agriculture and large producers (Aragão et al., Reference Aragão, Feitosa, Moraes and Corrêa2008). However, great losses are typically expected because of frequent outbreaks of pests, notably the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). In recent years, this microlepidopteran has become the most destructive pest of Brassicaceae both in Brazil and globally (Yu & Nguyen, Reference Yu and Nguyen1992; Talekar & Shelton, Reference Talekar and Shelton1993; Shelton et al., Reference Shelton, Perez, Tang, Vandenberg, Sivapragasm, Loke, Hussan and Lim1997; Medeiros, Reference Medeiros2002; Liang et al., Reference Liang, Chen and Liu2003). Among the current control measures, chemical methods are the most widely adopted for this pest, with pyrethroid and organophosphate compounds most commonly used in the past (Castelo Branco & Medeiros, Reference Castelo Branco and Medeiros2001; Monnerat et al., Reference Monnerat, Leal-Bertioli, Bertioli, Butt and Bordat2004). However, due to the high selection pressure induced by the indiscriminate use of insecticides, coupled with the high genetic plasticity inherent to this species, P. xylostella has developed resistance to virtually all classes of insecticides, including those based on Bacillus thuringiensis Berliner, thus hindering its control (Shelton et al., Reference Shelton, Sances, Hawley, Tang, Boune, Jungers, Collins and Farias2000; Mota-Sanchez et al., Reference Mota-Sanchez, Bills, Whalon and Wheeler2002; Mohan & Gujar, Reference Mohan and Gujar2003; Sarfraz & Keddie, Reference Sarfraz and Keddie2005; Khaliq et al., Reference Khaliq, Attique and Sayyed2007; Zago et al., Reference Zago, Siqueira, Pereira, Picanço and Barros2013).
In November 2009, the use of chlorantraniliprole was released in Brazil for the control of P. xylostella. This insecticide belongs to a new chemical class, the anthranilic diamides, which are used to control almost all economically important species of Lepidoptera. Chlorantraniliprole acts through a novel mechanism, activating the ryanodine receptors associated with calcium channels in muscle fibres to stimulate the release of the internal calcium stores in muscle cells of the insect in a non-regulated manner, resulting in feeding cessation, lethargy, muscle paralysis, and ultimately death (Cordova et al., Reference Cordova, Benner, Sacher, Rauh, Sopa, Lahm, Selby, Stevenson, Flexner, Gutteridge, Rhoades, Wu, Smith and Tao2006; Lahm et al., Reference Lahm, Stevenson, Selby, Freudenberger, Cordova, Flexner, Bellin, Dubas, Smith, Hughes, Hollingshaus, Clark and Benner2007; Teixeira et al., Reference Teixeira, Gut, Wise and Isaacs2009). Furthermore, the compound presents a low toxicity to mammals, as demonstrated by an oral acute LD50 greater than 5000 mg kg−1 for mice, and a good selectivity with regard to non-target arthropods, making it suitable for use in integrated pest management programmes (Lahm et al., Reference Lahm, Stevenson, Selby, Freudenberger, Cordova, Flexner, Bellin, Dubas, Smith, Hughes, Hollingshaus, Clark and Benner2007; Brugger et al., Reference Brugger, Cole, Newman, Parker, Scholz, Suvagia, Walker and Hammond2010).
Recently, studies of susceptibility baselines in Brazil showed that populations of P. xylostella were very homogenous and susceptible to chlorantraniliprole (Silva et al., Reference Silva, Siqueira, Silva, Campos and Barros2012), proving this compound to be an excellent tool for managing the resistance already evolved to other insecticide classes. Nonetheless, high levels of resistance to chlorantraniliprole have recently been reported for P. xylostella populations from China (Wang & Wu, Reference Wang and Wu2012). The resistance to this diamide was characterized by Wang et al. (Reference Wang, Khakame, Ye, Yang and Wu2012), who found that resistance was partially recessive and instable, suggesting an associated fitness cost. Adaptive costs in resistant individuals may impair the establishment of resistance in the field in the absence of an insecticide (Gassmann et al., Reference Gassmann, Carrière and Tabashnik2009), even reverting the resistance depending on the nature of such costs.
