Introduction
The carob moth, Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae) is one of the most important pests of pomegranate in Iran. This species is widely distributed throughout the pomegranate growing regions of Iran, especially in Yazd, one of the main pomegranate production areas in Iran. This pest has a multivoltine life cycle throughout Iran (Mozaffarian et al., Reference Mozaffarian, Sarafrazi, Nouri Ganbalani and Ariana2007). In autumn, fourth and fifth instar larvae enter facultative diapause inside the damaged fruits where they overwinter (Al-Izzi et al., Reference Al-Izzi, Al-Maliky, Younis and Jabbo1985). (Our observations in this research show that the fourth (a few) and fifth instar larvae enter diapause but only the last instar larvae could tolerate harsh conditions and finally pupate.)
Many insects distributed in the temperate zones enter winter diapause to overcome seasonal environmental stresses. Diapause is an adaptation assisting the survival of insects through periods of harsh environmental conditions and adverse seasons. Diapause is a genetically determined and an endocrine-mediated dormancy that occurs at a specific developmental stage according to the insect species (Denlinger, Reference Denlinger, Lee and Denlinger1991). Diapause is characterized by arrested development, suppressed metabolism, increased energy reserves and usually increased resistance to water loss and protection from low temperature (Denlinger, Reference Denlinger, Storey and Storey2000). Surviving long periods without eating is a challenge, and this is precisely the challenge that most diapausing insects confront. Managing metabolic resources is critical for insects during diapause when food sources are limited or unavailable. Insects use two strategies to mitigate the energetic costs of diapause: accumulation of reserves prior to diapause and metabolic depression during diapause (Hahn & Denlinger, Reference Hahn and Denlinger2011). It is well known that temperature has a pervasive effect on insects. Cold hardiness or cold tolerance has been defined as the capacity of a species to survive long- or short-term exposure to low temperature. This capacity is influenced by several factors such as developmental stage, genetic potential, season, duration of exposure and nutritional status (Lee, Reference Lee, Lee and Denlinger1991). Many strategies have been developed by insects to survive harsh environmental conditions. Cold hardiness and diapause are essential components of winter survival for most insects in temperate zones, but in many cases the relationship between these two are not clear. Cold hardiness can be achieved independently of diapause but it is often a component of the diapause syndrome, and the expression of diapause frequently extends the insect cold-hardening capacity (Denlinger, Reference Denlinger, Lee and Denlinger1991). Cold tolerance strategies of insects have generally been divided into two major categories: freeze-tolerant and freeze-intolerant insects. Freeze-tolerant insects tolerate the formation of extra-cellular ice within the body, whereas freeze-intolerant insects avoid the lethal effects of freezing by lowering the temperature at which the spontaneous freezing of body water occurs (Baust & Rojas, Reference Baust and Rojas1985; Zachariassen, Reference Zachariassen1985). This value is termed ‘the supercooling point’ (SCP) and is experimentally determined by detecting the released latent heat of fusion as body water freezes. The physiology of diapause has been thoroughly studied in many insects, and numerous studies have documented the impact of accumulation of low-molecular weight carbohydrates and polyols on diapause initiation in many species of insects (Denlinger, Reference Denlinger, Lee and Denlinger1991; Goto et al., Reference Goto, Fuji, Suzuki and Sakai1998, Reference Goto, Li, Kayaba, Outani and Suzuki2001; Kostal et al., Reference Kostal, Sula and Simek1998; Han et al., Reference Han, Gan, Kong and Ge2008; Behroozi et al., Reference Behroozi, Izadi, Samih and Moharamipour2012; Bemani et al., Reference Bemani, Izadi, Mahdian, Khani and Samih2012; Sadeghi et al., Reference Sadeghi, Izadi and Mahdian2012). Such compounds function as colligative (Zachariassen, Reference Zachariassen1985) and/or non-colligative cryoprotectants (Kostal et al., Reference Kostal, Slachta and Simek2001) enhance the level of cold hardiness and thus increase the chance of winter survival (Lee, Reference Lee, Lee and Denlinger1991; Storey & Storey, Reference Storey, Storey, Lee and Denlinger1991). The capacity of polyol accumulation may change seasonally and many species of insects initiate polyol synthesis at low temperatures (Nordin et al., Reference Nordin, Cui and Yin1984).
