Introduction
Arthropods represent approximately 80% of the species of the Animalia kingdom. Among these, insects have the highest richness and a considerable number of species live in rainforests (Zhang, Reference Zhang2011). Despite the deforestation, the Atlantic Forest is one of the largest rainforests in the Americas, with substantial species richness and endemism rates (Myers et al., Reference Myers, Mittermeier, Mittermeier, Fonseca and Kent2000; Galindo-Leal & Câmara, Reference Galindo-Leal, Câmara, Galindo-Leal and Câmara2003; Ribeiro et al., Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirot2009; Scarano & Ceotto, Reference Scarano and Ceotto2015). Its latitudinal range is around 29° extending along the Brazilian coast and adjacent regions in Argentina and Paraguay (Ribeiro et al., Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirot2009). This wide latitudinal extension of the Atlantic Forest and the significant oscillation in geographical relief result in different climate types and vegetation physiognomies across its domain area (Galindo-Leal & Câmara, Reference Galindo-Leal, Câmara, Galindo-Leal and Câmara2003; Pinto & Brito, Reference Pinto, Brito, Galindo-Leal and Câmara2003; Tabarelli et al., Reference Tabarelli, Pinto, Silva, Hirota and Bedê2005).
Several studies have produced evidence of the seasonal changes in abundance, richness, composition and diversity of tropical insects (Janzen & Schoener, Reference Janzen and Schoener1968; Pinheiro et al., Reference Pinheiro, Diniz, Coelho and Bandeira2002; Basset et al., Reference Basset, Novotny, Miller and Kitching2003; Grimbacher & Stork, Reference Grimbacher and Stork2009; Neves et al., Reference Neves, Oliveira, Espírito-Santo, Vaz-de-Mello, Louzada, Sanchez-Azofeifa and Fernandes2010; Ferreira et al., Reference Ferreira, Martins, Paixão and Silva2015). A specific group of insects, drosophilids, have become the object of considerable research addressing seasonal variations in the southern part of the Atlantic Forest in Brazil (Dobzhansky & Pavan, Reference Dobzhansky and Pavan1950; Saavedra et al., Reference Saavedra, Callegari-Jacques, Naap and Valente1995; De Toni et al., Reference De Toni, Gottschalk, Cordeiro, Hofmann and Valente2007; Bizzo et al., Reference Bizzo, Gottschalk, De Toni and Hofmann2010; Garcia et al., Reference Garcia, Hochmüller, Valente and Schmitz2012), where marked temperature and rainfall changes are observed throughout the year, characterized by four distinct seasons. The seasonality of drosophilids inhabiting the northern part of the Brazilian Atlantic Forest has been little investigated, despite the influence of rainfall on the region's seasonal features, while the temperature range is less extensive. As opposed to the southern portion of the biome, only two seasons are observed, the rainy and the dry (Colombo & Joly, Reference Colombo and Joly2010).
Seasonality is an important factor in survival strategies adopted by numerous organisms, playing an essential role in the knowledge about populations and the structure of tropical insect communities in a given area (Wolda, Reference Wolda1978a , Reference Wolda b ; Spitzer et al., Reference Spitzer, Novotny, Tonner and Leps1993). Seasonal patterns may interfere in population size, reproductive activity and the availability of food resources, among other aspects (Wolda, Reference Wolda1988). Although several insect species may be living in similar seasonal situations, they do not exhibit the same response pattern to environmental changes permanently. In other words, each species exhibits particular adaptations that underline their seasonal cycles (Tauber & Tauber, Reference Tauber and Tauber1981). From this perspective, understanding how seasonal changes impact ecological parameters of native as well as exotic insect species has fascinated researchers interested in evaluating the effects of biological invasions in natural environments (Sax et al., Reference Sax, Stachowicz, Brown, Bruno, Dawson, Gaines, Grosberg, Hastings, Holt, Mayfield, O'Connor and Rice2007).
