Introduction
Canola, Brassica napus L. (family Brassicaceae) is an important oilseed crop, which originated in either the Mediterranean region or the northern and western parts of Europe (Tsunoda, Reference Tsunoda1980). The diamondback moth, flea beetles, swede midge, cabbage seedpod weevil, pollen beetle, bertha armyworm, sucking insects, and noctuid pests can considerably limit canola production worldwide (Reddy, Reference Reddy2017). The cabbage aphid, Brevicoryne brassicae L. (Hem: Aphididae) is one of the most serious pests of this crop that creates large colonies on the leaves, stems, and buds of this crop and causes the twisting of leaves by sucking the phloem sap directly. It also interrupts photosynthesis by honeydew secretion and creating an environment for the growth of black mold fungus. This aphid could damage oilseed brassicas at flowering and pod founding stages (Ellis et al., Reference Ellis, Pink, Phelps, Jukes, Breeds and Pinnegar1998; Anwar & Shafique, Reference Anwar and Shafique1999). The seed loss is likely to occur 9–77%. Aphids also cause an 11% reduction in seed oil content (Kelm & Gadomski, Reference Kelm and Gadomski1995). On the other hand, they cause indirect damage via the transmission of viral diseases in plants, including the yellow mosaic virus, cauliflower mosaic virus, cucumber mosaic virus, onion yellow dwarf virus and cabbage black ringspot virus (Blackman & Eastop, Reference Blackman and Eastop2000). Chemical fertilizers may cause a population increase of herbivorous insects via modification of the nutritional quality of the host plant (Bentz et al., Reference Bentz, Reeves, Barbosa and Francis1995; Van Emden, Reference van Emden1995). Furthermore, chemical control by insecticides is the most effective and easiest way to control aphids (Verkerk et al., Reference Verkerk, Neugebauer, Ellis and Wright1998), but this method can lead to problems such as residues of pesticides in food and the environment, and pesticide resistance development in pests (Furk & Hines, Reference Furk and Hines1993). Therefore, more attention should be paid to other environmentally-safe and effective control methods for this pest. The use of biostimulants can limit the pest population and result in reduced reliance on its management on synthetic insecticides (Zehnder et al., Reference Zehnder, Kloepper, Yao and Wei.1997; Nardi et al., Reference Nardi, Pizzeghello, Schiavon and Ertani2015). A plant biostimulant is any microorganism or substance applied to plants or the rhizosphere with the aim to stimulate natural processes for the enhancement of nutrition efficiency, stress tolerance and/or crop quality, in spite of its nutritional content (du Jardin, Reference du Jardin2015). One group of more widely used biostimulants is humic substances. Several studies have demonstrated humic substances can improve plant growth and physiology (Bottomley, Reference Bottomley1914; Cacco & Dell'Agnola, Reference Cacco and Dell'Agnola1984; Dell'Agnola & Nardi, Reference Dell'Agnola and Nardi1987; Nardi et al., Reference Nardi, Arnoldi and Dell'Agnola1988; Pizzeghello et al., Reference Pizzeghello, Francioso, Ertani, Muscolo and Nardi2013), as well as plant defenses against biotic and abiotic stresses (Nardi et al., Reference Nardi, Pizzeghello, Schiavon and Ertani2015). Humic acid is a suspension derived from potassium-humates, which can be explored as a plant growth stimulant or soil conditioner for increasing natural resistance to plant diseases and pests (Scheuerell & Mahaffee, Reference Scheuerell and Mahaffee2004; Abd-El-Kareem, Reference Abd-El-Kareem2007).
Bio fertilizers like plant growth-promoting rhizobacteria (PGPR) can be classified as microbial biostimulants (du Jardin, Reference du Jardin2015). PGPR are able to mediate plant growth by diverse direct and indirect mechanisms (Glick, Reference Glick1995). Some of the mechanisms usually observed are the enhancement of nutrient availability; biological nitrogen fixation; protection from diseases and pests by the production of antibiotics, siderophores, hydrogen cyanide (de Medeiros et al., Reference de Medeiros, Silva, Mariano and Barros2005; Keel & Maurhofer, Reference Keel, Maurhofer, Weller, Thomashow, Loper, Paulitz, Mazzola, Mavrodi, Landa and Thompson2009), production of plant hormones and increasing stress tolerance (Glick et al., Reference Glick, Patten, Holgin and Penrose1999).
The construction of life tables is appropriate to study the dynamics related to the population growth potential, also called demographic parameters (Carey, Reference Carey1993; Southwood & Henderson, Reference Southwood and Henderson2000). Since the intrinsic rate of natural increase is a reflection of many factors, such as fecundity, survival, and generation time, it adequately summarizes the physiological qualities of an animal in relation to its capacity for population growth. Therefore, it is an appropriate index to evaluate the performance of an insect on different host plants as well as the host plant's antibiosis resistance to herbivorous insects (Smith, Reference Smith1989; Carey, Reference Carey1993; Southwood & Henderson, Reference Southwood and Henderson2000). Antibiosis (one of the basic modalities of host plant resistance) is a negative effect of the host plant on the biological parameters of pests (Hesler & Tharp, Reference Hesler and Tharp2005). Tolerance is another way of the host-plant resistance to pests and is defined as the plant's ability to endure an insect population that can damage a more susceptible host plant (Hesler & Tharp, Reference Hesler and Tharp2005). The application of humic substances and PGPR might lead to induced resistance of plants to some pests (Hosseini, Reference Hosseini2014; Mohamadi et al., Reference Mohamadi, Razmjou, Naseri and Hassanpour2017; Rashid & Chung, Reference Rashid and Chung2017). Induced resistance is defined as an enhancement of the plant's defensive capacity against a broad spectrum of pathogens and pests, acquired after appropriate stimulation (Broadway et al., Reference Broadway, Gongora, Kain, Sanderson, Monroy, Bennett, Warner and Hoffman1998). Yildirim & Unay (Reference Yildirim and Unay2011) reported the resistance induction of tomato plants by humic substances such as fulvic acid in combination with calcium nitrate against Liriomyza trifolii (Burgess). Moreover, induced resistance by PGPR has been found in corn to corn earworm, Helicoverpa zea Hübner (Bong & Sikorowski, Reference Bong and Sikorowski1991); in cucumber against cucumber beetles, Diabrotica undecimpunctata Barber (Zehnder et al., Reference Zehnder, Kloepper, Yao and Wei.1997); in cotton to cotton bollworm, Helicoverpa armigera Hübner (Qingwen et al., Reference Qingwen, Ping, Gang and Qingnian1998), and in bell pepper against the green peach aphid, Myzus persicae (Sulzer) (Mardani-Talaee et al., Reference Mardani-Talaee, Nouri-Ganblani, Razmjou, Hassanpour, Naseri and Asgharzadeh2016; Reference Mardani-Talaee, Razmjou, Nouri-Ganbalani, Hassanpour and Naseri2017).