The main purpose of the present study was to quantify several biochemical parameters that are associated with seasonal cold hardiness in a wild population of the carob moth larvae. Cold hardening was investigated by the quantitative assessment of SCPs and survival at subzero temperatures. Such integration aims to provide a better understanding of the overwintering strategy of this pest. Field data obtained in this study are used to verify and extend earlier laboratory experiments.
Material and methods
Insects
Diapausing (from October 2012 to March 2013) and non-diapausing (July 2013) larvae of E. ceratoniae were collected from damaged fruits which had fallen to the soil beneath the pomegranate trees or remained on the trees (overwintering sites), in Abarkooh, Iran (31°7′N, 53°165′E; alt. 1500 m).
Preparation of whole-body homogenates for chemical analysis
Total body sugars
Total body sugars (mono and disaccharides) were measured using a method described by Warburg & Yuval (Reference Warburg and Yuval1997). Larvae were carefully brushed to remove contaminating particles, weighed and homogenized in 200 μl of 2% Na2SO4. An additional 1300 μl chloroform–methanol (1:2) was added to the homogenate to extract the simple carbohydrates of the larvae. Individual homogenates were centrifuged for 10 min at 7150 g. To determine the amount of carbohydrates in adult insects, 300 μl was taken from the supernatant and mixed with 200 μl distilled water. The sample was reacted for 10 min at 90 °C with 1 ml anthrone reagent (500 mg anthrone dissolved in 500 ml concentrated H2SO4). Absorbances were measured at 630 nm on a spectrophotometer (T60U, Harlow Scientific, USA). The amount of sugar was determined from a standard curve, using glucose (Sigma) as a standard. This experiment was repeated with six larvae each month.
Glycogen
Glycogen content was determined from the pellet obtained from the centrifugation mentioned above. The pellet was washed in 400 μl of 80% methanol, thus possible remnants of sugar were removed. To extract the glycogen, 250 μl distilled water was added to the washed pellet, and the mixture was heated for 5 min at 70 °C. Subsequently, 200 μl of the solution was removed and reacted for 10 min at 90 °C with 1 ml anthrone reagent (600 mg anthrone dissolved in 300 ml concentrated H2SO4). Optical density was read at 630 nm on a spectrophotometer (T60U, Harlow Scientific, USA). The amount of glycogen in the sample was determined from a standard curve, using glycogen (Sigma) as a standard. This experiment was repeated with six individual larvae each month.
Low-molecular weight carbohydrates and polyols
Trehalose, glucose, glycerol and myo-inositol were measured using a method described by Khani et al. (Reference Khani, Moharamipour and Barzegar2007). Larvae were carefully brushed to remove the contaminating particles, weighed and homogenized in 1.5–2 ml of 80% ethanol. Homogenates were centrifuged for 15 min at 12,000 g. To determine the amount of sugar alcohols in larvae, the supernatant was taken and evaporated at 40 °C in a vacuum drying oven and then resuspended in 1 ml of high-performance liquid chromatography (HPLC) grade water. Just before the sample injection, the samples were further cleaned by passing through a 20 μm syringe filter. Sugars and alcohol sugars were analyzed by HPLC (Knauer, Berlin, Germany) using a carbohydrate column with 4 μm particle size (250 mm×4.6 mm, I.D., Waters, Ireland). The eluent was acetonitrile–water (70:30) and elution speed was 1 ml min−1. Separation was achieved at 40±1 °C. All aqueous solutions were degassed with helium. Aliquots of whole-body extracts (20 μl) were run along with standards of glucose, myo-inositol, glycerol and trehalose from 1500 to 5500 ppm. This experiment was repeated using six individual larvae each month.
Lipids
To determine the amount of lipids in larvae, 300 μl of the supernatant based on the method by Warburg & Yuval (Reference Warburg and Yuval1997) was evaporated at 35 °C in an oven. Samples from each tube were dissolved in 300 μl H2SO4. The samples were heated for 10 min at 90 °C, cooled, stirred and then 2700 μl vanillin reagent (600 mg vanillin+100 ml distilled water+400 ml 85% H3PO4) was added. The tubes were shaken for 30 min at room temperature. Absorbances were measured at 530 nm on a spectrophotometer (T60U, Harlow Scientific, USA). The amount of lipid was determined from a standard curve, using Triolein (Sigma) as a standard. This experiment was repeated on six larvae each month.
