Introduction
The cultivation of genetically modified (GM) plants is becoming increasingly important worldwide. In 2003, GM crops covered a surface of approximately 68 million hectares: they were grown mostly in the USA (42.8 million ha), Argentina (13.9 million ha) and Canada (4.4 million ha) (James, Reference James2003). The most important genetically engineered crops that have been introduced contained genes encoding insect resistance and herbicide tolerance; they were soybean, maize, cotton and canola. In 2003, according to an ISAAA report (James, Reference James2003), the increase of acreage covered with transgenic crops was about 15%, with a 40-fold increase with respect to 1996. Genetically engineered crops that encode toxin genes from bacteria such as Bacillus thuringiensis Berliner (Bt) have shown great promise for pest control (Hilder & Boulter, Reference Hilder and Boulter1999; Gatehouse & Gatehouse, Reference Gatehouse, Gatehouse, Rechcigl and Rechcigl2000; Mandaokar et al., Reference Mandaokar, Goyal, Shukla, Bisaria, Bhalla, Reddy, Chaurasia, Sharma, Altosaar and Ananda Kumar2000). However, the cultivation of GM crops, both for experimental and commercial aims, determined the need for an evaluation of the possible consequences for natural- and agro-ecosystems. Directive 2001/18/EC of the European Commission, since the introduction of Directive 90/220/EEC, introduced the basic principles for a correct evaluation of environmental risk, and it remains the basic methodology to use in risk assessment. In Italy, the rule n. 224 of 8 July 2003 considers information of public opinion as regards deliberate GMO emission in the environment (Sorlini et al., Reference Sorlini, Buiatti, Burgio, Cellini, Giovannelli, Lener, Massari, Perrino, Selva, Spagnoletti and Staiano2005). A general methodology for risk assessment, concerning GM organisms, was suggested by the Italian Ministry of Environment with the aim of reviewing the main risk sources and to propose a monitoring programme within a risk evaluation plan (Sorlini et al., Reference Sorlini, Buiatti, Burgio, Cellini, Giovannelli, Lener, Massari, Perrino, Selva, Spagnoletti and Staiano2005).
Similar to other plant protection technology, insect resistant transgenic plants can bear risks and benefits to the environment. The primary ecological concerns to the release of transgenic plants include those related to their possible invasiveness in ecosystems, out-crossing, horizontal gene transfer, development of pest resistance and effects on non-target organisms (Dutton et al., Reference Dutton, Romeis and Bigler2003; Knols & Dicke, Reference Knols and Dicke2003; Scholte & Dicke, Reference Scholte and Dicke2005). Such evaluations should be carried out with a case-by-case approach, considering the target crop, the genetic trait introduced and the environment (Groot & Dicke, Reference Groot and Dicke2002; Dutton et al., Reference Dutton, Romeis and Bigler2003; Scholte & Dicke, Reference Scholte and Dicke2005). Moreover, they need specific and detailed experimental activity both in the field and in the laboratory (Lövei & Arpaia, Reference Lövei and Arpaia2005). Negative effects of GM plants on non-target beneficial arthropods have been a major concern because these organisms play an important role in controlling pest populations and plant pollination (Jervis & Kidd, Reference Jervis and Kidd1996; Dutton et al., Reference Dutton, Romeis and Bigler2003). Some reviews were published on ecological risk assessment and potential non-target effects of GM plants on arthropod fauna (Groot & Dicke, Reference Groot and Dicke2002; Conner et al., Reference Conner, Glare and Nap2003; Dutton et al., Reference Dutton, Romeis and Bigler2003; Lövei & Arpaia, Reference Lövei and Arpaia2005). In particular, Lövei & Arpaia (Reference Lövei and Arpaia2005) presented a critical review of laboratory studies concerning the impact of GM plants on natural enemies.
Since the risk that GM plants pose on non-target organism is a function of exposure to the insecticidal protein and the toxicity of the substance towards the specific organism, one important component in the risk assessment is to determine exposure (Raps et al., Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001; Dutton et al., Reference Dutton, Klein, Romeis and Bigler2002, Reference Dutton, Romeis and Bigler2004; Scholte & Dicke, Reference Scholte and Dicke2005). Exposure will depend on the feeding behaviour of the organism in question, together with the expression of the transgene in the plant (Dutton et al., Reference Dutton, Obrist, D'Alessandro, Diener, Müller, Romeis and Bigler2004). Another open question is whether the transgene product could be transported in the phloem sap. Aphids and other pests are obligatory phloem sap feeders, and they represent important prey for beneficial insects such as predators and parasitoids. For these reasons, the open question whether Cry toxins are present in the phloem sap of transgenic plants and whether aphids ingest Bt-toxin is of great ecological relevance within the task of the evaluation of non-target effects of GM. The present study aimed to evaluate, by laboratory tests, the possible transfer of Bt-toxin from a transgenic plant (Bt-oilseed rape) to a non-target pest, Myzus persicae Sulzer (Hemiptera: Aphididae).