After almost 2 years of chlorantraniliprole marketing, reports of control failures in areas of Northeast Brazil led us to survey populations for resistance. A P. xylostella population (Camocim de São Félix – PE) highly resistant to this diamide was established in the laboratory for further characterization. Because it showed a relatively high decrease in resistance in only three generations of pressure relaxation, this study aimed to evaluate the existence of a cost associated with such a high resistance level in this population, by subjecting susceptible and resistant individuals to the absence and presence of sublethal concentrations of chlorantraniliprole.
Materials and methods
Insect rearing
Two populations of P. xylostella, a standard population maintained in the laboratory since 1998 without any insecticide contact and one population recently collected in an area of Brassica spp. cropping in the municipality of Camocim de São Félix – PE in December 2011, where there were reports of control failure by diamide-based insecticides, were used in this study. The two populations were maintained individually in the Laboratory of Insect–Toxicant Interactions and fed collard green leaves (Brassica oleracea var. Acephala) without contact with insecticides. The insects collected in Camocim de São Félix – PE were tested at generation one and are herein regarded as the resistant population, which was known to be resistant to pyrethroids, insect growth regulators, methomyl, and B. thuringiensis var. aizawai.
Susceptibility bioassays
The concentration–response curves for chlorantraniliprole (Prêmio®20SC, DuPont Brasil Ltda) were established for both populations through bioassays and from which sublethal concentrations were calculated for use in the ensuing trials. Collard green leaf discs (5 cm in diameter) were washed in 5% sodium hypochlorite, thoroughly rinsed in tap water, and treated with increasing concentrations of chlorantraniliprole solution by immersion for 30 s. After drying at room temperature, the discs were transferred to Petri dishes (60×15 mm) containing a filter paper (5 cm) moistened with distilled water. A total of nine concentrations (and three replicates each) of the insecticide in distilled water containing the emulsifier Triton X-100 (0.01%) were evaluated. The concentrations used for the susceptible population were 0.0009, 0.0019, 0.0039, 0.0078, 0.0156, 0.0312, 0.0625, 0.125, and 0.25 mg l−1, and the concentrations used for the resistant population were 14.06, 28.12, 56.25, 112.5, 225, 450, 900; 1800, and 3600 mg l−1. The LC50 values obtained for the susceptible and resistant populations were 0.0073 and 204.32 mg l−1, respectively. The control comprised collard green leaves treated with distilled water plus Triton X-100 (0.01%). Newly hatched larvae were obtained from eggs laid on a sheet by adult female moths over the course of 24 h. The sheet was then transferred to a plastic pot without fresh cabbage leaves. After hatching (three days on average), the larvae that left the pots were captured with the help of a soft-bristle brush, lifted by the silk threads and transferred to the Petri dishes; ten newly hatched larvae (0–24 h) were transferred to each Petri dish. All bioassays were kept inside a growth chamber at 25±1 °C, a relative humidity of 60±10%, and a 12-h photoperiod. The mortality was evaluated after 96 h of exposure by touching the larvae with a fine brush; the larvae were considered dead if no movement was observed. The mortality data were corrected using the mortality from the control treatment (Abbott, Reference Abbott1925) and subjected to a Probit analysis (Finney, Reference Finney1971) using the programme POLO-Plus (LeOra-Software, 2005). The resistance ratio and its 95% confidence interval were calculated according to the method described by Robertson et al. (Reference Robertson, Russell, Preisler and Savin2007).