Secondary plant chemicals, especially phenolic compounds are important resistance factors of plants, having unfavorable effects on insect growth and feeding behavior (Cipollini et al., Reference Cipollini, Stevenson, Enright, Eyles and Bonello2008). Furthermore, flavonoids are one of the largest phenolic compounds in plants that play a key role in plant defense against pests and diseases (Hondo et al., Reference Hondo, Yoshida, Nakagawa, Kawai, Tamura and Goto1992). Qingwen et al. (Reference Qingwen, Ping, Gang and Qingnian1998) reported polyphenol and terpenoid contents of cotton plants treated with Pseudomonas gladioli influenced the relative growth rate, consumption rate, and the digestibility of feed in H. armigera. Additionally, glucosinolates (GSLs) (a group of naturally-occurring thioglucosides) are the main secondary metabolites accumulated in Brassicaceae plants (Halkier & Gershenzon, Reference Halkier and Gershenzon2006), and are essential in the nutrition of the cabbage aphid (Tjallingii, Reference Tjallingii1976). Many studies have demonstrated that GSL accumulation can be ‘induced’ by various factors, such as insect attack (Lammerink et al., Reference Lammerink, MacGibhon and Wallace1984; Birch et al., Reference Birch, Griffiths and Smith1990; Bennett & Wallsgrove, Reference Bennett and Wallsgrove1994). Moreover, the presence of higher contents of GSLs in Brassicaceae plants induces plant resistance. GSLs are significant factors impairing the nutritional quality of the plant family of Brassicaceae and restricting their use as high-quality protein animal feed (Jezek et al., Reference Jezek, Haggett, Atkinson and Rawson1999).
Biostimulants can affect the amounts of defensive chemical components in plants against herbivorous insects. Therefore, the aim of this research was to evaluate the effects of humic acid as an organic fertilizer and PGPR as a bio-fertilizer on the contents of phenolic compounds and GSLs in canola leaves and the resultant effects on antibiosis and tolerance parameters of canola for B. brassicae, which could then be used in the integrated management of this pest.
Materials and methods
Plant collection
The seeds of tested canola plants (cv. Jerry) were procured from Research, Education, Agriculture, and Natural Resources of Kerman, Iran, and were grown individually in 20-cm-diameter pots filled with a mixture of soil, sand, and manure (2:1:1). The plants were reared in a greenhouse (20–30°C, 60 ± 5% RH and natural photoperiod).
Insect colony
The used aphids in the experiments were acquired from the aphid colony reared in the laboratory of the plant protection department of Shahid Bahonar University, Kerman, Iran, in September 2017 and transferred to the potted plants under the above-defined conditions. Aphids were reared on canola plants (cv. Jerry) for four generations before starting the experiments.
Induction treatments
In this study, four different treatments were used on canola plants twice (two and six-leaf stages): (1) the use of an aqueous solution of distilled water for control; (2) addition of humic acid to the pots (2.5 mg kg−1/pot−1) with irrigation water; (3) the spraying of Roshdafza (commercial product of Biorun company) as PGPR on plants (3cc litre−1 water), which contain Pseudomonas fluorescens strain NBTR168, Azotobacter chroococcum strain NBTAzt2; and Azospirillum brasilense strain NBTAzoof (each of these bacteria with the rate of 2 × 107 CFU ml−1) (4) integrated application of both treatments (humic acid + PGPR).
Canola plants were exposed to cabbage aphid 48 h after the second application of the treatments.
Life table study
To evaluate the effects of studied treatments on the life history parameters of the cabbage aphid, experiment was carried out using clip cages (6 cm diameter and 1.5 cm depth) established on leaves of potted canola plants (N = 5 potted plant for each treatment) in a growth chamber (20 ± 1°C, 60 ± 5% RH and 16L: 8D).
48 h after the second application of treatments, adult apterous aphids individually were placed on the lower surface of a given leaf of the respective plant. Each clip cage was considered a replicate, and a total of 30 clip cages were established on canola plants in each treatment. After 24 h, aphid mother and all nymphs except one nymph were removed. Each cage was monitored daily until the maturity of the aphid to determine nymphal developmental time and survival rate of B. brassicae for each treatment. After maturity, daily observations were followed until each female aphid died. The numbers of nymphs produced per female aphid were recorded daily, and then nymphs were completely removed from the cages. The obtained data from this experiment was used for assessing the population growth parameters.
Tolerance experiment
This experiment was carried out with the four above-mentioned treatments (ten replications for canola plants without any infestation to cabbage aphid and ten replications for canola plants with artificial infestation). The plants reached the six-leaf stage at the onset of the experiment. In the ten later replications, each plant within a plastic cage was infested with five apterous adults of B. brassicae. Every day, the plants were checked, and the number of aphids per plant was regulated to five aphids. The experiment was terminated 21 days after infestation. Then, the percentage of reduction in growth parameters, including leaf area, chlorophyll content, shoot length (the height of plant from the surface of the soil), root length, fresh weight, and dry weight in the infested plants to non-infested plants for each treatment was calculated as:
Where RGP % is the percentage reduction of the growth parameter in infested plants to non-infested plants, GP N is the growth parameter in non-infested plants, and GP I is the growth parameter in infested plants.
Determination of total phenolic compounds
The level of total phenolic compounds in leaves of canola plants treated with humic acid and PGPR was measured based on Ronald & Laima (Reference Ronald and Laima1999) for which a 0.1 mg sample of the leaf was milled in 95% ethanol and permitted to extract for 24–72 h. Thereafter, to 1 ml of the sample, 1.5 ml of 95% ethanol was added and made up to a volume of 5 ml with distilled water. To this mixture, 0.5 ml of 50% Folin's reagent and 1 ml of 5% sodium carbonate was added and vortexed. The mixture was kept in the dark for 1 h. Then, the absorbance was measured at 725 nm using a spectrophotometer (Ronald & Laima, Reference Ronald and Laima1999). Plants samples treated with distilled water used as control treatment.
Determination of flavonoids
The leaves of canola (0.1 g) grown in studied treatments were weighed and placed in a porcelain mortar and pestle to be crushed. Then, 10 ml of acidified ethanol was slowly added to the contents of porcelain mortar. After grinding the plant samples, they were centrifuged at 8000 × g for 10 min. The extract was passed through Whatman filter paper No.1 and it was put in a hot water bath (80°C) for 10 min for measuring the flavonoids. After cooling of the extracts, the absorbance was read using a spectrophotometer in the wavelengths of 270 nm (Krizek et al., Reference Krizek, Brita and Mirecki1998).
Determination of total GSL content
The powder (0.1 g) of canola leaves under each treatment was transferred to a 10 ml glass tube with a lid. Then, using the method described by Ishida et al. (Reference Ishida, Kakizaki, Ohara and Morimitsu2011), GSL was extracted as follows. To the powder in glass tubes, 4.8 ml of 80% methanol kept at room temperature was added. After the addition of 0.2 ml of 5 mM sinigrin as an internal standard, the tubes were kept at 25°C for 30 min. and then shaken reciprocally for 30 min. in a shaker. The tubes were centrifuged at 1600 × g for 10 min. The supernatant was used as a crude extract.
Palladium colorimetric analysis of the total GSL content
Colorimetric analysis of the total GSL content was performed by simplifying the method described by Møller et al. (Reference Møller, Ploger, Sørensen and Sørensen1985). Purification with ion-exchange chromatography was omitted. To 0.2 ml of crude GSL extract, 0.3 ml of distilled water and 3 ml of 2 mM palladium chloride reagent, in which 3.54 mg PdCl2 had been dissolved in 1.68 ml of concentrated hydrochloric acid and diluted to 1000 ml with distilled water, were added and mixed. After incubation at 25°C for 1 h, absorbance at 425 nm was measured using a spectrophotometer.