This study evaluated the influence of seasonality on abundance, richness, composition and diversity of the drosophilid assemblage focused on species native to the Neotropical region and exotic ones in Atlantic Forest fragments on the north region of its distribution range.
Material and methods
Study sites
Adult drosophilids were collected in three preserved fragments of the Atlantic Forest in the state of Pernambuco, northeast Brazil: Refúgio Ecológico Charles Darwin (Darwin, 07°48′S; 34°57′W), Estação Experimental de Itapirema (Itapirema, 07°38′S; 34°56′W) and Estação Ecológica do Tapacurá (Tapacurá, 08°03′S; 35°13′W) (fig. 1). The first two fragments cover an area of approximately 60 hectares (ha) (Costa-Lima, Reference Costa-Lima1998; Mascarenhas et al., Reference Mascarenhas, Beltrão, Souza, Galvão, Pereira and Miranda2005), while Tapacurá stretches across 382 ha (Coelho, Reference Coelho1979). The three areas studied have similar phytogeographic characteristics, and are classified as dense ombrophilous forests, all of which are located in the Pernambuco subregion (IBGE, 2012).
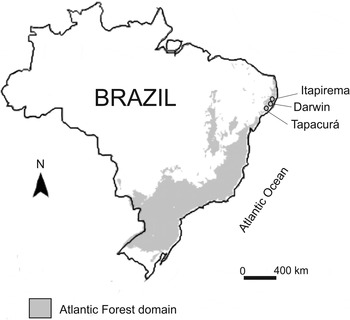
Fig. 1. Map of Brazil showing the Atlantic Forest domain in the country (grey area) and the three fragments where drosophilids were collected. Itapirema = Estação Experimental de Itapirema, Darwin = Refúgio Ecológico Charles Darwin and Tapacurá =Estação Ecológica do Tapacurá.
Climate in the region is type As according to the Köpen classification system, defined as tropical moist with dry summers and rainfall below 60 mm in the season of greater drought. The rainy season starts in April and ends in August, when almost 70% of all rain volume is recorded in a year. Annual rainfall exceeds 2000 mm. Mean temperature is approximately 25 °C, oscillating between 22 and 30 °C (INMET, 2016; LAMEPE, 2016).
Collection and identification of drosophilids
Two collections were carried out in the rainy (between June and August) and two in the dry (between January and March) seasons in each study fragment between March 2011 and June 2012. Ten traps baited with banana and constructed with plastic bottles according to Tidon & Sene (Reference Tidon and Sene1988) were placed in each fragment for 3 days. Traps were hung 1.5 m above the ground and 30 m away from one another along a linear transect located 500 m away from the fragment edge.
The drosophilids captured were characterized using taxonomic keys (Freire-Maia & Pavan, Reference Freire-Maia and Pavan1949; Poppe et al., Reference Poppe, Schmitz, Grimaldi and Valente2014) and species descriptions (Val & Sene, Reference Val and Sene1980; Vilela & Bächli, Reference Vilela and Bächli1990; Chassagnard & Tsacas, Reference Chassagnard and Tsacas1993; Bächli et al., Reference Bächli, Vilela, Escher and Saura2004; Culik & Ventura, Reference Culik and Ventura2009). Cryptic species were recognized after inspection of male genitalia. Drosophila melanogaster and D. simulans were identified based on the shape of the posterior salience of the genital arch (Salles, Reference Salles1948). The males of the willistoni subgroup of Drosophila were named considering the shape of the hypandrium (Burla et al., Reference Burla, Da Cunha, Cordeiro, Dobzhansky, Malogolowkin and Pavan1949; Malogolowkin Reference Malogolowkin1952; Rohde et al., Reference Rohde, Monteiro, Cabral, Silva, Oliveira, Montes and Garcia2010). Other cryptic species were documented after inspection male terminalia (Breuer & Pavan, Reference Breuer and Pavan1950; Magalhães & Björnberg, Reference Magalhães and Björnberg1957; Vilela, Reference Vilela1983; Vilela & Bächli, Reference Vilela and Bächli1990; Vilela et al., Reference Vilela, Silva and Sene2002). For the analysis of male terminalia, flies were prepared in potassium hydroxide (KOH) 10%, stained in acid fuchsin and dissected in glycerol (Bächli et al., Reference Bächli, Vilela, Escher and Saura2004). The number of females of cryptic species was estimated calculating each species’ sex ratio.