Sublethal effects of chlorantraniliprole on P. xylostella biology
Collard green leaf discs (8 cm in diameter) were immersed for 30 s in chlorantraniliprole solution corresponding to the LC1, LC10, and LC25 values estimated for each population. The concentrations were 0.001, 0.002, and 0.004 mg l−1 for the susceptible population and 35, 78, and 123 mg l−1 for the resistant population. After drying, the leaf discs were transferred to Petri dishes containing filter paper moistened with distilled water. The control consisted of leaf discs treated with distilled water plus Triton X-100 (0.01%). For each Petri dish, 12 newly hatched larvae (0–24 h) were transferred, and each treatment consisted of 15 repetitions. After 96 h of exposure, the treated and untreated leaf discs were replaced daily with fresh leaves without insecticide until the larvae reached the pupal stage. The parameters evaluated were the daily larval survivorship, larval period, larval weight measured at 6 days (the period before the onset of pupae formation), pupal period, and viability. The pupae were weighed at 24 h after formation, transferred to acrylic test tubes, and observed until adult emergence. The emerged adults were sexed to determine the sex ratio, and couples were transferred to transparent, plastic cylindrical cages (12 cm in diameter×15 cm in height) with a side vent closed with voile fabric. Collard green leaf discs (8 cm in diameter) on filter paper were offered as a substrate for oviposition and replaced daily. The cages were closed at the bottom with a sponge soaked in water to maintain the humidity. The adults were offered cotton swabs soaked in a 10% honey solution. Ten replicates per treatment were performed, and the total number of eggs per female, hatching larvae, and adult longevity were evaluated. The experiment was conducted at 25±1 °C, 60±10% relative humidity, and a photoperiod of 12 h.
Data analysis
Data regarding the daily larval survivorship were compared by a log-rank test using the Kaplan–Meyer method. The other parameters were compared by a non-parametric analysis, as most of the data did not adhere to normality assumptions. The Kruskal–Wallis test was performed to assess the treatment effects within each population. When necessary, multiple comparison tests for pairwise comparisons were performed using the Wilcoxon test, followed by a sequential Bonferroni correction (Rice, Reference Rice1989). Comparisons between populations were performed using the Wilcoxon test (U test), adopting α=0.05 in all cases. All of the analyses were performed using SAS statistical software (SAS Institute, 2001).
Results
Concentration–response curves
The LC50 values estimated for the susceptible and resistant populations were 0.0073 mg l−1 and 204.32 mg l−1 chlorantraniliprole, respectively. The resistant population was highly resistant (resistance ratio=27,793 times) to chlorantraniliprole compared with the laboratory population (Table 1). The stability of resistance was evaluated from the first generation and drastically decreased up to the third generation (resistance ratio=4690 times) (Table 1), when the colony was accidentally lost. Regardless, the data suggest instability with regard to the resistance to chlorantraniliprole.
Table 1. Susceptibility of Plutella xylostella populations to chlorantraniliprole after 96 h exposure.
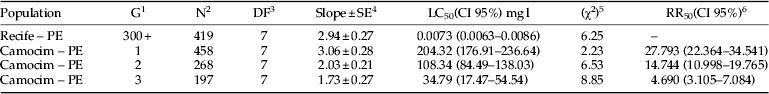
1 Generation tested.
2 Total number of treated insects.
3 Degree of freedom.
4 Standard error.
5 Chi-square test (P>0.05).
6 Resistance ratio: ratio of the LC50 estimative between the resistant and susceptible populations calculated through the ‘lethal ratio test’ (Robertson et al., Reference Robertson, Russell, Preisler and Savin2007).
