Introduction
Macrocyclic lactones for veterinary endectocides that are currently used worldwide include avermectin (ivermectin, doramectin, and eprinomectin) and milbemycin (moxidectin). In particular, ivermectin has been used all over the world; it not only controls gastrointestinal nematodes and arthropod parasites of livestock but also gets rid of pest flies by residual active ingredient in feces (Miller et al., Reference Miller, Kunz, Oehler and Miller1981; Benz, Reference Benz1985; Drummond, Reference Drummond1985). However, parasiticide residues in dung are not selective. They kill non-target insects, which would otherwise accelerate degradation of the dung (Wall & Strong, Reference Wall and Strong1987; Madsen et al., Reference Madsen, Nielsen, Holter, Pedersen, Jespersen, Vagn Jensen, Nansen and Grønvold1990; Floate et al., Reference Floate, Colwell and Fox2002, Reference Floate, Wardhaugh, Boxall and Scherratt2005). In particular, dung beetles provide important ecosystem services in soil fertility and pest control by transporting and decomposing dung in pastures (Bornemissza, Reference Bornemissza1970; Nichols et al., Reference Nichols, Spector, Louzada, Larsen, Amezquita and Favila2008). Adverse effects of ivermectin residues on the larval survival and reproduction of dung beetles have been reported by Ridsdill-Smith (Reference Ridsdill-Smith1988), Wardhaugh & Rodriguez-Menendez (Reference Wardhaugh and Rodriguez-Menendez1988), Houlding et al. (Reference Houlding, Ridsdill-Smith and Bailey1991), Fincher (Reference Fincher1992), Holter et al. (Reference Holter, Sommer, Grønvold and Madsen1993), Lumaret et al. (Reference Lumaret, Galante, Lumbreras, Mena, Bertrand, Bernal, Cooper, Kadiri and Crowe1993), Sommer et al. (Reference Sommer, Grønvold, Holter, Madsen and Nansen1993), Krüger & Scholtz (Reference Krüger and Scholtz1997), Dadour et al. (Reference Dadour, Cook and Neesam1999), Errouissi et al. (Reference Errouissi, Alvinerieb, Galtierb, Kerboeufc and Lumaret2001), Iwasa et al. (Reference Iwasa, Nakamura, Fukaki and Yamashita2005, Reference Iwasa, Maruo, Ueda and Yamashita2007), and Pérez-Cogollo et al. (Reference Pérez-Cogollo, Rodríguez-Vivas, Delfín-González, Reyes-Novelo and Ojeda-Chi2015). Interference with dung degradation throughout reduction of abundance and activity of dung beetles could damage the productivity of pasture.
Eprinomectin was recently introduced to the animal health industry, and it has a broad spectrum of activity and high efficacy for cattle, including lactating cows (Schoop et al., Reference Schoop, Demontigny, Fink, Williams, Egerton, Mrozik, Fisher, Skelly and Turner1996a, Reference Schoop, Egerton, Eary, Haines, Michael, Mrozik, Eskola, Fisher, Slayton, Ostlind, Skelly, Fulton, Barth, Costa, Gregory, Campbell, Seward and Turnerb). Furthermore, eprinomectin can be applied to milking cows with zero milk holding time because eprinomectin has lower transferability than other avermectins (Williams et al., Reference Williams, Stuedemann, Bairden, Kerboeuf, Ciordia, Hubert, Broussard, Plue, Alva-Valdes, Baggott, Pinkall and Eagleson1997; Reist et al., Reference Reist, Forbes, Bonfanti, Beretta and Pfister2011; Gokbulut et al., Reference Gokbulut, Naturali, Rufrano, Anastasio, Yalinkilinc and Veneziano2012; Mason et al., Reference Mason, Pomroy, Lawrence and Schot2012). Effects of eprinomectin on non-target coprophagous insects have been studied in only a few papers in some countries (Wardhaugh et al., Reference Wardhaugh, Longstaff and Morton2001; Floate et al., Reference Floate, Colwell and Fox2002; Lumaret et al., Reference Lumaret, Errouissi, Galtier and Alvinerie2005; Iwasa & Sugitani, Reference Iwasa and Sugitani2014), and no information is available about its toxic effects on medium- and large-sized dung beetles. This study was performed to estimate the effects of eprinomectin by a pour-on application on the survival, reproduction, and feeding activity of the dung beetles Onthophagus lenzii Harold and Copris ochus Motschulsky (Coleoptera: Scarabaeidae), which are important dung-degrading species in Japan.