Voucher specimens were deposited in the drosophilid collection of the Laboratório de Genetica, Centro Acadêmico de Vitória, Universidade Federal de Pernambuco, Brazil. The species were also categorized as native to the Neotropical region and exotic (Gottschalk et al., Reference Gottschalk, Hofmann and Valente2008).
Ecological analyses
Richness and abundance of each species were recorded during each field excursion. These parameters were used to estimate the Shannon–Wiener diversity (H′) and the Smith and Wilson's evenness (E var) indices in the software Ecological Methodology (Kenney & Krebs, Reference Kenney and Krebs2000).
Species accumulation curves were constructed for each fragment surveyed and compared using the jackknife 1 species estimator, calculated in the software Biodiversity Pro, version 2 (McAleece et al., Reference McAleece, Lambshead, Paterson and Gage1997).
Similarity trees were constructed using the Jaccard and Morisita indices in the software PAST, version 1.94b (Hammer et al., Reference Hammer, Harper and Ryan2001). The temporal and spatial distribution patterns of the Neotropical and exotic species were characterized based on the relative abundance of individuals. The Chi-square (χ2) test was used to compare absolute richness and abundance considering the two seasons (dry and rainy), the three survey sites (Darwin, Itapirema and Tapacurá) and the species native to the Neotropical region and the exotic ones. The null hypothesis assumed was that these categories of comparison do not differ significantly.
Results
In total, 40,911 drosophilids of 36 species and 6 genera (Drosophila, Neotanygastrella, Rhinoleucophenga, Scaptodrosophila, Zaprionus and Zygothrica) were collected. Drosophila was the richest genus, with 29 species, followed by Rhinoleucophenga, with 3. The other genera included one species each (table 1). The Neotropical and exotic drosophilids captured were represented by 29 and 7 species, respectively. Exotic species accounted for 75.44% of total abundance recorded.
Table 1. List of drosophilid species native to the Neotropical region and exotic ones (*) in the rainy and dry seasons for the sampling excursions carried out in three fragments of the Atlantic Forest, north of their distribution.

N, number of individuals; S obs, species richness observed; H′, Shannon–Wiener heterogeneity index; E var, Smith–Wilson evenness index.
Spatial variation
The highest richness was recorded in Darwin (27 species), followed by Tapacurá (25 species) and Itapirema (22 species). The jackknife 1 estimator revealed that the richness values observed are similar to the values estimated for each fragment, when 35, 29 and 27 species were expected in Darwin, Tapacurá and Itapirema, respectively. Abundance followed an opposite pattern as that of richness, when Itapirema had the largest number of individuals, followed by Tapacurá and Darwin (table 1). The analysis of species composition estimated by the Jaccard index showed that species clustered for fragment surveyed (fig. 2a), indicating that the drosophilid assemblages living in the three sites were different.

Fig. 2. Similarity tree based on the Jaccard (a) and Morisita (b) indices for the drosophilids collections carried out in the rainy and dry seasons in the Atlantic Forest domain, in Itapirema (Estação Experimental de Itapirema), Darwin (Refúgio Ecológico Charles Darwin) and Tapacurá (Estação Ecológica do Tapacurá).
Temporal variation
No significant difference was observed in richness between seasons: 34 species were recorded in the rainy season, 28 in the dry (χ2 = 0.581, df = 1, P = 0.5250). Species richness was higher in the rainy season in all localities (table 1), though with no significant difference (χ2 = 0.286, df = 2, P = 0.8670). Similarly, no differences were observed in richness of native (χ2 = 0.750, df = 1, P = 0.3865) and exotic (χ2 = 0.070, df = 1, P = 0.7815) species between seasons.