Data analysis
The raw life-history data of all individuals of B. brassicae were analyzed using the TWOSEX-MSChart program (Chi, Reference Chi2017) based on the age-stage, two-sex life table theory (Chi & Liu, Reference Chi and Liu1985). The age-stage specific survival rate (s xj) (where x is the age and j is the stage), age-stage specific fecundity (f xj), age-specific survival rate (l x), age-specific fecundity (m x), and age-specific maternity (l xmx) were evaluated from the daily records of the survival and fecundity of all individuals in the cohort. Furthermore, the fertility life table parameters including the intrinsic rate of natural increase (r), net reproductive rate (R 0), mean generation time (T), finite rate of increase (λ), and doubling time (DT) were calculated.
The intrinsic rate of increase was estimated using the iterative bisection method from the Euler–Lotka formula with age indexed from 0 (Goodman, Reference Goodman1982):
The means and standard errors of life table parameters were determined using the bootstrap technique (Efron & Tibshirani, Reference Efron and Tibshirani1993; Huang & Chi, Reference Huang and Chi2012) with 100,000 resampling. The bootstrap method is embedded in the computer program TWOSEX-MSChart. The paired bootstrap test was used to evaluate the differences between treatments.
Data of the tolerance experiment, determination of total phenolics, flavonoids and GSLs were assessed for normality with the Kolmogorov–Smirnov test and were analyzed using the one-way analysis. Arcsin square root transformation was applied for percentage data to handle the heterogeneity of variance prior to data analysis. Then, multiple comparisons were made using the Tukey test (SPSS, 2015).
Result and discussion
There was no significant difference in the nymph period of B. brassicae among treatments (F = 8.49; df = 3, 116; P > 0.05; table 1). However, tested treatments significantly influenced longevity (F = 137.14; df = 3, 116; P < 0.05), reproductive period (F = 404.93; df = 3, 116; P < 0.05), and fecundity of this aphid (F = 545.14; df = 3, 116; P < 0.05) (table 1). The longevity of adult females under PGPR and PGPR + humic acid treatments was significantly lower compared with control treatment (table 1). Moreover, no significant difference was found in adult longevity between humic acid and the other studied treatments. The reproductive period and fecundity of B. brassicae on plants treated with humic acid, PGPR, and PGPR + humic acid were significantly lower than those on plants without fertilizer treatment (control) (table 1). There are several reports on the significant effects of humic compounds and PGPR on the biological characteristics of other aphids such as Aphis gossypii Glover reared on cucumber (Fahimi et al., Reference Fahimi, Ashouri, Ahmadzadeh, Hoseini Naveh, Asgharzadeh and Maleki2014; Hosseini, Reference Hosseini2014) and M. persicae reared on bell pepper (Mardani-Talaee et al., Reference Mardani-Talaee, Razmjou, Nouri-Ganbalani, Hassanpour and Naseri2017). In our research, the lowest number of nymphs produced per female was calculated for PGPR treatment but did not differ significantly from the PGPR + humic acid treatment (table 1). The lower fecundity of B. brassicae on bacterial treatments indicated a minor suitability of canola plants treated with PGPR than the others for the cabbage aphid. Moreover, Fahimi et al. (Reference Fahimi, Ashouri, Ahmadzadeh, Hoseini Naveh, Asgharzadeh and Maleki2014) reported that Pseudomonas fluorescens strains UTPF68 and PF169 could decrease the average progeny produced by A. gossypii adults on cucumber. Our results showed that the adult pre-reproduction period (APRP) of each reproduced female was zero in the four tested treatments and all females that emerged started producing immediately. The APRP is calculated based on the adult stage. It assumes all females emerged at the same time. Therefore, no significant differences were observed in the APRP of B. brassicae among the tested treatments.
Table 1. Mean (±SE) nymph period, adult longevity, reproductive period and fecundity of Brevicoryne brassicae L. on canola plants treated with humic acid and PGPR.

Means within a column followed by different letters are significantly different according to the paired bootstrap test at 5% significance level.
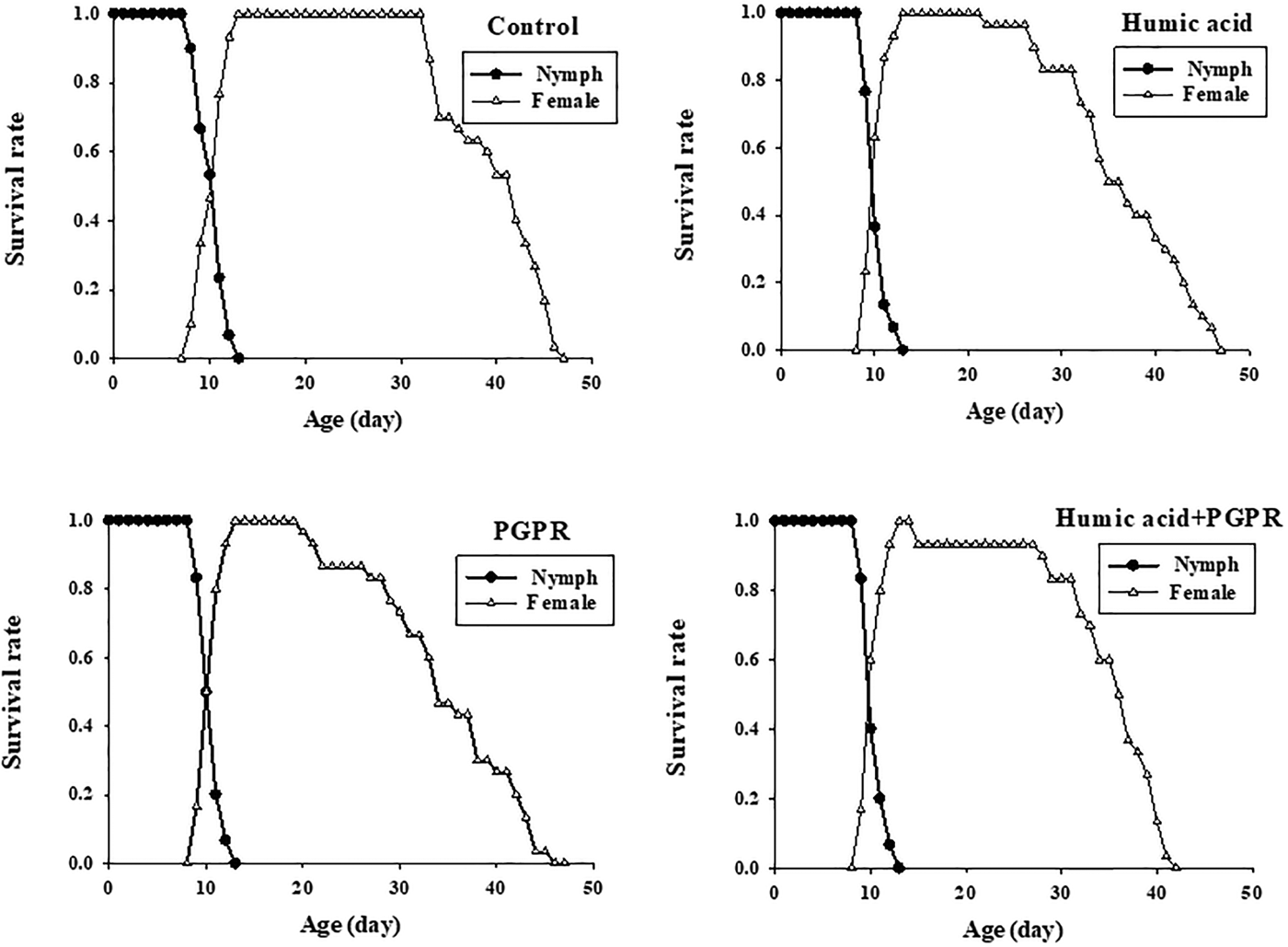
The age-stage specific survival rate (s xj) represents the probability that a nymph of B. brassicae will survive to age x and stage j (fig. 1). The variable development rates among individuals in the cohort resulted in an overlapping of the stage-specific survivorship curves. The age-stage-specific survival rate (s xj) curve showed a similar pattern in all the different fertilizer treatments. When all stages are pooled, the age-specific survival rate (lx) gives a simplified overview of the survival history of the whole cohort (fig. 2). The l x curve indicated that survival was approximately 100% in the nymph period, and then it declined slowly until the death of the last adult. This could be because of increased secondary metabolites in the plant and the composition of the epicuticular lipids (Eigenbrode & Espelie, Reference Eigenbrode and Espelie1995). Death of the last female under treatments of humic acid, PGPR, PGPR + humic acid, and control occurred at days of 47, 46, 41, and 47, respectively.