Proteins
The residue from the polyol assay was resuspended in a solution of 1% SDS containing 0.4% sodium hydroxide, 2% sodium carbonate and 0.18% sodium tartarate and left overnight to solubilize the protein. After centrifugation, the protein content was estimated using a modified Lowry method (Markwell et al., 1978). Bovine serum albumin (Sigma) was used as a standard. This experiment was repeated with six individual larvae each month.
Determination of SCPs
The SCPs of individual larvae (n=20–25 larvae for each month) were measured using a thermocouple (NiCr-Ni probe) connected to an automatic temperature recorder, Testo 177-T4 (Testo, Germany), so that the cooling could be recorded at 0.5 min intervals, and the data were read using the Comsoft 3 Software. The specimens were attached to the thermocouple by adhesive tape and placed inside a programmable refrigerated test chamber (Gotech, GT-7005-A, Taiwan), the temperature of which was lowered at a rate of 0.5 °C min−1, starting at 20 °C and ending at −25 °C. The temperature at which an abrupt temperature increase occurred with the liberation of the latent heat of freezing was recorded as the SCP (Khani & Moharamipour, Reference Khani and Moharamipour2011).
Determination of low-temperature survival
Larvae were collected monthly from October to March (for diapausing larvae) and in September (for non-diapausing larvae) and transferred (n=20–25) to a programmable refrigerated test chamber (Gotech, GT-7005-A, Taiwan) and the temperature lowered at a rate of 0.5 °C min−1, from 20 °C to the desired treatment temperature (−5, −10 and −15±0.5 °C). The larvae were maintained at these temperatures for 24 h and then slowly (0.5 °C min−1) heated to 25 °C and maintained at the same temperature for 24 h. Live and dead larvae were counted, and the larvae showing no movement were considered to be dead (Khani & Moharamipour, Reference Khani and Moharamipour2011).
Water content and body weight
The dry weight was obtained after drying the larvae for 1 day in an oven at 65 °C. Total water content (w/w) was calculated by subtracting dry weight from fresh weight and dividing by fresh weight (Lehmann et al., Reference Lehmann, Lyytinen, Sinisalob and Lindstrom2012). This experiment was repeated with six individual larvae each month.
Weather data
Environmental temperature data were obtained from the Data Processing Center of the Iran Meteorological Organization (fig. 1). The station was located near the sampling site.

Fig. 1. Seasonal changes in average, minimum and maximum ambient temperature in Abarkooh between October 2012 and March 2013.
Statistical analysis
Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by a post hoc Tukey's test (P=0.05). Data were initially tested for normality (Kolmogorov–Smirnov test) and homoscedasticity (Levene's test) before subjecting them to ANOVA.
Results
The mean ambient temperature in Abarkuh, Iran was 21.0 °C in October, declining to 15.4 °C in November, 8.3 °C in December, 5.7 °C in January and 1.3 °C in February, before increasing to 12.9 °C in March. The minimum monthly temperature was below 0 °C from November to the end of March, with the lowest in January (−9 °C) (fig. 1).
Seasonal changes in low-molecular weight carbohydrates and polyols contents
As it is evident from table 1, two major carbohydrates in the last instar larvae of E. ceratoniae were found to be trehalose and myo-inositol. Glucose and glycerol were present at low levels in the overwintering larvae. Levels of trehalose and myo-inositol changed significantly during the overwintering period but difference in the levels of glucose and glycerol were not significant. Trehalose and myo-inositol contents were at the lowest levels (0.17 and 0.003 mg g−1 fresh body weight, respectively) in the first month of diapause (October). As the temperature decreased, levels of trehalose and myo-inositol increased and reached their highest levels of 0.50 and 0.068 mg g−1 fresh body weight, respectively, in the coldest month of the year, February. Trehalose and myo-inositol showed more than 2.9 and 22.6 times increase from the onset of diapause (October) through February. There were significant difference between the levels of trehalose and myo-inositol in diapausing (February) and non-diapausing (September) larvae (table 1). Trehalose and myo-inositol contents in diapausing larvae (February) were 3.12 and 17.0 times higher, respectively, than in non-diapausing (September) larvae. No significant differences were found between the levels of glucose and glycerol in diapausing and non-diapausing larvae. In addition, no significant differences were found between the levels of trehalose and myo-inositol in pupae that were derived from non-diapausing larvae (August) and pupae that were derived from diapausing larvae (April) (table 2).