Abundance was five times as high during the dry season, with significant statistical difference (χ2 = 18,368.472, df = 1, P < 0.0001). In all localities, the number of individuals recorded was higher in this season (Itapirema: χ2 = 15,231.761, df = 1, P < 0.0001; Darwin: χ2 = 3073.778, df = 1, P < 0.0001; Tapacurá: χ2 = 1488.783, df = 1, P < 0.0001). In the dry season, exotic species were more abundant than Neotropical species, independently of fragment surveyed (χ2 = 14,837.545, df = 1, P < 0.0001). However, in the rainy season the species native to the Neotropical region were more abundant (χ2 = 425.696, df = 1, P < 0.0001) (fig. 3).

Fig. 3. Relative abundance of drosophilid species native to the Neotropical region and exotic ones in the rainy and dry seasons, in three Atlantic Forest fragments: Itapirema (Estação Experimental de Itapirema), Darwin (Refúgio Ecológico Charles Darwin) and Tapacurá (Estação Ecológica do Tapacurá).
Rare species (with abundance values below 1%) represented 77.78% of the richness, but only 1.53% of the abundance. Eight species had relative abundance above 1%, four of which were exotic (D. malerkotliana, Z. indianus, D. simulans and S. latifasciaeformis) and four were Neotropical (D. willistoni, D. sturtevanti, D. paulistorum and D. prosaltans) (fig. 4). Together, these species accounted for 98.47% of the total abundance.

Fig. 4. Ranking based on abundance of 36 drosophilid species collected in three fragments of the Atlantic Forest. 1 = Drosophila malerkotliana, 2 = D. willistoni, 3 = Zaprionus indianus, 4 = D. sturtevanti, 5 = D. simulans, 6 = Scaptodrosophila latifasciaeformis, 7 = D. paulistorum, 8 = D. prosaltans, 9 = D. mercatorum, 10 = D. nebulosa, 11 = D. neocardini, 12 = D. melanogaster, 13 = D. fumipennis, 14 = D. ararama, 15 = Rhinoleucophenga punctulata, 16 = D. sp7, 17 = D. cardinoides, 18 = D. pictilis, 19 = D. polymorpha, 20 = D. ellisoni, 21 = D. sp6, 22 = D. ananassae, 23 = D. sp2, 24 = D. sp5, 25 = Neotanygastrella tricoloripes, 26 = Zygothrica orbitalis, 27 = D. zottii, 28 = D. sp9, 29 = D. sp10, 30 = D. sp1, 31 = D. sp3, 32 = R. sp1, 33 = D. sp4, 34 = D. sp8, 35 = R. capixabensis, 36 = D. kikkawai.
Concerning the most abundant exotic species, i.e. D. malerkotliana, Z. indianus and S. latifasciaeformis were more intensively captured in the dry season (χ2 = 1527.360, df = 1, P < 0.0001; χ2 = 886.261, df = 1, P < 0.0001; χ2 = 232.865, df = 1, P < 0.0001, respectively) (table 1). The three species were more representatively collected in this season, in all fragments studied. But the opposite behaviour was observed for D. simulans, which was more abundant during high rainfall periods (χ2 = 1131.867, df = 1, P < 0.0001) (fig. 5a).

Fig. 5. Seasonal variation of exotic (a) and native drosophilid species to the Neotropical region (b) in three fragments of the Atlantic Forest: Itapirema (Estação Experimental de Itapirema), Darwin (Refúgio Ecológico Charles Darwin) and Tapacurá (Estação Ecológica do Tapacurá).