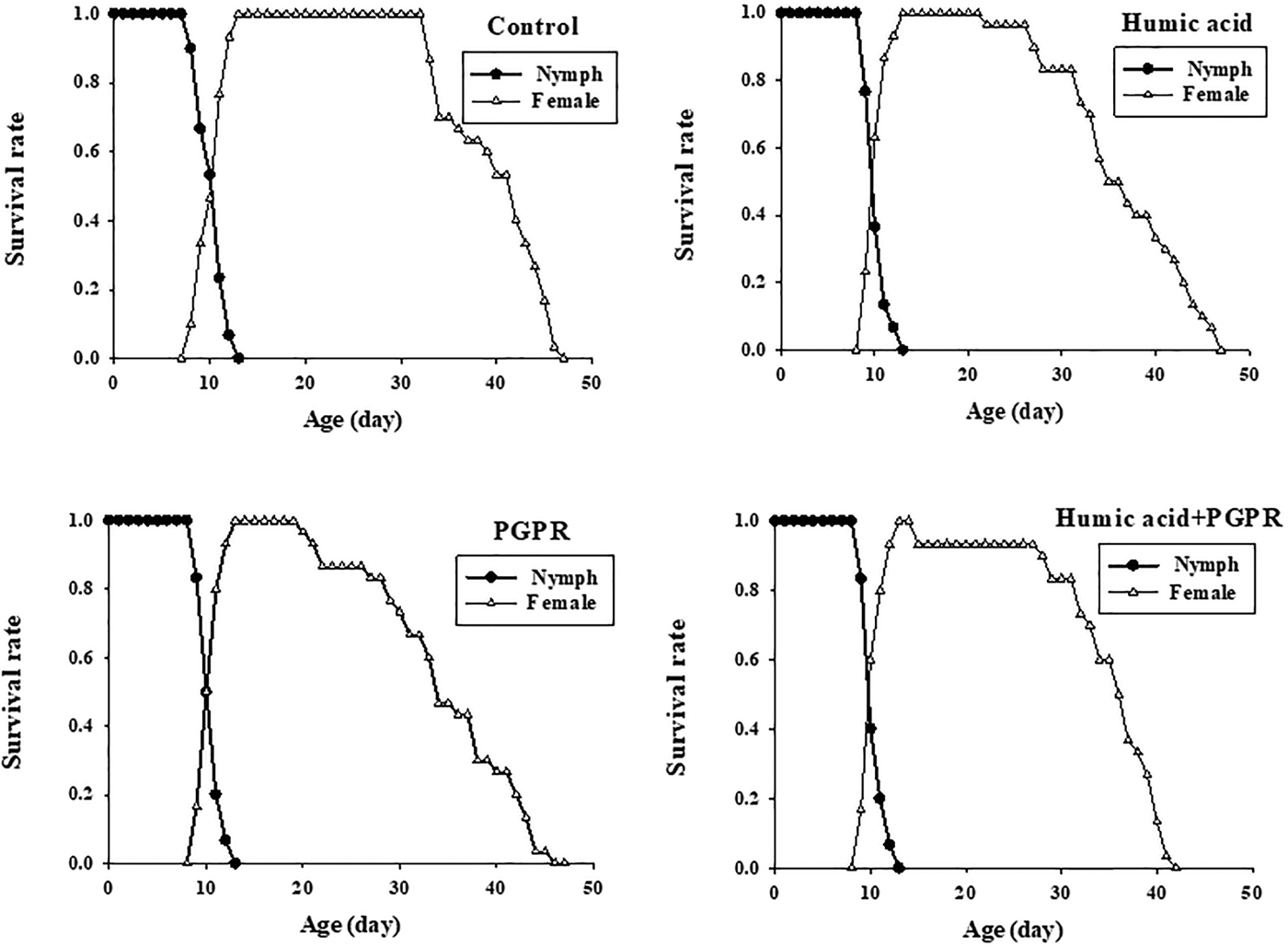
Fig. 1. Age-stage specific survival rate (s xj) of Brevicoryne brassicae L. on canola plants treated with humic acid and PGPR.