Larval survivorship
The treatment with sublethal concentrations of chlorantraniliprole did not affect the survivorship of the susceptible population (log-rank test: χ2=6.07, DF=3, P=0.1082) (fig. 1A), and only the LC10 (log-rank test: χ2=11.59, DF=1, P=0.0007) and LC25 (log-rank test: χ2=14.51, DF=1, P=0.0001) treatments significantly reduced the survival of the resistant population compared with the control (fig. 1B). The control treatment showed a significantly higher survival rate of the resistant larvae than the control for the susceptible larvae (log-rank test: χ2=4.03, DF=1, P=0.0445), an outcome also observed for the LC1 treatment (log-rank test: χ2=5.00, DF=1, P=0.0253) (fig. 2). Survivorship at the highest concentrations, LC10 and LC25, did not differ between the populations (log-rank test: χ2=2.39, DF=1, P=0.1219 and χ2=0.24, DF=1, P=0.6217, respectively) (fig. 2).

Fig. 1. Larval survivorship of susceptible (A) and resistant (B) Plutella xylostella exposed to sublethal concentrations of chlorantraniliprole. Different letters means significance by the log-rank test (P<0.05). Temperature: 25±1 °C, RH: 60±10%, and 12 h photophase.

Fig. 2. Differential larval survivorship between susceptible and resistant Plutella xylostella exposed to sublethal concentrations of chlorantraniliprole. Different letters means significance by the log-rank test (P<0.05). Temperature: 25±1 °C, RH: 60±10%, and 12 h photophase.
Larval weight and period
Sublethal treatments with chlorantraniliprole reduced larval weight in both populations. The LC10 treatment (Wilcoxon test: adjusted α=0.01, χ2=10.33, DF=1, P=0.0013) and LC25 treatment (Wilcoxon test: adjusted α=0.0083, χ2=13.17, DF=1, P=0.0003) significantly reduced larval weight in the susceptible population when compared with the control though the larval weight did not differ between these LC values (Wilcoxon test: adjusted α=0.05, χ2=0.0004, DF=1, P=0.9835). LC1 did not differ from the control (Wilcoxon test: adjusted α=0.025, χ2=0.79, DF=1, P=0.37). Although all of the LC treatments led to a significant reduction in larval weight in the resistant population compared with the control (Wilcoxon test: adjusted α=0.0083, 0.01, 0.0125, respectively. LC1: χ2=15.04, DF=1, P=0.0001; LC10 and LC25: χ2=20.25, DF=1, P<0.0001), the larval weight of the resistant population at the LC10 and LC25 treatments were similar (Wilcoxon test: adjusted α=0.05, χ2=0.0017, DF=1, P=0.9669) (Table 2). The larvae from the resistant population showed lower weights (Wilcoxon test: Controls: χ2=10.06, DF=1, P=0.0015; LC1 values: χ2=20.25, DF=1, P<0.0001; LC10 values: χ2=18.78, DF=1, P<0.0001; LC25 values: χ2=15.20, DF=1, P<0.0001) and longer periods of development (Wilcoxon test: Controls: χ2=13.99, DF=1, P=0.0002; LC1 values: χ2=15.07, DF=1, P=0.0001; LC10 values: χ2=15.73, DF=1, P<0.0001; LC25 values: χ2=17.92, DF=1, P<0.0001) compared with the susceptible population in all of the treatments (Table 2). Regarding the larval period, no significant differences were observed between the treatments LC1 and LC10 in the susceptible population (Wilcoxon test: adjusted α=0.025, χ2=1.45, DF=1, P=0.2284) as well as between LC10 and LC25 treatments (Wilcoxon test: adjusted α=0.05, χ2=0.72, DF=1, P=0.3946). Only the LC1 and LC25 treatments significantly differed (Wilcoxon test: adjusted α=0.0125, χ2=6.86, DF=1, P=0.0088), whereas LC10 (Wilcoxon test: adjusted α=0.0166, χ2=15.56, DF=1, P<0.0001) and LC25 (Wilcoxon test: adjusted α=0.025, χ2=13.80, DF=1, P=0.0002) significantly differed from LC1 in the resistant population. The larval periods for the LC1 (Wilcoxon test: adjusted α=0.0166, χ2=6.04, DF=1, P=0.0139), LC10 (Wilcoxon test: adjusted α=0.01, χ2=11.46, DF=1, P=0.0007) and LC25 treatments (Wilcoxon test: adjusted α=0.0083, χ2=17.96, DF=1, P<0.0001) differed significantly from the control treatment in the susceptible population as well as in the resistant population (Wilcoxon test: adjusted α=0.0083, 0.01, 0.0125, respectively. LC1: χ2=16.89, DF=1, P<0.0001; LC10 and LC25: χ2=21.41, DF=1, P<0.0001) (Table 2).