Materials and methods
Plants and insects
Transgenic oilseed rape plants (Brassica napus L. cv. ‘Westar’ lines GT 2-4), expressing a truncated, synthetic version of the cry1Ac gene from Bacillus thuringiensis var. kurstaki active against Lepidoptera under the constitutive CaMV 35S promoter (Harper et al., Reference Harper, Mabon, Leffel, Halfhill, Richards, Moyer and Stewart1999), were employed for this study. Transgenic oilseed rape expressed, as markers, a fluorescence gene (GFP) and a kanamycin resistance gene (nptII). The corresponding near-isogenic line was employed as control. For the remainder of the manuscript, these two hybrids will be referred to as (Bt+) and (Bt−). All plants were planted in plastic pots (15 cm diameter) containing a 1:1 (v/v) peat:sand sterile potting mix. Plants were placed in a greenhouse set at 24±4°C, 50±10% relative humidity (RH). Plants were fertilized and irrigated weekly. For all experiments, plants were used when they had reached a height of 70 cm (7–10 leaf stage).
A stock colony of M. persicae was maintained in the laboratory according to Lanzoni et al. (Reference Lanzoni, Accinelli, Bazzocchi and Burgio2004). Aphid populations were then transferred for the experiments on (Bt+) and (Bt−) oilseed rape plants. Two aphid strains were employed in the laboratory tests: the first one (climatic chamber strain) was reared on (Bt+) and (Bt−) oilseed rape plants at ‘Dipartimento di Scienze e Tecnologie Agroambientali’ laboratories in separate growth chambers at 20±1°C, 50±10% RH, and L:D 16:8 h photoperiod. The second strain (greenhouse strain) was reared on (Bt+) and (Bt−) oilseed rape plants at ‘Istituto Nazionale di Apicoltura’, Bologna greenhouses (mean temperature=25°C, range: 10–35°C; mean RH=60%, range: 25–100%).
Bt-toxin analysis
Cry1Ac levels in plants and herbivores were determined using a double sandwich enzyme-linked immunosorbent assay (ELISA) QuantiPlate kit Cry1Ab/Cry1Ac (EnviroLogix Inc. Portland, Maine). Spectrophotometric measurements were conducted with a microtitre plate reader (Labsystem Multiscan, Dasit, Italy) at 405 nm.
Plant expression of Cry1Ac was quantified by analysing leaf material (50±3 mg±SD). Leaf pieces from randomly selected (Bt+) and (Bt−) oilseed rape plants were weighted, homogenized in a 5 ml extraction buffer, centrifuged and the extract was diluted 1:50 with an extraction buffer.
Phloem sap from (Bt+) and (Bt−) oilseed rape plants was collected from leaves (two leaves per plant). Leaves were cut and immediately soaked in an EDTA buffer (20 mm ethylendiaminetetraacetate-Na2-salt-dihydrate, pH 7). After a 24-h exudation period, samples were frozen at −80°C until analysis. This operation was performed twice.
To quantify Cry1Ac toxin in herbivores, protein was extracted from samples of 200–400 adult aphids reared on (Bt+) and (Bt−) plants, weighted, homogenized in a 200 μl extraction buffer with no dilution and analysed (for sample weights, see table 1).
Table 1. Results of the ELISA test of two Myzus persicae strains (G, greenhouse strain; Cc, climatic chamber strain) fed on isogenic (Bt−) or transgenic (Bt+) oilseed rape.

Bt-toxin concentrations were expressed in ppb (μg Cry1Ac kg−1 of fresh weight). All samples were centrifuged for 5 min at 13,000 rpm before they were introduced into the ELISA plate. The statistical comparison of the concentration of Cry1Ac toxin in aphid and phloem sap with the limit of detection of ELISA was carried out by means of a t-test (Berthouex & Brown, Reference Berthouex and Brown2002).
Results
A mean concentration of 64.3±2.9 (mean±SD) ppb of Cry1Ac was found in (Bt+) oilseed rape leaves (fig. 1). Bt toxin was present also in phloem sap, with a mean concentration of 2.7±1.46 ppb (mean±SD), which is 24-fold lower than in leaves. A t-test calculated on the positive samples above the limit of detection (LOD) (=1.2 ppb) for Cry1Ac reported in the ELISA kit, showed that the concentration of Bt-toxin was significantly (P<0.05) higher than the LOD.