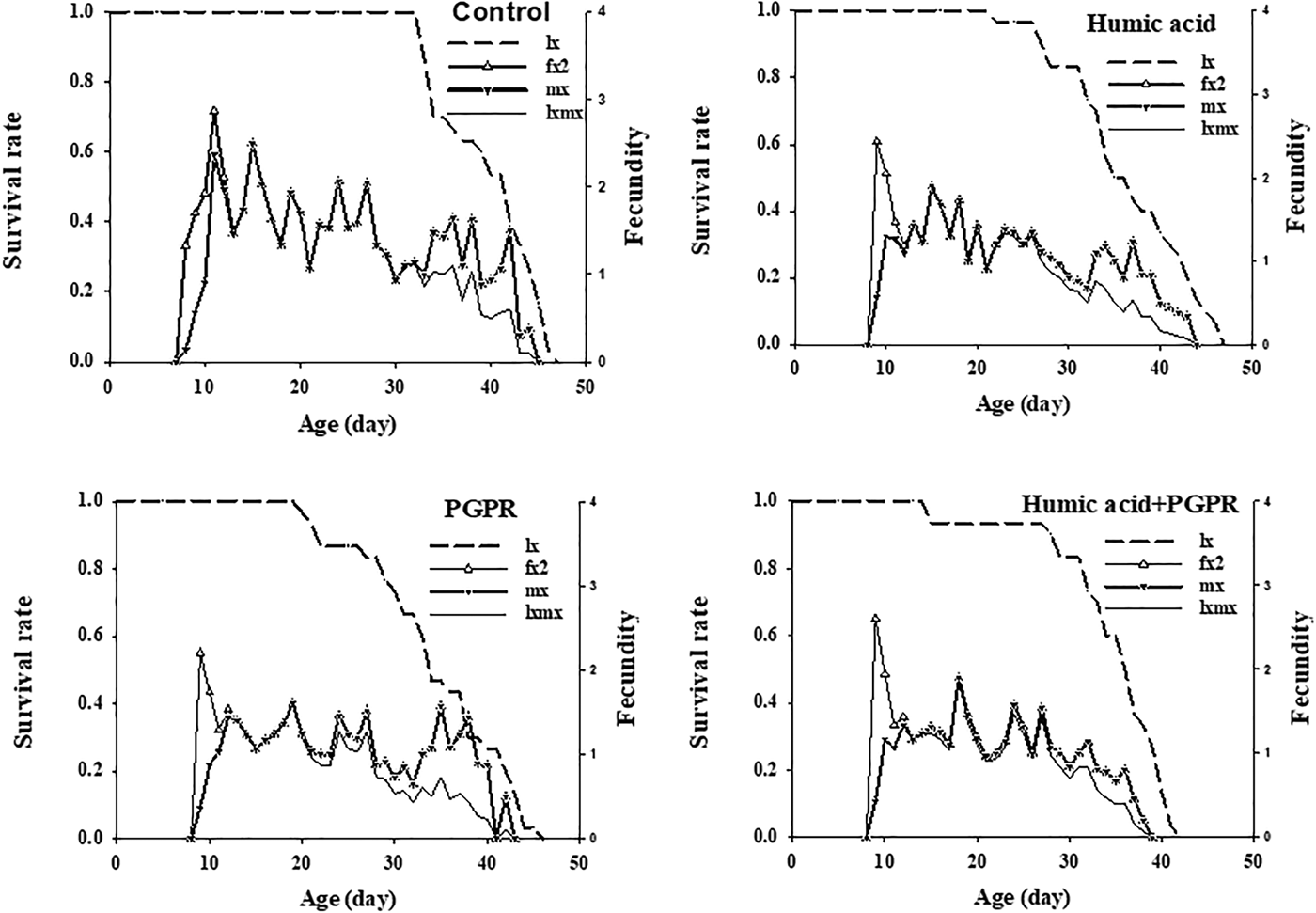
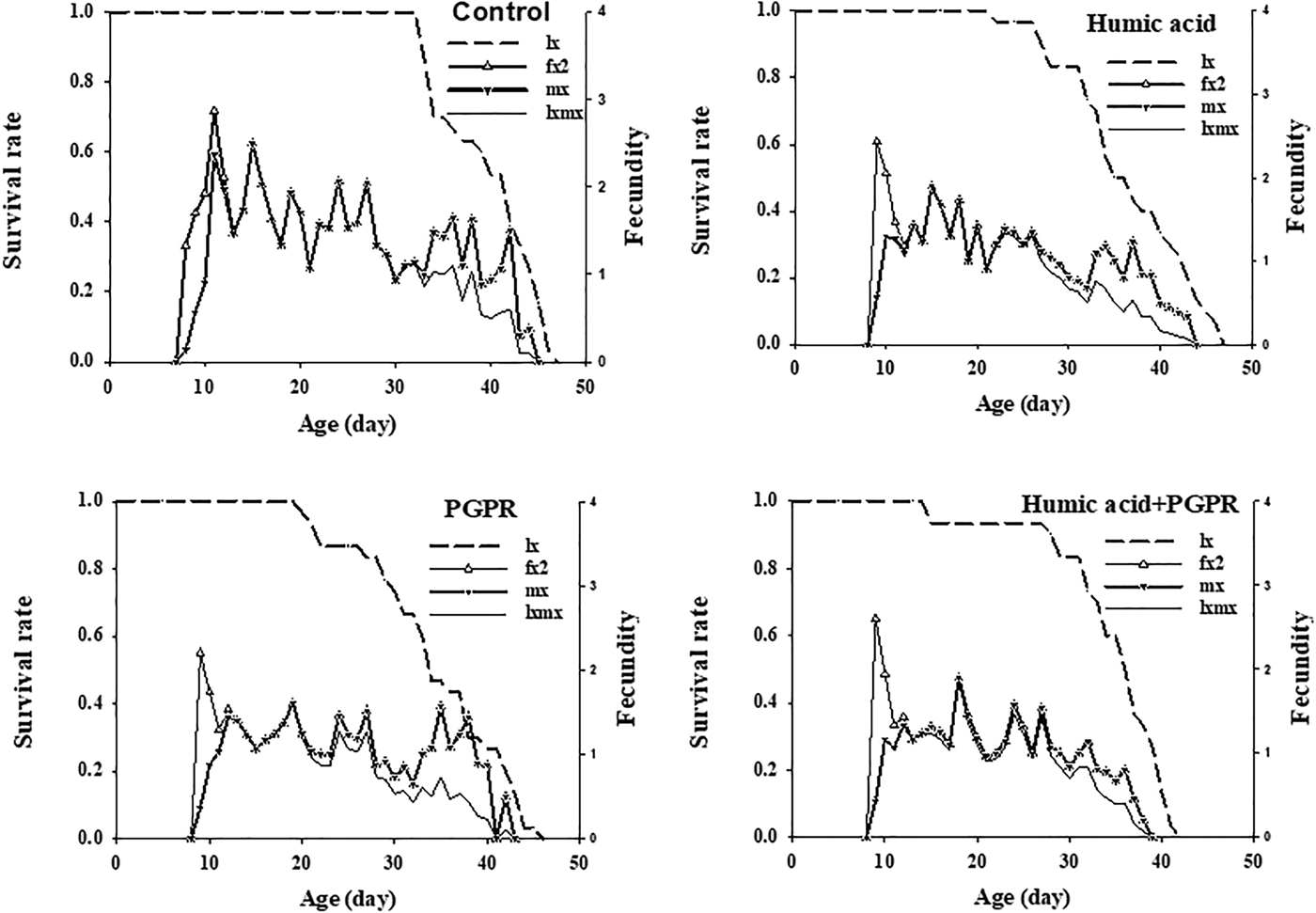
Fig. 2. Age-specific survival rate (l x), female age-specific fecundity (f x2), age-specific fecundity (m x), and age-specific maternity (l xmx) of Brevicoryne brassicae L. on canola plants treated with humic acid and PGPR.
The highest f x2 peak in treatments of humic acid, PGPR, PGPR + humic acid, and control was 2.4, 2.2, 2.6, and 2.9, respectively. The age-specific fecundity (m x) and the age-specific maternity (l xmx) of B. brassicae are also shown in fig. 2. The highest peaks of m x and l xmx were recorded as 1.9 on humic acid (at 15 day), 1.6 on PGPR (at 19 day), 1.9 on PGPR + humic acid (at 18 day) and 2.5 on control (at 15 day).