Table 2. Medians (ML–MU) of larvae weight and larval period of susceptible and resistant Plutella xylostella exposed to sublethal concentrations of chlorantraniliprole.

1 Values with different letters within column are significantly different based on Kruskal–Wallis test followed by Wilcoxon test and Bonferroni correction.
* Differs statistically between populations by Wilcoxon test (P<0.05).
Pupae weight, period, and viability
The weights of the pupae from the larvae treated with the insecticide did not differ significantly from the control weight within each population (Kruskal–Wallis test: susceptible: χ2=0.97, DF=3, P=0.80; resistant: χ2=4.19, DF=3, P=0.24) (Table 3). Although no differences were observed for the weight between the control of both the susceptible and resistant populations (Wilcoxon test: χ2=2.55, DF=1, P=0.1102), the resistant pupae weighed on average less than the susceptible pupae at LC1 (Wilcoxon test: χ2=6.29, DF=1, P=0.0121), LC10 (χ2=4.04, DF=1, P=0.0442), and LC25 (χ2=4.74, DF=1, P=0.0294) (Table 3). None of the treatments significantly affected the pupal period of the susceptible population by Kruskal–Wallis test (χ2=2.91, DF=3, P=0.4045) or the resistant population (Wilcoxon test: adjusted α=0.0083 for all pairwise comparisons). However, the duration of pupal development in the resistant population was longer than for the susceptible pupae, both between the controls (χ2=21.13, DF=1, P<0.0001) and between the concentrations evaluated (Wilcoxon test: LC1 values: χ2=16.78, DF=1, P<0.0001; LC10 values: χ2=20.52, DF=1, P<0.0001; LC25 values: χ2=20.84, DF=1, P<0.0001) (Table 3). There was no difference in the pupal viability among the treatments (Kruskal–Wallis test: susceptible: χ2=1.50, DF=3, P=0.6805; resistant: χ2=1.69, DF=3, P=0.6372) or between the populations (Wilcoxon test: Controls: χ2=0.99, DF=1, P=0.3183; LC1 values: χ2=0.14, DF=1, P=0.7011; LC10 values: χ2=1.16, DF=1, P=0.2798; LC25 values: χ2=2.47, DF=1, P=0.1159) (Table 3).
Table 3. Medians (ML–MU) of pupal period, pupae weight and viability of susceptible and resistant Plutella xylostella populations from larvae exposed to sublethal concentrations of chlorantraniliprole.

1 Values followed by the same letter within column are not significant different by the Kruskal–Wallis test (P>0.05).
* Differs statistically between populations by Wilcoxon test (P<0.05).