Table 1. Changes in carbohydrate contents of non-diapausing (September), early diapausing (October) and diapausing larvae of Ectomyelois ceratoniae during 2012–2013.

Means within a column followed by the same letter are not significantly different (P>0.05, Tukey's test).
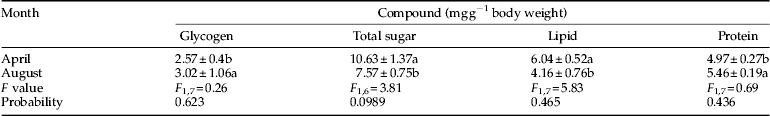
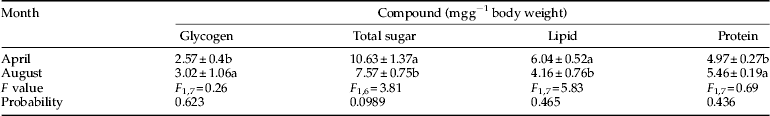
Table 2. Carbohydrate contents of pupae of Ectomyelois ceratoniae that were derived from diapausing larvae (April) and pupae that were derived from non-diapause larvae (August) during 2012–2013.

Means within a column followed by different lowercase letters are significantly different (one-way ANOVA, P<0.05).
Seasonal changes in carbohydrate content
The total sugar content increased from 5.22 mg g−1 fresh body weight in November as the mean ambient temperature decreased below 15.4 °C, and reached the maximum level of 12.82 mg g−1 fresh body weight in February as the mean ambient temperature reached its lowest level of 1.3 °C. At the end of overwintering, as the mean ambient temperature increased from February to March, the total sugar level decreased and reached a level of 7.92 mg g−1 fresh body weight in March (table 3). During the first month of overwintering (October), glycogen content was at the highest level of 2.94 mg g−1 fresh body weight and began to decrease from October as the temperature fell below 21.0 °C, and reached the lowest level of 1.12 mg g−1 fresh body weight in the coldest month of the year, February. Glycogen content increased at the end of overwintering and reached its highest level of 4.38 mg g−1 fresh body weight in March (table 3). Decrease in glycogen content was proportional to the increase in the total body sugar, trehalose and myo-inositolcontent. Glycogen and total body sugar content in the non-diapausing larvae (September) (2.73 and 6.41 mg g−1 fresh body weight, respectively) was significantly different from the diapausing larvae (February) (1.12 and 12.82 mg g−1 fresh body weight, respectively) (table 3). In addition, no significant difference was found in the level of glycogen between pupae that were derived from non-diapausing larvae (August) and pupae that were derived from diapausing larvae (April), but the total sugar content in pupae that were derived from diapausing larvae (10.63 mg g−1 fresh body weight) was significantly higher than in pupae that were derived from non-diapausing larvae (7.57 mg g−1 fresh body weight) (table 4).
Table 3. Changes in chemical contents of non-diapausing (September), early diapausing (October) and diapausing larvae of Ectomyelois ceratoniae during 2012–2013.

Means within a column followed by the same letter are not significantly different (P>0.05, Tukey's test).
Table 4. Chemical contents of pupae of Ectomyelois ceratoniae that were derived from diapausing larvae (April) and pupae that were derived from non-diapause larvae (August) during 2012–2013.
Means within a column followed by different lowercase letters are significantly different (one-way ANOVA, P<0.05).
Seasonal changes in lipid content
Lipid content was at its lowest level, 1.02 mg g−1 fresh body weight, in October and reached its highest level, 4.09 mg g−1 fresh body weight, in November with a mean ambient temperature of 15.4 °C. Lipid content steadily decreased with decrease in ambient temperature and reached the lowest level of 1.47 mg g−1 fresh body weight in January (table 3). Lipid content of diapausing larvae (1.79 mg g−1 fresh body weight) was significantly higher than in non-diapausing larvae (1.15 mg g−1 fresh body weight) (table 3). The difference between lipid content of pupae that were derived from diapausing larvae (6.04 mg g−1 fresh body weight) and pupae that were derived from non-diapausing larvae (4.16 mg g−1 fresh body weight) was also significant (table 4).