Drosophila willistoni, D. sturtevanti, D. paulistorum and D. prosaltans were significantly more abundant in the rainy season (χ2 = 275.179, df = 1, P < 0.0001; χ2 = 112.901, df = 1, P < 0.0001; χ2 = 111.284, df = 1, P < 0.0001; χ2 = 58.243, df = 1, P < 0.0001, respectively) (table 1). The relative abundance of the four species was higher in this season, in all localities. The exception was D. sturtevanti in Tapacurá (fig. 5b).
Drosophila malerkotliana was the most abundant species recorded in this study, with more than 60% of the flies collected. It was the dominant species in the dry season, when it accounted for almost 70% of the individuals captured, against 21% in the rainy season. During this period D. willistoni was the main species, representing more than 40% of the total number of drosophilids observed, as opposed to <7% of the flies captured in the period of drought (table 1).
The similarity tree constructed using the Morisita index formed clusters for season (fig. 2b). The rainy season had higher diversity indices (H′ = 2.639, E var = 0.119), compared with the period of drought (H′ = 1.731, E var = 0.076). This was also observed for the fragment surveyed, when analysed separately (table 1).
Discussion
Seasonality in tropical regions is often marked by contrasting seasons concerning rainfall volumes, when temperature does not vary considerably (Peel et al., Reference Peel, Finlayson and McMahon2007). In this study, we evaluated areas of similar characteristics in the northern Atlantic Forest, observing that drosophilids are significantly more abundant in the dry season. It was in this period that exotic species were more numerous, while native ones were more abundant in the rainy season. No significant differences were observed in richness between seasons and between the fragments surveyed. Also, the highest diversity indices were recorded in the rainy period.
The average number of drosophilids captured per trap was similar to the values recorded in the northern Atlantic Forest (Garcia et al., Reference Garcia, Silva, Monteiro, Oliveira, Montes and Rohde2014; Monteiro et al., Reference Monteiro, Garcia, Oliveira and Rohde2016) and higher compared with studies carried out in the southern part of the biome (Gottschalk et al., Reference Gottschalk, De Toni, Valente and Hofmann2007; Döge et al., Reference Döge, Valente and Hofmann2008; Cavasini et al., Reference Cavasini, Buschini, Machado and Mateus2014). Richness values were near those observed in other parts of the Atlantic Forest (De Toni & Hofmann, Reference De Toni and Hofmann1995; Garcia et al., Reference Garcia, Valiati, Gottschalk, Rohde and Valente2008; Penariol & Madi-Ravazzi, Reference Penariol and Madi-Ravazzi2013). These comparisons and the jackknife 1 values that were similar to the observed ones reveal that the sampling strategy adopted was efficient.
The similarity tree constructed using the Jaccard index clustered collections for study areas, indicating that drosophilid assemblages are different in the sites surveyed, even though these were geographically close. Differences in species composition in Atlantic Forest fragments in somewhat distant sites have been observed in other investigations about drosophilids (De Toni et al., Reference De Toni, Gottschalk, Cordeiro, Hofmann and Valente2007; Gottschalk et al., Reference Gottschalk, De Toni, Valente and Hofmann2007; Döge et al., Reference Döge, Valente and Hofmann2008). The fact that the three fragments analysed in this study had heterogeneous assemblages and similar responses concerning the parameters investigated indicates that our results may reflect the patterns expected for these organisms in the northern Atlantic Forest.
Although a larger number of drosophilid species have been recorded in the rainy season, the seasonal differences in richness were not significant. In other areas of the northern Atlantic Forest (Monteiro et al., Reference Monteiro, Garcia, Oliveira and Rohde2016) as well as in the southern part of the biome (Torres & Madi-Ravazzi, Reference Torres and Madi-Ravazzi2006; Garcia et al., Reference Garcia, Hochmüller, Valente and Schmitz2012), higher richness values of these drosophilids were also observed in the rainy season. Concerning other arthropod groups, such as coleopterans, isopterans and arachnids, no significant differences were reported for richness in terms of seasonality in tropical forests (Vasconcellos, Reference Vasconcellos2003; Dias et al., Reference Dias, Brescovit, Couto and Martins2006, Anu et al., Reference Anu, Sabu and Vineesh2009).