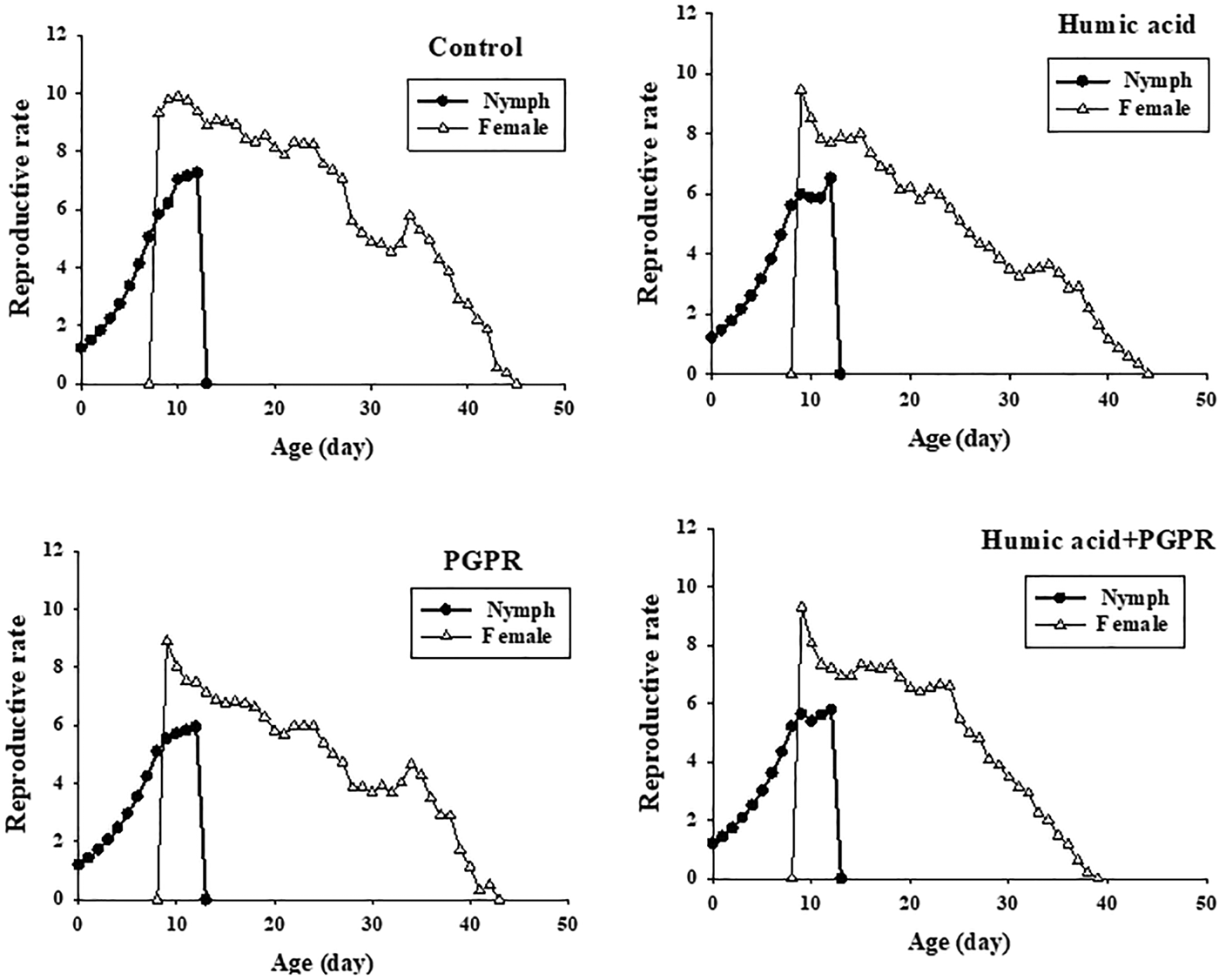
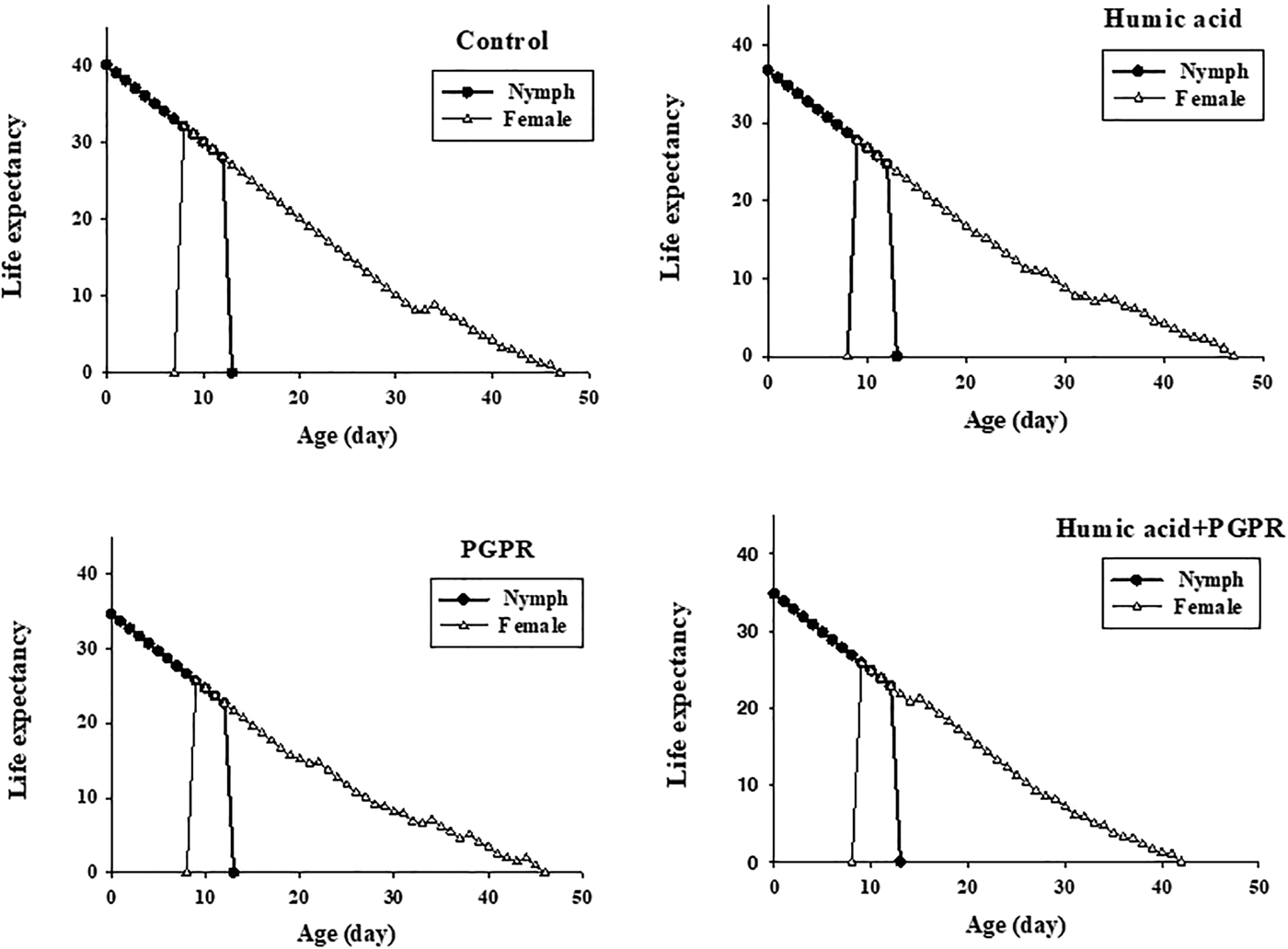
The reproductive value (vxj) gives the expected contribution of individuals of age x and stage j (fig. 3). At age 0, the reproductive values (v 01), were the same as the finite rates on the four treatments, i.e. 1.211 day−1 on humic acid, 1.198 day−1 on PGPR, 1.201 day−1 on PGPR + humic acid and 1.224 day−1 on control (fig. 3). The values of v xj peaks on these treatments increased to 9.452 day−1, 8.896 day−1, 9.30 day−1, and 9.880 day−1 after the emergence of female adults (fig. 3). It shows that individuals at the peak reproductive phases can contribute much more than a newborn nymph. The age-stage life expectancy (e xj) (where x is the age and j is the stage) shows the expected lifespan for an individual of age x and stage j (fig. 4). The life expectancies of B. brassicae at age 0 (e 01) were 36.7, 34.7, 34.8, and 40.1 days, on treatments of humic acid, PGPR, PGPR + humic acid and control, respectively, and, at the stage of aphid maturity, 27.7, 25.7, 25.8, and 32.1 days (fig. 4).