Seasonal changes in protein content
Protein level in the coldest month of the year, February, reached the highest level of 6.11 mg g−1 fresh body weight which was significantly different from the protein content at the onset of overwintering (October), but no significant difference in the level of protein was detected in other months (table 3). Protein content of diapausing larvae (6.11 mg g−1 fresh body weight) was significantly higher than in non-diapausing larvae (4.29 mg g−1 fresh body weight) (table 3). The difference between protein content of pupae that were derived from diapausing larvae (4.97 mg g−1 fresh body weight) and pupae that were derived from non-diapausing larvae (5.46 mg g−1 fresh body weight) was also significant (table 4).
Seasonal changes in SCPs and cold hardiness
Whole-body SCPs of diapausing larvae decreased significantly from November (−12.7 °C) to February and reached a minimum of −17.3 °C in February (table 5). At the onset of overwintering (early October), when the mean ambient temperature was approximately 21 °C, the collected larvae had a mean SCP of −12.3 °C, while the diapausing larvae which were collected from overwintering sites in early November, had a mean SCP of −12.7 °C. As ambient temperature increased, the SCP increased from February onwards and reached −11.26 °C in March (table 5). SCPs of diapausing larvae (−17.3 °C) were significantly lower than those of non-diapausing larvae (−12.0 °C) (table 5). The difference between SCP of pupae that were derived from diapausing larvae (−15.9 °C) and pupae that were derived from non-diapausing larvae (−10.4 °C) was also significant (table 2).
Table 5. Changes in low-temperature survival and SCPs of non-diapausing (September), early diapausing (October) and diapausing larvae of Ectomyelois ceratoniae during 2012–2013.

Means within a column followed by the same lowercase letter (a, b, c, d) are not significantly different (one-way ANOVA, P<0.05).
The highest cold hardiness was found in diapausing larvae, which had 100% survival following exposure to −5 °C/24 h and 50% survival following exposure to −10 °C/24 h. At both tested temperatures cold hardiness increased as ambient temperature decreased and reached its highest level in the coldest month of the year, February. This increase was expressed as a greater capacity to survive at −5 °C/24 h and −10 °C/24 h. Survival incidence decreased in March as the ambient temperature increased (table 5). All larvae died after 24 h at −15 °C. Increased cold hardiness was proportional to decreases in SCP. In comparison between diapausing and non-diapausing larvae, the survival of diapause destined larvae at −5 °C (100%) was significantly higher than that of non-diapausing larvae (50%). Survival of diapausing larvae at −10 °C (50%) was also significantly higher than that of non-diapausing larvae (0%) (table 5). In addition, the difference between the survival of pupae that were derived from diapausing larvae (100%) and pupae that were derived from non-diapausing larvae (75.66%) exposed to −5 °C/24 h was significant but all pupae that were derived from non-diapausing and diapausing larvae died following exposure at −10 °C/24 h (table 2).
Seasonal changes in water content and larval weight
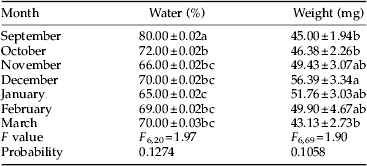
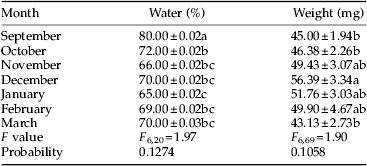
There were significant differences in water content and larval body weight during diapause from October to March. Diapausing larvae had the lowest water content, 65%, in mid diapause (January) and the lowest weight of 43.13 mg at the end of diapause (March) (table 6). In comparison between diapausing and non-diapausing larvae, the difference in water content was significant (62 and 80%, respectively) but the difference in the larval weight was not significant (49.9 and 45 mg, respectively) (table 6). No significant difference was detected between weight of pupae that were derived from non-diapausing and diapausing larvae (table 2).
Table 6. Changes in water content and weight of non-diapausing (September), early diapausing (October) and diapausing larvae of Ectomyelois ceratoniae during 2012–2013.
Means within a column followed by the same letter are not significantly different (P>0.05, Tukey's test).