Sex ratio, oviposition period, fecundity, fertility, and longevity of adults
The sex ratio was not affected by the treatments (Kruskal–Wallis test: susceptible: χ2=0.59, DF=3, P=0.8984; resistant: χ2=1.33, DF=3, P=0.7214) and did not differ between the populations (Wilcoxon test: Controls: χ2=1.05, DF=1, P=0.3037; LC1 values: χ2=0.34, DF=1, P=0.5576; LC10 values: χ2=0.3893, DF=1, P=0.5326; LC25 values: χ2=0.0981, DF=1, P=0.7541) (Table 4). The LC25 treatment increased the oviposition period of the resistant population compared with the susceptible population (Wilcoxon test: χ2=4.37, DF=1, P=0.0364) (Table 4), although the total number of eggs per female was not significantly different among the treatments for each population (Kruskal–Wallis test: susceptible: χ2=5.51, DF=3, P=0.1376; resistant: χ2=1.66, DF=3, P=0.6445). The number of eggs laid in the control treatments was significantly lower for the resistant than for the susceptible females (Wilcoxon test: χ2=4.54, DF=1, P=0.0330) (Table 4). The percentage of eggs hatching did not differ among the treatments for each population (Kruskal–Wallis test: susceptible: χ2=1.49, DF=3, P=0.6834; resistant: χ2=2.53, DF=3, P=0.4693) yet did differ between the control treatments (Wilcoxon test: χ2=5.75, DF=1, P=0.0164), being significantly higher for the resistant population (Table 4). The chlorantraniliprole treatments significantly affected the longevity of the resistant male moths, which was smaller than the longevity of the control treatment (Wilcoxon test: adjusted α=0.0125, 0.01, 0.0083, respectively. LC1: χ2=7.04, DF=1, P=0.0079; LC10: χ2=10.38, DF=1, P=0.0013 and LC25: χ2=12.97, DF=1, P=0.0003) (Table 5). The resistant male insects exhibited a significantly greater longevity in comparison with the susceptible insects for the control (Wilcoxon test: χ2=3.70, DF=1, P=0.0543) and LC1 treatments (Wilcoxon test: χ2=6.05, DF=1, P=0.0139), whereas the resistant females showed a higher longevity than the susceptible females when exposed to LC25 (Wilcoxon test: χ2=5.01, DF=1, P=0.0251) (Table 5).
Table 4. Medians (ML–MU) of sexual ratio, egg-laying period, fecundity, and hatchability of susceptible and resistant Plutella xylostella populations from larvae exposed to sublethal concentrations of chlorantraniliprole.

1 Values followed by the same letter within column are not significant different by the Kruskal–Wallis test (P>0.05).
* Differs statistically between populations by Wilcoxon test (P<0.05).
Table 5. Medians (ML–MU) of adult male and female longevity of susceptible and resistant Plutella xylostella populations from larvae exposed to sublethal concentrations of chlorantraniliprole.
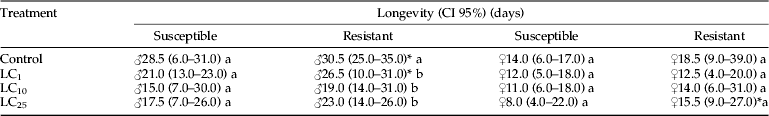
1 Values with different letters within column are significantly different based on Kruskal–Wallis test followed by Wilcoxon test and Bonferroni correction.
* Differs statistically between populations by Wilcoxon test (P<0.05).
Discussion
Resistance is a major threat to the lifespan of an insecticide, particularly when used against P. xylostella, an insect that exhibits extensive plasticity. Despite the short commercial use of chlorantraniliprole in Brazil, resistance has built up very rapidly in P. xylostella field populations to alarmingly high levels of resistance (27,703-fold in this case), particularly in areas of Northeast Brazil and in association with control failure. These levels of resistance were much higher than those reported by other recent works (Troczka et al., Reference Troczka, Zimmer, Elias, Schorn, Bass, Emyr Davies, Field, Williamson, Slater and Nauen2012; Wang & Wu, Reference Wang and Wu2012; Wang et al., Reference Wang, Khakame, Ye, Yang and Wu2012), suggesting a very rapid evolution in the field because of the misuse of chlorantraniliprole, reducing its efficacy. Since its release, chlorantraniliprole has been virtually the only insecticide used by growers to treat their fields, imposing a high selection pressure in most areas of Brassica cultivation. Despite the previous resistance to pyrethroids, insect growth regulators, abamectin, indoxacarb (Oliveira et al., Reference Oliveira, Siqueira, Oliveira, Silva and Michereff Filho2011; Santos et al., Reference Santos, Siqueira, Silva and Farias2011), and B. thuringiensis (Zago et al., Reference Zago, Siqueira, Pereira, Picanço and Barros2013), such reliance on chlorantraniliprole was due to its high efficacy (Silva et al., Reference Silva, Siqueira, Silva, Campos and Barros2012). Although no mechanisms of resistance have been elucidated in this population, Troczka et al. (Reference Troczka, Zimmer, Elias, Schorn, Bass, Emyr Davies, Field, Williamson, Slater and Nauen2012) showed that a single mutation in the P. xylostella ryanodine receptor is the major factor associated with the resistance to diamides. High levels of resistance are usually associated with target site alterations and are unstable in many cases, which is likely to be associated with the resistance to diamides in the Brazilian populations. Indeed, we found that resistance was not stable, sharply decreasing to lower values and suggesting a resistance-associated fitness cost. This hypothesis was supported by the increase in the larval cycle and weight reduction in the resistant insects in the absence of chlorantraniliprole and at the LC1 exposure. The same pattern of reduction was observed for pupal weight, particularly when comparing the susceptible and resistant insects in the presence of the insecticide, suggesting a higher impact on the development of the resistant insects under exposure.