In the Cerrado, a biome characterized by a dry season and a more intense water deficit compared with the northern Atlantic Forest, seasonal differences in richness have been observed for drosophilids, when the highest number of species was recorded in times of higher rainfall (Tidon, Reference Tidon2006; Mata et al., Reference Mata, Roque and Tidon2008; Roque et al., Reference Roque, Mata and Tidon2013). In the Atlantic Forest, humidity is more consistently preserved in periods of low rainfall (Por et al., Reference Por, Imperatriz-Fonseca and Lencioni2005). Therefore, reduced rain volumes do not seem to represent a limiting factor to drosophilid richness in this environment.
Contrasting with our results, several other studies about tropical insects have demonstrated the higher abundance of individuals in the rainy season (Owen & Chanter, Reference Owen and Chanter1970; Wolda, Reference Wolda1978a ; Denlinger, Reference Denlinger1980; Smythe, Reference Smythe, Leigh, Rand and Windsor1982; Frith & Frith, Reference Frith and Frith1985, Hammond, Reference Hammond, Knight and Holloway1990; Hill, Reference Hill1993; Novotny & Basset, Reference Novotny and Basset1998; Devries & Walla, Reference Devries and Walla2001). Although our findings did not reproduce this model when Neotropical and exotic species are considered together, the pattern is observed when only Neotropical species are included in the analysis. Probably, trophic resources are more readily available in the rainy season (Buril et al., Reference Buril, Melo, Alves-Araújo and Alves2013), which is an advantage for native species (David et al., Reference David, Allemand, van Herrewege, Cohet, Ashburner, Carson and Thompson1983; Döge et al., Reference Döge, Valadão and Tidon2015). In turn, it may be supposed that exotic species were more successful when invading these natural areas, by using the resources available in the dry season.
There is no single pattern to describe the seasonal abundance of exotic and native drosophilids in different parts of the world. Our findings reflect the configuration observed by Srinath & Shivanna (Reference Srinath and Shivanna2014), for instance, who investigated the drosophilid fauna in India, recording greater abundance of native species in the rainy season. This was also observed in Brazil, more specifically in the Cerrado (Mata et al., Reference Mata, Roque and Tidon2008) and in the southern part of the Atlantic Forest (Torres & Madi-Ravazzi, Reference Torres and Madi-Ravazzi2006). Concerning exotic species, Bombin & Reed (Reference Bombin and Reed2016), confirm our results, noting that these flies are more abundant during drought periods in North America, similarly to what was reported by Mata et al. (Reference Mata, Roque and Tidon2008) in Brazil.
The diversity indices (H′ and E var) were higher in the rainy season. In the southern Atlantic Forest, these indices have exhibited a trend towards increasing values during the dry period (De Toni & Hofmann, Reference De Toni and Hofmann1995; De Toni et al., Reference De Toni, Gottschalk, Cordeiro, Hofmann and Valente2007; Gottschalk et al., Reference Gottschalk, De Toni, Valente and Hofmann2007). In this study, the high dominance of exotic species in the dry season justifies the lower E var values recorded. The greater richness and evenness of abundance values when rainfall volumes are increased explain the higher H′ values observed in this season.
Approximately 80% of the richness of the drosophilid assemblage analysed was formed by rare species, which are represented by those whose frequency is below 1%. This pattern is regularly observed for Neotropical arthropods (Novotny & Basset, Reference Novotny and Basset2000; Coddington et al., Reference Coddington, Agnarsson, Miller, Kuntner and Hormiga2009), and it has been described in studies about drosophilids from the Atlantic Forest (Schmitz et al., Reference Schmitz, Hofmann and Valente2010, Cavasini et al., Reference Cavasini, Buschini, Machado and Mateus2014) and other biomes, such as the Amazon Forest (Acurio et al., Reference Acurio, Rafael and Dangles2010), the Cerrado (Roque et al., Reference Roque, Mata and Tidon2013) and the Caatinga (Oliveira et al., Reference Oliveira, Rohde, Garcia, Montes and Valente2016). From the ecological and evolutionary standpoints, rare species are those that have become more specialized to a few environments (Dobzhansky & Pavan, Reference Dobzhansky and Pavan1950).