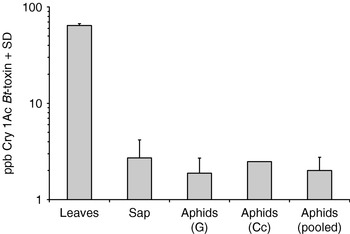
Fig. 1. Mean (+SD) Cry1Ac Bt-toxin concentration (μg/Kg fresh weight) measured in transgenic oilseed rape leaves and sap and in two Myzus persicae strains fed on Bt-oilseed rape (G=Greenhouse strain; Cc=Climatic chamber strain).
In our analysis, four aphid samples (of a total of four examined) fed on Bt-oilseed rape in the greenhouse, and one aphid sample (of a total of eight examined) fed on Bt-oilseed rape in the climatic chamber, contained a detectable amount of Cry1Ac (table 1). The concentration of Cry1Ac was 1.9±0.8 ppb (mean±SD) for the greenhouse strain and 2.5±1.3 ppb (mean±SD) for the climatic chamber strain (fig. 1). The concentration of the toxin in the positive aphid samples was 2.0±0.8 ppb (mean±SD); this value was significantly higher (t-test, P<0.05) than the LOD. This value is quite similar to that reported for the concentration of toxin in Bt-oilseed rape phloem sap. Expression of Cry1Ac in the plants on which aphid strains had fed was confirmed. These results seem to corroborate that Bt-toxin can pass through from Bt-oilseed rape to aphids.
Discussion
Phloem sap from Bt-oilseed rape showed a detectable amount of Cry1Ac; however, a strong difference in the Cry1Ac concentration between leaves and sap was observed. The present results showed that Cry1Ac can be present in Bt-oilseed rape phloem sap, but it is unknown if the toxin is expressed in phloem tissue or if it is simply translocated in the sap. Raps et al. (Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001) did not detect Cry1Ab in Bt-maize sap by microcapillary technique; on the contrary, Cry1Ab toxin was detected by EDTA-method at 1 ppb concentration, but the authors hypothesized that the traces of toxin could originate from damaged cells (Raps et al., Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001). The detection of Cry1Ac toxin in phloem from Bt-oilseed rape in our experiments can not originate from damaged cells, as the exudation from petioles prevents the damage of mesophyll cells. On the other hand, in transgenic tobacco plants expressing the snowdrop lectin (GNA) under the control of the CaMV 35S promoter, the toxin was detected in phloem, xylem cells, parenchyma cells, mesophyll cells cortex and pith tissue (Shi et al., Reference Shi, Wang, Powell, Van Damme, Hilder, Gatehouse, Boulter and Gatehouse1994). In the same way, soybean trypsin inhibitor expression (SKTI), driven by the CaMV 35S promoter, enhances the resistance in transgenic potato or rice against aphids such as M. persicae, Aulacorthum solani (Kaltenbach) (Hemiptera: Aphididae) or Nilaparvata lugens Stål (Hemiptera: Delphacidae), indicating a translocation of toxins from phloem cells to sap (Down et al., Reference Down, Gatehouse, Hamilton and Rossignol1996; Gatehouse et al., Reference Gatehouse, Down, Powell, Sauvion, Rahbé, Newell, Merryweather, Hamilton and Gatehouse1996; Lee et al., Reference Lee, Lee, Koo, Chun, Lim, Mun, Song and Cho1999). However, notwithstanding the control of CaMV 35S promoter in the expression of Cry1Ab in maize, no evidence exists of the translocation of Cry1Ab into the phloem sap in maize. One reason could be due to high Cry1Ab molecular size (65–68 kDa) (Raps et al., Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001), but this would not explain the transport of the Cry1Ac toxin (60 kDa) in oilseed rape phloem sap. Another explanation could be the lack in maize of specific signal sequences for the transport to phloem sap. Concerning the present results, specific information about the mechanism undergoing the presence of Bt-toxin in phloem sap is still to be assessed.
Concentrations of Cry1Ac of about 2 ppb in adult M. persicae, feeding on Bt-oilseed rape, were easily and reliably detected. The detection of the toxin was appreciable in all greenhouse samples and in 12.5% of climatic chamber samples. Previous studies about Bt effect on non-target insects demonstrated that no or little Cry1Ab could be detected on Rhopalosiphum padi (Linnaeus) (Hemiptera: Aphididae) fed on Bt-maize (Raps et al., Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001; Dutton et al., Reference Dutton, Obrist, D'Alessandro, Diener, Müller, Romeis and Bigler2004). However, available studies about the effects of Bt-crops on aphids are still few. Field tests evidenced no short-term effects of Bt-maize on aphids, and laboratory tests evidenced the lack of direct toxic effects (Lozzia et al., Reference Lozzia, Furlanis, Manachini and Rigamonti1998).