The elongation of the larval stage and reduced weight were more impacting on resistant than susceptible insects at all of the concentrations, despite of the significance observed among treatments for the susceptible insects. These results, together with those for survival and longevity, indicate that the resistant population shows a higher sensitivity to sublethal effects than the susceptible population. Such alterations of the larval period and weight were also reported in the larvae of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) (Xu et al., Reference Xu, Xu, Xu and Gu2010; Lai & Su, Reference Lai and Su2011). Han et al. (Reference Han, Zhang, Shen, Liu, Ren and Gao2012) observed several sublethal effects of chlorantraniliprole on P. xylostella after treatment with LC10 and LC25 values, including a decreased pupation rate, pupal weight, adult emergence, fecundity, and egg hatching and an extended period of pre-oviposition, although a few differences were observed in the present work. The discrepancies between our study and that of Han and collaborators are potentially associated with the age of larvae used in the experiment – they used third-instar larvae, whereas we used neonate larvae, which possibly allowed the recovery of the insects during their development (Lai & Su, Reference Lai and Su2011). Han et al. (Reference Han, Zhang, Shen, Liu, Ren and Gao2012) and Lai & Su (Reference Lai and Su2011) found no significant effect of chlorantraniliprole on the longevity of P. xylostella and S. exigua adults, respectively, when the larvae were treated with sublethal concentrations of this insecticide. A similar result was observed in the susceptible population, although the males from the resistant population showed significantly reduced longevity. This finding suggests a higher adaptive cost of resistant insects to sublethal concentrations of chlorantraniliprole and a differential effect on both sexes. Differential responses of sexes were also observed for mating behaviour of Cydia pomonella L. (Knight & Flexner, Reference Knight and Flexner2007), since males was more affected by chlorantraniliprole exposure than females.
The lower larval weight, longer period of pupae and larvae, and reduced fecundity of the resistant individuals in the absence of chlorantraniliprole suggest an increased fitness cost in these individuals, which is commonly associated with insecticide resistance (Jia et al., Reference Jia, Liu, Zhu, Liu, Gao and Shen2009; Yu-ping et al., Reference Yu-ping, Yong-yue, Ling and Guang-wen2010; Sun et al., Reference Sun, Liang and Gao2012). However, positive traits were also observed, such as an increased larval survival, egg hatchability, and male longevity, suggesting a possible physiological mechanism of compensation (Yin et al., Reference Yin, Wu, Li, Zhang and Xu2009). The increased longevity of males may suggest an advantage to resistant individuals by increasing their chance of mating and copulation and fertilization ability, even though these aspects are affected by resistance in some cases (Alyokhin & Ferro, Reference Alyokhin and Ferro1999; Wyss et al., Reference Wyss, Young, Shukla and Roe2003). Although such effects as reduced mating success in males resistant to B. thuringiensis have been addressed for P. xylostella by Groeters et al. (Reference Groeters, Tabashnik, Finson and Johnson1993), the effect of chlorantraniliprole on these traits requires further attention.