The occurrence of intraspecific variations in temporal abundance patterns of several insect groups has been well documented (Wolda & Broadhead, Reference Wolda and Broadhead1985; Wolda, Reference Wolda1989; Wolda et al., Reference Wolda, O'Brien and Stockwell1998; Noguera et al., Reference Noguera, Zaragoza-Caballero, Chemsak, Rodríguez-Palafox, Ramirez, González-Soriano and Ayala2002; Wiwatwitaya & Takeda, Reference Wiwatwitaya and Takeda2005; Kishimoto-Yamada et al., Reference Kishimoto-Yamada, Itioka, Sakai and Ichie2010). In this study, among the most abundant exotic drosophilids, D. malerkotliana, Z. indianus and S. latifasciaeformis were more considerably recorded in the dry season, while D. simulans was more abundant in the rainy period. Of these, low abundance of S. latifasciaeformis has been recorded in other areas of the Atlantic Forest (De Toni et al., Reference De Toni, Gottschalk, Cordeiro, Hofmann and Valente2007; Bizzo et al., Reference Bizzo, Gottschalk, De Toni and Hofmann2010), making a comparison with our results more difficult.
The pattern observed for D. simulans has been recorded in the southern Atlantic Forest (Schmitz et al., Reference Schmitz, Valente and Hofmann2007, Döge et al., Reference Döge, Valente and Hofmann2008; Bizzo et al., Reference Bizzo, Gottschalk, De Toni and Hofmann2010; Garcia et al., Reference Garcia, Hochmüller, Valente and Schmitz2012). Ecological findings point to the higher sensitivity of this species to water deficit, compared with other drosophilids (David et al., Reference David, Allemand, Capy, Chakir, Gibert, Pétavy and Moreteau2004). Bombin & Reed (Reference Bombin and Reed2016) also observed significant positive correlation of D. simulans with high rainfall periods in the USA. Studies have shown that this species is strongly influenced by the availability of trophic resources (Döge et al., Reference Döge, Valadão and Tidon2015). It is possible that, in the fragments surveyed in this study, these resources are more plentiful during the rainy season (Buril et al., Reference Buril, Melo, Alves-Araújo and Alves2013), which may be favourable to this species.
Contrasting with the pattern observed, D. malerkotliana and Z. indianus have been recorded more abundantly during periods of more intense rains in their native sites, in Asia (Srinath & Shivanna, Reference Srinath and Shivanna2014) and Africa (Prigent et al., Reference Prigent, Le Gall, Mbunda and Veuille2013), respectively. This pattern is also observed in the southern part of the Atlantic Forest (Tidon-Sklorz & Sene, Reference Tidon-Sklorz and Sene1992; De Toni et al., Reference De Toni, Gottschalk, Cordeiro, Hofmann and Valente2007; Bizzo et al., Reference Bizzo, Gottschalk, De Toni and Hofmann2010). These data demonstrate that these species do not present a uniform seasonal fluctuation pattern concerning rainfall along their distribution range in the biome. It is possible that other abiotic factors such as temperature (which is comparatively low and varies more broadly in the southern Atlantic Forest) could explain these oscillations. Low temperatures may limit population sizes of Z. indianus in the southern Atlantic Forest (Garcia et al., Reference Garcia, Valiati, Gottschalk, Rohde and Valente2008). At 18 °C, this species’ biological cycle may extend for up to 1 month (Nava et al., Reference Nava, Nascimento, Stein, Haddad, Bento and Parra2007), which is too long for a colonizing drosophilid (Atkinson, Reference Atkinson1979). Similarly, D. malerkotliana is also influenced by temperature, with reduced fertility below 20 °C and total interruption of its development at 15 °C (Medeiros et al., Reference Medeiros, Martins and David2003).