Raps et al. (Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001) demonstrated that R. padi, a grain aphid infesting corn, ingested or contained no or very low concentrations of Cry1Ab toxin when fed on Bt-corn; because R. padi is an important prey for beneficial insects in corn, the authors concluded that Cry1Ab was unlikely to cause any harm to the predator guild. Shi et al. (Reference Shi, Wang, Powell, Van Damme, Hilder, Gatehouse, Boulter and Gatehouse1994) detected CaMV 35S promoter activity in different tissues of transgenic tobacco plants, therefore, Cry proteins can also be expected even in phloem cells of other Bt-transformed crops, even if the expression of a protein in a phloem cell does not imply the translocation in phloem sap due to the size and structure of the protein. GNA can be expressed in transgenic tobacco and rice plants in a phloem specific manner, using a gene construct containing the GNA coding sequence driven by the promoter from the rice sucrose synthase gene RSs1 (Gatehouse & Gatehouse, Reference Gatehouse, Gatehouse, Rechcigl and Rechcigl2000). The RSs1 promoter directs the expression of a gus reporter gene in the phloem tissue of leaves, stems, petioles and roots of transgenic tobacco plants with no detectable expression in other tissues (Shi et al., Reference Shi, Wang, Powell, Van Damme, Hilder, Gatehouse, Boulter and Gatehouse1994). Transgenic plants containing these constructs are effective against phloem-feeding homopteran insect pests (Gatehouse & Gatehouse, Reference Gatehouse, Gatehouse, Rechcigl and Rechcigl2000). Transgenic rice containing an RSs1-GNA construct has been shown to accumulate GNA in vascular and epidermal tissue (Sudhakar et al., Reference Sudhakar, Fu, Stoger, William, Spence, Bown, Bharathi, Gatehouse and Christou1998). Expression of GNA from a constitutive promoter (maize ubiquitin) gave similar results (Gatehouse & Gatehouse, Reference Gatehouse, Gatehouse, Rechcigl and Rechcigl2000). However, available information in the literature is rather fragmentary, and it has not completely clarified the mechanism undergoing the presence of insecticidal toxins in the phloem sap.
The presence of the Cry1Ac toxin in phloem sap from Bt-oilseed rape, expressed under the control of the CaMV 35S promoter and in M. persicae, showed the importance of providing an estimate of the expected environmental concentration of Cry1Ac in the diets of non-target organisms eating aphids feeding on the transgenic crop. Moreover, our preliminary results improve the knowledge of a trophic system not as well-known as Bt-maize.
In the present study, all aphids reared in greenhouse conditions resulted as positive, while aphid colonies reared in climatic chambers exhibited only 12.5% positive samples. This difference could be due to environmental conditions influencing either plant physiology or insect behaviour, but this hypothesis should be investigated in further experiments. The present data could improve the knowledge of aspects concerning the transfer of the toxin to organisms belonging to higher trophic levels, including natural enemies that are indirectly exposed by feeding on herbivores (Dutton et al., Reference Dutton, Klein, Romeis and Bigler2002; Groot & Dicke, Reference Groot and Dicke2002; Romeis, Reference Romeis2004; Scholte & Dicke, Reference Scholte and Dicke2005). Moreover, detailed knowledge of the possible chronic effects, which involve the uptake of toxin along the food chain, is considered a basic aspect in the risk-assessment of GM crops (Romeis, Reference Romeis2004; Scholte & Dicke, Reference Scholte and Dicke2005).
In conclusion, the evidence that Bt-toxin is present in herbivores, in this case an aphid, could be useful to clarify functional aspects linked to possible consequences of Bt-crops on herbivore–natural enemy trophic systems. Besides, transfer of Cry1Ac from Bt-oilseed rape to aphid increases exposition of this toxin in the environment, and this possibility must be taken into account to evaluate the possible impact of transgenic crops on non-target organisms. Future work should address also bioassay with beneficial species (for example predators like coccinellids), including tests like Western blot to demonstrate if the toxin remains active in the aphid.
Acknowledgements
This work was supported by the Ministero dell'Ambiente e della Tutela del Territorio (Direzione generale per la salvaguardia ambientale). The authors are grateful to Francesco Cellini and Maria Carola Fiore (Metapontum Agriobios, Matera, Italy) for supplying Bt and isogenic oilseed rape seeds, Marcel Dicke (Laboratory of Entomology, Wageningen University) for critically reviewing the manuscript and Maria Luisa Dindo (DiSTA-Entomologia, Alma Mater Studiorum-Università di Bologna) for useful suggestions.