Materials and methods
Cattle used and dung collection
The experiments were conducted in the pasture of Yachiyo Farm southwest of Obihiro City, Hokkaido, Japan. Six Holsteins (breeding cows, aged 10–25 months) were selected. On 1 June 2015 (experiment 1) and 6 June 2016 (experiment 2), eprinomectin (Eprinex Topical; Merial, Haarlem, The Netherlands) was applied topically on the back from the withers to the tail head of cattle by pour-on formulation at the recommended dose of 500 µg kg−1 of body weight. Ten Holsteins (breeding cows, aged 10–35 months) were used as controls. The cattle were fed mainly with green grass composed of Kentucky bluegrass, timothy, and ladino clover.
Fresh dung pats were collected from treated and control groups on the day prior to treatment and at 1, 3, 7, 14, and 21 days after treatment. During collection, each dung pat was thoroughly mixed and stored at −20°C until used.
Determination of eprinomectin concentration
The concentration of eprinomectin in the feces was determined using high-performance liquid chromatography (HPLC) following the method of Payne et al. (Reference Payne, Hicks and Wehner1995) with some modifications. A 5 g dung sample on each date after treatment was analyzed. The extraction was carried out with homogenization (Physcotron, Microtec Co., Ltd) at 10,000 rpm for 1 min (instead of sonication), and the concentration was calculated from the calibration curve of a standard solution. The determination limit of concentrations was determined at 0.05 ppm.
The effect of eprinomectin on survival and reproduction of O. lenzii
O. lenzii is a medium-sized dung-burying beetle, which is distributed throughout Japan. A 50 g dung sample collected from treated (1, 3, and 7 days after treatment in experiment 1) and control cattle was placed on andosol (15 cm depth) in a plastic container (12 cm diameter × 18 cm depth). Three male–female pairs were placed on dung in each container, which was covered with gauze and maintained at 22°C and the light regime of 16L:8D. Dung samples were replaced with fresh ones once per week for 2 months. At that time, andosol from each container was sieved to collect and record the numbers of brood balls produced by the beetles. The balls were placed in a plastic cup (5 cm diameter × 3 cm depth) with andosol and kept at 22°C (light regime 16L:8D) until adult emergence. Rearing tests were replicated five times for each collection date of dung from treated and control cattle.
The effect of eprinomectin on survival and reproduction of C. ochus
C. ochus is the largest dung-burying beetle in Japan; it is distributed in Japan, Korea, and China. A 100 g sample of dung collected at 3 days after treatment in experiment 1 and control, and collected at 7 and 14 days after treatment in experiment 2 and control, were placed on andosol (30 cm depth) in a plastic container (30 cm diameter × 40 cm depth). One male–female pair was placed on dung in each container, and the rims of containers were wrapped in gauze. Feces were replaced with fresh samples once per week for 90 days; this period corresponds to the breeding season (July–September) of C. ochus in Japan. At that time, andosol from each container was sieved to collect the brood balls produced by the beetles every 30 days. The balls collected were placed in a plastic cup (12 cm diameter × 6 cm depth) with andosol and until adult emergence. Rearing tests of adults and larvae were replicated ten times for each collection date of dung from treated and control cattle at 22°C with a light regime of 16L:8D.
The effects of eprinomectin on feeding activity of O. lenzii and C. ochus
Each dung sample in the rearing container was inspected when it was replaced with fresh dung, and the level of feeding (F) was assessed on an arbitrary scale of 0–4 based on Wardhaugh & Rodriguez-Menendez (Reference Wardhaugh and Rodriguez-Menendez1988) with some modifications (i.e., 0 = no feeding; 1 = one feeding trace on surface of dung; 2 = feeding trace of two or more; 3 = most feces was eaten, but surface part of dung was left; 4 = all of the feces was decomposed into fiber or carried to a burrow).
Statistical analysis
The log-rank test was used to test for differences in the adult mortality between controls and treatments. Then, a multiple testing adjustment of P-values was applied using the Bonferroni method for each comparison between the groups. The Bonferroni correction required setting the significance level at 0.0083 (=0.05/6; O. lenzii in experiment 1) or 0.0167 (=0.05/3; C. ochus in experiment 2).