The P. xylostella females that were resistant to chlorantraniliprole presented significantly longer periods of oviposition at the highest concentration evaluated; however, this aspect is apparently not relevant in this case because it was not followed by increased fecundity (the total number of eggs laid per female did not differ from the susceptible population). Indeed, previous works have shown that prior exposure or the expression of resistance genes may not have an impact on fitness but can also increase or decrease the fitness of insects (Fournier et al., Reference Fournier, Pralavorio, Coulon and Berge1988; Haubruge & Arnaud, Reference Haubruge and Arnaud2001; James & Price, Reference James and Price2002; Ako et al., Reference Ako, Borgemeister, Poehling, Elbert and Nauen2004). In the present work, the biology of both the susceptible and resistant populations was impacted due to the exposure to chlorantraniliprole and was most intense in the resistant population, most likely because of the lower biological characteristics shown by comparing both populations not exposed to the insecticide. Han et al. (Reference Han, Zhang, Shen, Liu, Ren and Gao2012) found that the exposure to sublethal concentrations of chlorantraniliprole affects the population dynamics of susceptible P. xylostella by reducing the growth of the next generation, regardless of exposure. Although this hypothesis was addressed in this study, it is likely that the same outcome may occur with the resistant population and in a more intense manner due to the biological characteristics observed.
The alleles that confer resistance are generally associated with negative effects on the fitness of pests in the absence of the insecticide, and these alleles typically become rare in populations in the absence of selection pressure (Hoffmann & Parsons, Reference Hoffmann and Parsons1991; Hollingsworth et al., Reference Hollingsworth, Tabashnik, Johnson, Messing and Ullman1997). Tabashnik et al. (Reference Tabashnik, Finson, Groeters, Moar, Johnson, Luo and Adang1994) showed that a population of P. xylostella highly resistant to Bt was rapidly reversed in the absence of insecticide and that the loss of resistance was associated with an increased fitness cost. Despite the very few generations assessed in the present study, the reversion of resistance to chlorantraniliprole was observed and appears to be associated with reduced P. xylostella fitness. Moreover, the resistance-associated fitness costs appeared to be inherent to the individuals in the presence and absence of chlorantraniliprole, which also exerted negative effects on the biological parameters of the susceptible individuals at a sublethal exposure, which was more pronounced in the resistant insects. However, the resistance to chlorantraniliprole did not consistently cause a disadvantage to the P. xylostella individuals, and some positive effects were observed both in the absence and in the presence of chlorantraniliprole when compared with the susceptible individuals.
Because of the high resistance to chlorantraniliprole observed in the field and the existence of negative effects on P. xylostella biology inherent to this resistance, the rotation of insecticides with different modes of action should be adopted as a practice for the management of P. xylostella to decrease the frequency of resistant genes and thereby restore susceptibility in field populations (Georghiou, Reference Georghiou, Georghiou and Saito1983; Roush & McKenzie, Reference Roush and McKenzie1987). No cross-resistance was observed between chlorantraniliprole and the other insecticides used to control P. xylostella in Brazil (Silva et al., Reference Silva, Siqueira, Silva, Campos and Barros2012). Spinosad, abamectin, and chlorfenapyr, for instance, are good options for managing the P. xylostella populations in the Brassica fields of tropical areas where the insect presents many generations per year. If such a practice is accompanied by reduced diamide application, the frequency of resistance to these compounds may be reduced in the short term, restoring insect susceptibility.
Acknowledgements
This research was supported in part by ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)’. We would also like to acknowledge CAPES for granting the scholarship to the first author, enabling this research. We further thank the anonymous reviewers for their valuable comments and suggestions on the manuscript.