Discussion
Diapause is an adaptive arrest of development that helps synchronize the active stages with suitable environmental conditions and thus increases the survival potential during unfavorable periods of the year (Hodek, Reference Hodek2012). Insects overwintering in the temperate zones commonly exhibit the strategies of diapause and cold tolerance which enhance survival and therefore fitness (Pullin, Reference Pullin1992). Diapause is characterized physiologically by a hormonally mediated suppression of metabolism, development and reproduction. Cold tolerance is not so easily defined because of its range of contributory physiological mechanisms such as accumulation of polyol cryoprotectants, masking of ice nucleators and production of antifreeze proteins, any combination of which may be evident in a single species (Zachariassen, Reference Zachariassen1985).
During overwintering of the carob moth larvae, trehalose and myo-inositol contents increased and reached their highest levels in the coldest month of the year, February. Increase in the levels of these carbohydrates was proportional to increase in total simple sugar, protein and lipid and decrease in glycogen. This finding strongly suggests the temperature-dependent interconversion between glycogen and low-molecular weight carbohydrates and polyols in overwintering larvae of E. ceratoniae under field conditions. Polyols are derived usually from glycogen reserves in overwintering insects (Storey & Storey, Reference Storey, Storey, Lee and Denlinger1991). Pullin et al. (Reference Pullin, Bale and Fontaine1991) suggested that the diapause-mediated ‘suppression of some metabolic pathways in advance of low-temperature exposure may prevent the damaging imbalance which may occur when enzyme activities change relative to each other as temperature decreases’. Winter accumulation of low-molecular weight sugars and/or polyols has been well documented in many overwintering insects from polar and temperate regions (Goto et al., Reference Goto, Li, Kayaba, Outani and Suzuki2001; Khani et al., Reference Khani, Moharamipour and Barzegar2007; Han et al., Reference Han, Gan, Kong and Ge2008; Behroozi et al., Reference Behroozi, Izadi, Samih and Moharamipour2012; Bemani et al., Reference Bemani, Izadi, Mahdian, Khani and Samih2012; Sadeghi et al., Reference Sadeghi, Izadi and Mahdian2012). Our results also showed that the diapausing larvae of the carob moth accumulate high levels of trehalose and myo-inositol. Analysis of these carbohydrates in the whole body showed an almost 3- and 17-fold increase in trehalose and myo-inositol content, respectively, between diapausing and non-diapausing larvae, but the levels of these compounds were more or less the same in pupae that were derived from non-diapausing larvae and pupae that were derived from diapausing larvae. The increase in the level of trehalose and myo-inositol was proportional to a decrease in SCP and increase in cold hardiness. In response to or in preparation for low temperatures, insects produce low-molecular weight carbohydrates and polyols (Lee, Reference Lee, Lee and Denlinger1991). So, it could be concluded that the increase in carbohydrate content is most likely a response to a temperature cue and acts in the prediction of cryoprotectants. This finding is in agreement with the results of Goto et al. (Reference Goto, Li, Kayaba, Outani and Suzuki2001) (the cabbage armyworm, Mamestra brassicae), Khani et al. (Reference Khani, Moharamipour and Barzegar2007) (the codling moth, Cydia pomonella), Han et al. (Reference Han, Gan, Kong and Ge2008) (the pine caterpillar Dendrolimus tabulaeformis), Behroozi et al. (Reference Behroozi, Izadi, Samih and Moharamipour2012) (the pistachio white leaf borer, Ocneria terebinthina), Sadeghi et al. (Reference Sadeghi, Izadi and Mahdian2012) (the common pistachio psylla, Agonoscena pistaciae) and Bemani et al. (Reference Bemani, Izadi, Mahdian, Khani and Samih2012) (the pistachio fruit hull borer, Arimania comaroffi). The data in the present study indicated that trehalose and myo-inositol were the dominant sugars accumulated in the overwintering larvae of E. ceratoniae. As cryoprotectants, trehalose and myo-inositol play an important role in the overwintering strategy of this pest. Numerous studies reported these sugars as cryoprotectants in some insect species (Storey & Storey, Reference Storey, Storey, Lee and Denlinger1991; Kostal et al., Reference Kostal, Slachta and Simek2001; Khani et al., Reference Khani, Moharamipour and Barzegar2007; Han et al., Reference Han, Gan, Kong and Ge2008; Behroozi et al., Reference Behroozi, Izadi, Samih and Moharamipour2012; Sadeghi et al., Reference Sadeghi, Izadi and Mahdian2012; Bemani et al., Reference Bemani, Izadi, Mahdian, Khani and Samih2012). Accumulation of low-molecular weight carbohydrates has been correlated with an increase in insect supercooling ability and chill tolerance (Lee, Reference Lee, Lee and Denlinger1991). One of the possible functions of such compounds in insect cold hardiness is the colligative effect on supercooling ability. It is believed that large polyol content provides depression of the SCP (Storey & Storey, Reference Storey, Storey, Lee and Denlinger1991).