The generalized linear mixed models (GLMMs) with Poisson error and log-link function were used to test the numbers of brood balls and feeding levels, and the GLMMs with binomial error and log-link function were used to test the adult emergence rates. The numbers of brood balls and adult emergence rates were response variables, days of dung post-treatment were explanatory variables, and replicates were random effects. In the feeding activity, feeding levels on each date were the response variables; days after the beginning of rearing and type of dung were explanatory variables, and replicates were random effects. Significance levels for comparisons on these tests were adjusted by Tukey's method. All tests were performed using R 3.2.4. Software (R Development Core Team 2016).
Results
Determination of eprinomectin concentration
Eprinomectin in cattle dung attained maximal concentration at 1 day after treatment, followed by marked declines at 3 and 7 days, and it was not detected at 14 days or later in experiments 1 and 2 (fig. 1). No eprinomectin was detected in control dung of both control experiments.

Fig. 1. Eprinomectin concentrations in dung (ppm of wet weight) after treatment (500 µg kg−1) in experiments 1 and 2. No eprinomectin was detected in both controls (experiments 1 and 2).
The effects of eprinomectin on adult survival, brood ball production, emergence, and feeding activity of O. lenzii
Adult mortalities of O. lenzii in dung at 7 days post-treatment were not significantly different from those in control dung from 14 to 56 days after the beginning of rearing (fig. 2). However, in dung at 1 and 3 days post-treatment, adult mortalities significantly increased from 21 to 56 days, and attained 100% at 42 and 35 days after the start of rearing, respectively (fig. 2; χ2 = 39.8, P < 0.0083; χ2 = 33.7, P < 0.0083). The numbers of brood balls per female were 17.0 in dung from control cattle, but no brood balls were constructed in dung at 1-day post-treatment. In dung at 3 days post-treatment, the numbers of brood balls were significantly reduced (table 1; Z = −5.2, P < 0.001), and an equivalent level to the control was restored at 7 days post-treatment. The adult emergence rate was 41.9% in dung from control cattle, but that in dung at 3 and 7 days post-treatment was significantly reduced (table 1; Z = −5.04, P < 0.001).

Fig. 2. Accumulated adult mortality of O. lenzii in dung from control and eprinomectin treated cattle in experiment 1. Different letters indicate significant differences (P < 0.0083) among control and treatments (log-rank test followed by the Bonferroni correction).
Table 1. Number of brood balls constructed and adult emergence rates of O. lenzii in dung from control and eprinomectin-treated cattle in experiment 1.

Each value is a mean of five replicates involving three pairs of each parent.
Values in parentheses are total numbers of brood balls.
In each column, means not associated with the same letter differ at P < 0.05 among control and treatments (GLMM followed by Tukey's multiple comparisons).
Fig. 3 shows the estimates of accumulated feeding activity of O. lenzii per dung pat from control and treated cattle. There were no significant differences in feeding rates at 7 days post-treatment. However, in dung from 1 and 3 days post-treatment, feeding rates were significantly suppressed compared with control (Z = −4.7, P < 0.001; Z = −3.1, P < 0.05, respectively).

Fig. 3. Accumulated feeding activity (F) of O. lenzii in dung from control and eprinomectin treated cattle in experiment 1. Different letters indicate significant differences (P < 0.05) among control and treatments (GLMM followed by Tukey's multiple comparisons).
The effects of eprinomectin on adult survival, brood ball production, emergence, and feeding activity of C. ochus
Table 2 shows the adult mortality, numbers of brood balls constructed, and adult emergence rate of C. ochus at 3, 7, and 14 days post-treatment in dung from control and treated cattle in experiments 1 and 2. In dung from control cattle in experiments 1 and 2, accumulated adult mortalities of C. ochus were 10 and 20%, respectively, until 90 days after the beginning of rearing. However, in dung from 3 days post-treatment (experiment 1), accumulated adult mortalities were 90% at 60 days and 100% at 90 days after the beginning of rearing, showing a marked difference with controls (60 days: χ2 = 18.1, P < 0.0083; 90 days: χ2 = 30.2, P < 0.0083, respectively). Meanwhile, normal adult mortalities, which were equivalent to the level of controls, were restored at 7 and 14 days post-treatment (experiment 2) at all dates after the beginning of rearing. The numbers of brood balls constructed per female in control dung were 4.3 (experiment 1) and 4.4 (experiment 2), but there were no brood balls in dung at 3 days (experiment 1) post-treatment. The numbers of brood balls in dung at 7 days post-treatment were significantly reduced (experiment 2; Z = −2.7, P < 0.05), and an equivalent level to the control was restored at 14 days post-treatment. In dung from control cattle, adult emergence rates were 100% (experiment 1) and 71.6% (experiment 2). However, there was no oviposition in dung at 3 days post-treatment, and all offspring died at an early stage of development (egg or first instar of larva) in dung at 7 and 14 days post-treatment (data not shown).