Fig. 3. Age-stage reproductive value (V xj) of Brevicoryne brassicae L. on canola plants treated with humic acid and PGPR.

Fig. 4. Age-stage specific life expectancy (e xj) of Brevicoryne brassicae L. on canola plants treated with humic acid and PGPR.
The population growth parameters are appropriate indexes to compare pest performance under different conditions in host plants (Carey, Reference Carey1993; Southwood & Henderson, Reference Southwood and Henderson2000). The net reproductive rate (R 0) and the intrinsic rate of population increase (r) are the two key demographic parameters used to evaluate the fitness of populations across diverse climatic and food-related conditions (Liu et al., Reference Liu, Li, Gong and Wu2004). The value of the net reproductive rate (R 0) under fertilizer treatments of humic acid, PGPR, and PGPR + humic acid was significantly lower compared with no-fertilizer treatment (control) (F = 545.14; df = 3, 116; P < 0.05; table 2). In our study, this could be attributed to the lower fecundity of B. brassicae under the mentioned fertilizer treatments than control treatment. Furthermore, Mohamadi et al. (Reference Mohamadi, Razmjou, Naseri and Hassanpour2017) reported that R 0 of Tuta absoluta (Meyrick) was significantly lower on tomato plants treated with PGPR (Pseudomonas fluorescens) and humic fertilizer than on untreated plants. It may be related to the promoted plant growth and induced systemic resistance. The lowest values of the intrinsic rate of increase (r) and finite rate of increase (λ) of B. brassicae were achieved in the PGPR treatment (0.181 and 1.198 day−1, respectively) and the highest values of these parameters were observed in control (0.202 and 1.224 day−1, respectively) (F = 150.28; df = 3, 116; P < 0.05 and F = 149.74; df = 3, 116; P < 0.05; respectively) (table 2). Mardani-Talaee et al. (Reference Mardani-Talaee, Nouri-Ganblani, Razmjou, Hassanpour, Naseri and Asgharzadeh2016) showed that r of M. persicae on bell pepper treated with Bacillus subtilis and Glomus Intraradices × Pseudomonas fluorescens was significantly lower than on control (no treatment). In the current study, there was no significant difference in the generation time (T) of B. brassicae among the tested treatments (F = 49.98; df = 3, 116; P > 0.05; table 2). However, the DT was longest in treatments of PGPR and PGPR + humic acid and shortest in control treatment (F = 153.20; df = 3, 116; P < 0.05; table 2).
Table 2. Mean (±SE) life table parameters of Brevicoryne brassicae L. on canola plants treated with humic acid and PGPR.

R 0, net reproductive rate; r m, intrinsic rate of increase; λ, finite rate of increase; T, mean generation time; DT, doubling time.
Means within a column followed by different letters are significantly different according to the paired bootstrap test at 5% significance level.
Our results demonstrated that the population growth of B. brassicae under treatments containing PGPR was limited mostly by the shorter reproductive period and poor fecundity. Furthermore, the decreased performance of the cabbage aphid on plants treated with PGPR was related to the lower age-stage life expectancy (e xj) of individuals used in the life table study in this treatment. In fact, PGPR led to the lowest intrinsic rate of increase, finite rate of increase, and the reproductive value (v xj) of B. brassicae on canola plants, and, hence, it appeared to be the most suitable induction treatment to reduce the population growth of this pest among the tested treatments. Therefore, it can be concluded that the PGPR cause antibiosis resistance in canola plants to cabbage aphid.
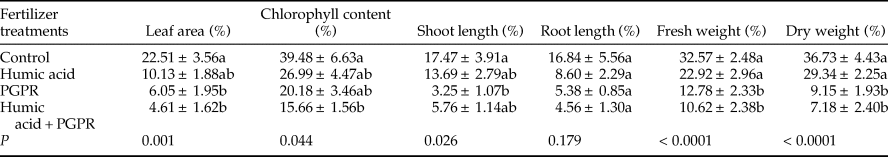
In this experiment, the analysis of growth parameters data showed significant differences in leaf area (F = 6.76; df = 3, 36; P = 0.001), chlorophyll content (F = 5.49; df = 2, 98; P = 0.044), shoot length (F = 3.47; df = 3, 36; P = 0.026), fresh weight (F = 13.15; df = 3, 26; P < 0.0001), and dry weight (F = 14.77; df = 3, 36; P < 0.0001). No significant differences were found in root length among the tested treatments (F = 1.73; df = 3, 36; P = 0.179; table 3).
Table 3. The mean (±SE) percentage reduction of growth parameters in aphid-infested canola plants treated with humic acid and PGPR.
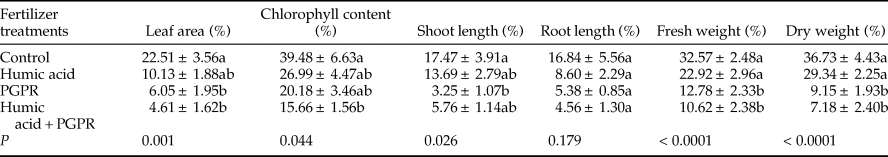
Means followed by a different letter within a column are significantly different (Tukey's HSD test; P < 0.05).
In this study, the percentage reduction of the leaf area under PGPR and PGPR + humic acid treatments (6.05 and 4.61, respectively) was calculated to be significantly lower compared with control treatment (22.51) (table 3). Furthermore, the percentage reduction of Chlorophyll content was lowest on PGPR + humic acid treatment (15.66) and the highest on control treatment (39.48) (table 3). The least and the maximum percentage reduction of shoot length was determined in treatments of PGPR (3.25) and control (17.47), respectively (table 3). In addition, the percentage reduction of fresh weight and dry weight was significantly lower in treatments of PGPR and PGPR + humic acid rather than humic acid and control (table 3).