Concerning D. malerkotliana, which was the most abundant species in this work, studies have shown its opportunistic character, taking over trophic resources at least 24 h before other drosophilids. This aspect, besides its short life cycle (Martins, Reference Martins, Bierregaard, Gascon, Lovejoy and Mesquita2001) and the likely occupation of sites that are inaccessible to other species are characteristics that may lend competitive advantages to D. malerkotliana, particularly in times when food resources are more limited.
The greater abundance of Neotropical species in the rainy season may be attributed mainly to species of the willistoni (D. willistoni and D. paulistorum) and of the saltans (D. sturtevanti and D. prosaltans) subgroups. In a study about the first subgroup, Garcia et al. (Reference Garcia, Silva, Monteiro, Oliveira, Montes and Rohde2014) had already observed the identical seasonal pattern in other fragments in the northern Atlantic Forest, which has also been reported for the southern part of the biome (Dobzhansky & Pavan Reference Dobzhansky and Pavan1950; Franck & Valente, Reference Franck and Valente1985; Tidon-Sklorz & Sene, Reference Tidon-Sklorz and Sene1992; Saavedra et al., Reference Saavedra, Callegari-Jacques, Naap and Valente1995; De Toni et al., Reference De Toni, Gottschalk, Cordeiro, Hofmann and Valente2007; Garcia et al., Reference Garcia, Hochmüller, Valente and Schmitz2012). Dobzhansky (Reference Dobzhansky1957) and Spassky et al. (Reference Spassky, Richmond, Pérez-Salas, Pavlovsky, Mourão, Hunter, Hoenigsberg, Dobzhansky and Ayala1971) highlight the fact that humidity is a limiting factor for these species, which may explain their higher abundance values in the rainy season.
Drosophila sturtevanti exhibited one single seasonal pattern along the whole extension of the Atlantic Forest, with greater abundance in the rainy season (Torres & Madi-Ravazzi Reference Torres and Madi-Ravazzi2006; De Toni et al., Reference De Toni, Gottschalk, Cordeiro, Hofmann and Valente2007). Torres & Madi-Ravazzi (Reference Torres and Madi-Ravazzi2006) observed a positive correlation of this species with rainfall. Considering D. prosaltans, few individuals have been collected in the southern part of the Atlantic Forest, preventing any discussion about a seasonal pattern for this species along the biome.
In the dry season, when trophic resources (especially fruit) are less readily available in the northern Atlantic Forest (Buril et al., Reference Buril, Melo, Alves-Araújo and Alves2013), generalist species have greater survival success, which explains their dominance in this period. Drosophila malerkotliana, Z. indianus, S. latifasciaeformis and D. simulans are generalist species (Yassin et al., Reference Yassin, Gidaszewski, Albert, Hiver, David, Orgogozo and Debat2012), and the first three have larger populations during the dry period. These findings demonstrate that the seasonal pattern observed in this study is explained by differences in abundance between native and exotic species, indicating the adoption of adaptation strategies by these groups.
Acknowledgements
The authors were grateful to the following funding agencies: Fundação de Amparo à Ciência e Tecnologia do estado de Pernambuco (FACEPE), Pró-Reitoria de Pesquisa e Pós-Graduação (PROPESQ) da Universidade Federal de Pernambuco (UFPE), Pró-Reitoria de Pesquisa e Pós-Graduação (PRPPG) da Universidade Federal Rural de Pernambuco (UFRPE) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). They also thank Mr Roberto Siqueira, Mr Paulo Martins and Mr Manoel Américo de Carvalho Fonseca for granting permission to collect drosophilids in Refúgio Ecológico Charles Darwin, Estação Ecológica do Tapacurá and Estação Experimental de Itapirema, respectively and Dr Elisângela Lúcia de Santana Bezerra for help with botanical information.