For the stressful period without food (often lasting many months) diapause destined insects prepare in time by accumulating reserves and substances needed for resistance to future hazardous changes of environmental conditions (Hodek, Reference Hodek2012). Sufficient reserves must be sequestered to both survive the diapause period and enable postdiapause development that may involve metabolically expensive functions such as metamorphosis or long-distance flight (Hahn & Denlinger, Reference Hahn and Denlinger2011). Lipid and glycogen are two major forms of energy reserves and their patterns of utilization can differ during diapause (Han & Bauce, Reference Han and Bauce1998). Our results showed a fourfold increase in lipid content in overwintering larvae of E. ceratoniae at the onset of diapause in early November. Lipid content steadily decreased from December to February and reached the lowest level in February. This suggests that overwintering larvae of E. ceratoniae have the ability to reserve energy in the form of lipid and utilize it during diapause. Lipid has been reported as an energy resource during diapause in some other insects (Han et al., Reference Han, Gan, Kong and Ge2008). However, Goto et al. (Reference Goto, Fuji, Suzuki and Sakai1998) demonstrated that lipid content of overwintering larvae of Enosima leucotaniella (Lepidoptera: Pyralidae) did not differ at various acclimation temperatures. Kostal et al. (Reference Kostal, Sula and Simek1998) showed that glycogen content decreased substantially toward the end of diapause in the Mediterranean tiger moth, Cymbalophora pudica (Lepidoptera: Arctiidae), whereas the decrease in lipid content was not significant. Similar results were reported by Behroozi et al. (Reference Behroozi, Izadi, Samih and Moharamipour2012) (the pistachio white leaf borer, O. terebinthina), Sadeghi et al. (Reference Sadeghi, Izadi and Mahdian2012) (the common pistachio psylla, A. pistaciae) and Bemani et al. (Reference Bemani, Izadi, Mahdian, Khani and Samih2012) (the pistachio fruit hull borer, A. comaroffi). Glycogen content also decreased from the onset of overwintering and reached its lowest level in February. In March, by increasing the ambient temperature and activity of the overwintering larvae, glycogen content increased significantly. Glycogen was thus considered the main metabolic fuel and source of cryoprotectants during diapause, whereas lipids were the main source of energy and of the constituents for larval–pupal metamorphoses. Since the loss of cryoprotectants in spring is linked to the termination of diapause in many insects (Tsumuki, Reference Tsumuki, Hoshi and Yamashita1990), decreases in trehalose and myo-inositol content of overwintering larvae of the carob moth and on the other hand, increase in glycogen content at the onset of spring may be an indication of diapause termination.
Our results indicated approximately 1.5 times increase in protein content of the carob moth larvae from September to February. Insects can also be protected against injury from ice crystal formation by synthesizing antifreeze proteins and ice-nucleating agents (Duman et al., Reference Duman, Bennett, Sformo, Hochstrasser and Barnes2004; Michaud & Denlinger, Reference Michaud, Denlinger, Morris and Vosloo2004). Up-regulation of heat shock proteins is a mechanism permitting enhanced cold tolerance during diapause (Yocum, Reference Yocum2001). Heat shock proteins are a superfamily of molecular chaperones characteristically up-regulated in response to stress conditions and frequently associated increased cold hardiness during diapause (Aruda et al., Reference Aruda, Baumgartner, Reitzel and Tarrant2011). It could be concluded from our results that significant increases in protein content of overwintering larvae of the carob moth is a response to low-temperature stress to enhance cold tolerance.