Table 2. Accumulated adult mortality, number of brood balls constructed, and adult emergence rates of C. ochus in dung from control and eprinomectin-treated cattle in experiments 1 and 2.

Each value is a mean of ten replicates involving one pair of parents each.
Values in parentheses are total numbers of brood balls.
*Significantly different vs. control in experiment 1 (log-rank test: P < 0.05).
In each column, means not associated with the same letter differ among control and treatments in experiment 2 (adult mortalities: log-rank test followed by the Bonferroni method, P < 0.0167, numbers of brood balls: GLMM followed by Tukey's multiple comparisons, P < 0.05).
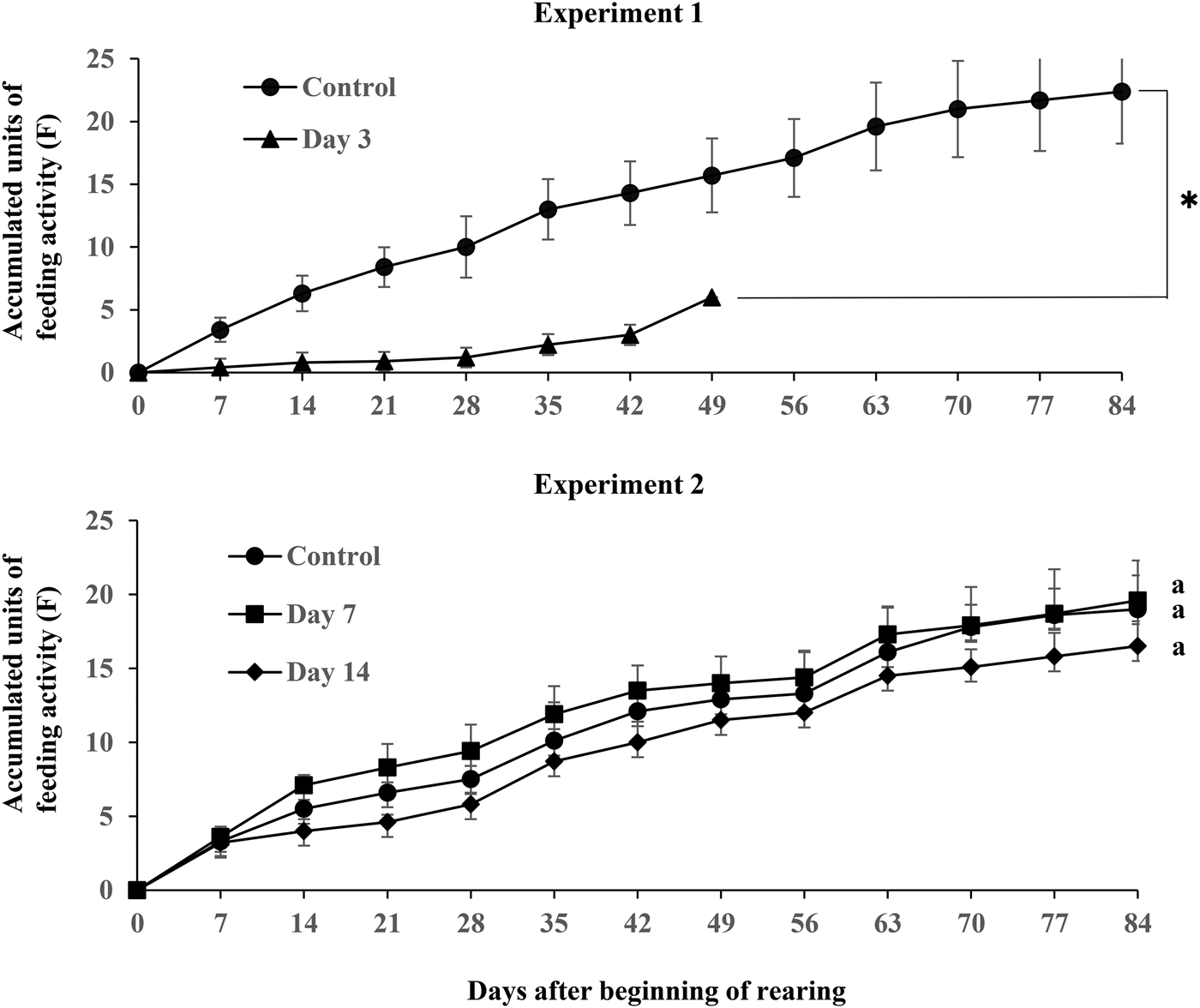
Fig. 4 shows the estimates of accumulated feeding activity of C. ochus per dung pat from control and treated cattle (experiments 1 and 2). The feeding levels of C. ochus in dung at 3 days post-treatment were significantly suppressed from 7 to 49 days after the beginning of rearing between control and treatment (experiment 1; Z = −7.6, P < 0.001). However, there were no differences in the feeding levels at 7 and 14 days post-treatment between control and treatment (Z = 0.3, P = 0.95; Z = −1.3, P = 0.35, respectively).