Our research indicated that PGPR application resulted in a greater tolerance level of canola plants for B. brassicae. PGPR can accelerate plant growth by helping to escape from attacks by some pathogenic microorganisms and pests (Pineda et al., Reference Pineda, Zheng, Van Loon, Pieterse and Dicke2010). Cakmakci et al. (Reference Cakmakci, Erat, Erdo.an and Donmez2007) showed the considerable effects of PGPR on plant growth parameters such as shoot fresh weight, plant height, and leaf area in wheat and spinach. Cheng et al. (Reference Cheng, Park and Glick2007) reported inoculation of the Pseudomonas putida strain UW4, containing the ACC deaminase enzyme in the presence of salt, significantly improved canola growth. Generally, PGPR can increase plant growth through the production of hormones such as auxins, gibberellins, and cytokinins (Bhattacharyya & Jha, Reference Bhattacharyya and Jha2012). The positive effect of an integrated application of PGPR and humic acid on plant growth indices was evident in our study. Furthermore, Ahmad et al. (Reference Ahmad, Daur, Al-Solaimani and Yasir2016) concluded that an integrated use of humic acid and PGPR was an effective approach to improve canola nourishment and yield. Some research has shown that humic acid application could increase the growth indices such as the dry weight, fresh weight, and shoot length of maize (Eyheraguibel et al., Reference Eyheraguibel, Silvestre and Morard2008) and pepper (Gulser et al., Reference Gulser, Sonmez and Boysan2010). The effect of humic acid was dependent on the generation of reactive oxygen species (ROS) in the inducement of root growth and the spread of lateral root (Cordeiro et al., Reference Cordeiro, Catarina, Silveira and De Souza2011).
Our results showed that fertilizer treatments significantly influenced the amount of total GSL in leaves of canola (F = 12.29; df = 3, 36; P < 0.001). The level of total GSL was significantly higher in treatments of PGPR and PGPR + humic acid rather than humic acid and control (table 4). Earlier studies had shown a significant negative correlation between the GSL content of Brassica species and populations of aphids feeding on them such as Lipaphis erysimi (Kaltenbach) (Labana et al., Reference Labana, Ahjua, Gupta and Brar1983; Malik et al., Reference Malik, Anand and Srinivasachar1983).
Table 4. The mean (±SE) amount of total Glucosinolate, total phenol, and flavonoids in aphid-infested leaves of canola plants treated with humic acid and PGPR.
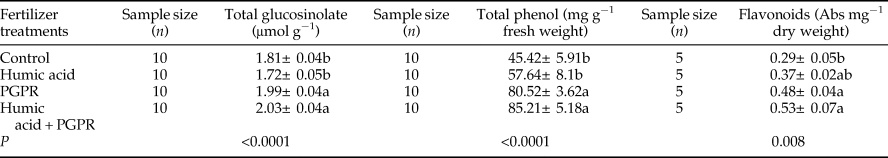
Means followed by a different letter within a column are significantly different (Tukey's HSD test; P < 0.05).
In the current study, the amount total phenolic compounds and flavonoids varied significantly among different fertilizer treatments (F = 10.12; df = 3, 36; P < 0.001 and F = 5.64; df = 3, 16; P = 0.008; respectively). The level of total phenolic compounds in leaves of canola was significantly higher under treatments of PGPR and PGPR + humic acid rather than humic acid and control (table 4). Moreover, the flavonoids in treatments of PGPR and PGPR + humic acid were significantly more than control (table 4). The amount of flavonoids in humic acid treatment did not differ significantly from the other tested treatments.
Chamam et al. (Reference Chamam, Sanguin, Bellvert, Meiffren, Comte, Wisniewski-Dye, Bertrand and Prigent-Combaret2013) demonstrated that phenolic compounds such as flavonoids and hydroxycinnamic derivatives were the main rice metabolites affected in response to Azospirillum bacteria. Also, foliar application of Pseudomonas fluorescens strain Pf1 could increase phenolic compounds of peanut plants (Meena et al., Reference Meena, Radhajeyalakshmi, Marimuthu, Vidhyasekaran, Doraiswamy and Velazhahan2000). The productions of secondary metabolites in plants are usually enhanced in response to environmental tensions such as herbivores and pathogens (Bourgaund et al., Reference Bourgaund, Gravot, Milesi and Gontier2010). Hence, phenolics are biologically effective secondary metabolites, negatively impacting development, on development, reproduction, and population growth parameters of the aphids (Wójcicka, Reference Wójcicka2010). Negative associations between the presence of phenolic compounds in plant species and aphid's invasion have been recorded for some aphid species (Havlíčková, Reference Havlíčková1995; Sandström et al., Reference Sandström, Telang and Moran2000). Chassy et al. (Reference Chassy, Bui, Renaud, Van Horn and Mitchell2006) reported that humic acid increased flavonoids, total phenol, and ascorbic acid in organic tomatoes. Hanafy Ahmed et al. (Reference Hanafy Ahmed, Nesiem, Hewedy and Sallam2010) showed that the amount of sugars, amino acids, proteins, and total phenol content in green bean plants increased after humic acid application. Furthermore, tomato plants treated with humic substances had a negative effect on populations of Liriomyza trifolii (Burgess) (Yildirim & Unay, Reference Yildirim and Unay2011).
In our research, humic acid alone (without integrated with PGPR) was not significantly effective on the resistance of canola to cabbage aphid. This could be attributed to the level of consumption, source, and type of humic substances (Arancon et al., Reference Arancon, Edwards, Lee and Byrne2006). Our results indicated that canola plants treated with PGPR having a high level of phenolic compounds were more resistant to the cabbage aphid. Mardani-Talaee et al. (Reference Mardani-Talaee, Nouri-Ganblani, Razmjou, Hassanpour, Naseri and Asgharzadeh2016) demonstrated that phenolics in leaves of bell pepper could decrease fecundity and the growth rate of the M.persicae population. Previous research suggested that the PGPR was able to induce resistance responses against insects by promoting plant growth and distinctive alterations in biochemical profiles and plant molecular mechanisms (Zehnder et al., Reference Zehnder, Kloepper, Yao and Wei.1997; Rashid & Chung, Reference Rashid and Chung2017). Zebelo et al. (Reference Zebelo, Song, Kloepper and Fadamiro2016) reported that cotton root colonization by PGPR could induce systemic resistance to S. exigua due to increased plant hormones. In addition, induced systemic resistance has been found in cotton plants against H. armigera (Rajendran et al., Reference Rajendran, Samiyappan, Raguchander and Saravanakumar2007), and in tomato plants against whitefly (Hanafi et al., Reference Hanafi, Traore, Schnitzler, Woitke, Hanafi and Schnitzler2007) due to PGPR application. The relative growth rate and the relative consumption rate of H. armigera larvae were reduced in cotton plants treated with Pseudomonas gladioli because of an increase in the content of polyphenol and terpenoids in cotton (Qingwen et al., Reference Qingwen, Ping, Gang and Qingnian1998). In fact, rhizobacteria can increase plant health and resistance to herbivore insects by triggering systemic defense responses (Rashid & Chung, Reference Rashid and Chung2017).
In summary, we concluded that canola plants treated by PGPR were the least suitable host for the B. brassicae. Therefore, PGPR could induce resistance in canola against the cabbage aphid. The research could provide valuable information for integrated management of B. brassicae in the canola fields. More attention should be paid to field experiments to obtain more accurate results with respect to the performance of this pest.
Acknowledgements
The authors appreciate Shahid Bahonar University (Iran) for financial support of this research.