As it is evident from our results, last instar larvae of the carob moth overwinter in a supercooled state and avoid freezing of their body fluid. Mean SCP (approximately −17 °C) of diapausing larvae is about 5 °C lower than that of non-diapausing larvae. Gradually decreasing temperatures in autumn and winter resulted in a continuing reduction of the SCP and enhancement of cold hardiness. The comparison between diapausing and non-diapausing larvae of the carob moth showed a nearly 1.5 decrease in SCP and 50 increase in cold tolerance when larvae were exposed to −10 °C/24 h. Fifty percent mortality in diapausing larvae exposed to −10 °C/24 h in comparison to 100% mortality of non-diapausing larvae highlights the enhanced cold hardiness. The significant lower SCP of diapausing larvae versus non-diapausing larvae strongly supports this conclusion. This finding is in agreement with earlier laboratory experiments (Neven, Reference Neven1999; Watanabe & Tanaka, Reference Watanabe and Tanaka1999; Li et al., Reference Li, Oguchi and Goto2002; Khani et al., Reference Khani, Moharamipour and Barzegar2007; Hou et al., Reference Hou, Lin and Han2009; Bemani et al., Reference Bemani, Izadi, Mahdian, Khani and Samih2012).
SCP is the temperature at which an insect's internal fluid freezes (Morey et al., Reference Morey, Hutchison, Venette and Burkness2012). In conjunction with the SCP, the lower lethal temperature can be used to categorize an insect's response to cold by briefly exposing the insect to temperatures around the mean SCP and measuring mortality (Morey et al., Reference Morey, Hutchison, Venette and Burkness2012). If most mortality occurs at the SCP, the insect is freeze-intolerant, below the SCP it is freeze-tolerant, and above the SCP it is chill-intolerant (Lee, Reference Lee, Denlinger and Lee2010). Since overwintering larvae of the carob moth could not tolerate temperatures lower than the SCP (−15 °C used in this study) the overwintering larvae are considered to be chill-intolerant. Variation in parameters related to cold hardiness (SCPs and low-temperature survival) during the cool winter season (January through March) shows that overwintering larvae of the carob moth could adjust their cold hardiness to the environmental conditions. This relationship between SCP and lower lethal temperature was recognized earlier in some pests such as C. pomonella (Neven, Reference Neven1999; Khani & Moharamipour, Reference Khani and Moharamipour2011), Phyllonorycter ringoniella (Li et al., Reference Li, Oguchi and Goto2002), Chilo suppressalis (Hou et al., Reference Hou, Lin and Han2009), O. terebinthina (Behroozi et al., Reference Behroozi, Izadi, Samih and Moharamipour2012), Aulacophora nigripennis (Watanabe & Tanaka, Reference Watanabe and Tanaka1999) and A. comaroffi (Bemani et al., Reference Bemani, Izadi, Mahdian, Khani and Samih2012). Two aspects that play important roles in the cold tolerance of diapausing larvae of the carob moth were found to be completely missing in the non-diapausing larvae. These were: (a) a down-regulation of ice nucleators resulting in SCP depression and (b) an accumulation of winter polyols, i.e. trehalose and myo-inositol (Slachta et al., Reference Slachta, Berkova, Vambera and Kostal2002).
Overwintering insects commonly reduce their water content and, in some cases, this has been interpreted as conferring increased cold hardiness by increasing the concentration of solutes (Slachta et al., Reference Slachta, Berkova, Vambera and Kostal2002). Our result showed that overwintering larvae of E. ceratoniae lost approximately 11% of their body water between September and January. This may enhance cold tolerance of diapausing larvae by increasing the concentration of solutes. Kostal & Simek (Reference Kostal and Simek2000) found that overwintering adults of Pyrrhocoris apterus lose 10–15% of their body water between September and January.
In conclusion, our results document some biochemical adaptations for winter survival in larvae of the carob moth. Large amounts of metabolic reserves (glycogen and lipid) accumulated in the beginning of diapause and decreased concomitant with diapause development. Most probably, overwintering larvae of the carob moth have the ability to reserve energy in the form of lipid and utilize it during overwintering. Low-molecular weight carbohydrates and polyols may play a role in winter survival and adaptation of the carob moth larvae to cold by providing cryoprotection. The SCP decreased in early winter and reached a minimum in February. As the ambient temperature increased, the SCP increased from March onwards. The carob moth larvae were found to be a chill-intolerant insect since the overwintering larvae died at temperatures above the SCP.
Acknowledgement
The authors are grateful to the research vice presidency of Vali-e-Asr University of Rafsanjan for the grant to Dr Izadi.