Fig. 4. Accumulated feeding activity (F) of C. ochus in dung from control and eprinomectin treated cattle in experiments 1 and 2. *Significantly different vs. control in experiment 1 (GLMM: P < 0.05). Values followed by same letters within each day after the beginning of rearing are not significantly different in experiment 2 (GLMM followed by Tukey's multiple comparisons: P < 0.05).
Discussion
In previously published studies eprinomectin pour-on formulation was found to cause dung contamination for 1–29 days post-treatment with a peak at 2 or 3 days post-treatment (Lumaret et al., Reference Lumaret, Errouissi, Galtier and Alvinerie2005; Iwasa & Sugitani, Reference Iwasa and Sugitani2014). Present results which showed a peak at 1 day post-treatment are different from those of above literatures. Moreover, the maximum detectable level of eprinomectin in this study was 3–5 times higher than those of preceding studies (Lumaret et al., Reference Lumaret, Errouissi, Galtier and Alvinerie2005; Iwasa & Sugitani, Reference Iwasa and Sugitani2014). Cook et al. (Reference Cook, Dadour and Ali1996) and Iwasa et al. (Reference Iwasa, Maruo, Ueda and Yamashita2007, Reference Iwasa, Suzuki and Maruyama2008) reported that concentrations of ivermectin and moxidectin in dung can be affected by the diet of cattle. In the present study, the site and diet were the same as in a preceding study (Iwasa & Sugitani, Reference Iwasa and Sugitani2014). Sommer et al. (Reference Sommer, Steffansen, Overgaard Nielsen, Grønvold, Vagn Jensen, Brøchner Jespersen, Springborg and Nansen1992) suggested that ivermectin concentrations in cattle dung increased by the metabolization of organic matter in field exposed pats. It seems likely that the difference of concentrations of eprinomectin residues in dung was attributed to other factors such as contents of organic matter and moisture in dung which were affected by weather and the condition of cattle (age, body weight, diarrhea). It is, therefore, necessary to clarify the relation between the quality of dung and eprinomectin residues.
Other works have shown that dung from cattle treated with ivermectin had no effect on the survival of adult beetles which were small-to-medium sizes, such as Caccobius, Euoniticellus, Liatongus, and Onthophagus (Fincher, Reference Fincher1992; Lumaret et al., Reference Lumaret, Galante, Lumbreras, Mena, Bertrand, Bernal, Cooper, Kadiri and Crowe1993; Iwasa et al., Reference Iwasa, Nakamura, Fukaki and Yamashita2005, Reference Iwasa, Maruo, Ueda and Yamashita2007). In large dung beetles, however, ivermectin residues in dung caused a high mortality for newly emerged adults of Copris hispanus Linnaeus, Onitis belial Fabricius (Wardhaugh & Rodriguez-Menendez, Reference Wardhaugh and Rodriguez-Menendez1988), and matured adults of Copris acutidens Motschulsky and C. ochus (Iwasa et al., Reference Iwasa, Maruo, Ueda and Yamashita2007). In dung from cattle treated with eprinomectin, there was also no effect on survival of matured adults of Caccobius jessoensis Harold and Liatongus minutus Motschulsky (Iwasa & Sugitani, Reference Iwasa and Sugitani2014). Wardhaugh et al. (Reference Wardhaugh, Longstaff and Morton2001) reported that dung from eprinomectin-treated cattle had no effect on matured adult survival of Onthophagus taurus Schreber, but mortality of newly emerged adults increased in dung at 3 days post-treatment. The present results for adult mortalities of two species (figs 1 and 2 and table 2) show that high concentrations (0.45–1.5 ppm) of eprinomectin after treatment at the recommended pour-on dose cause a high adult mortality in O. lenzii and C. ochus. We suggest that the effect of eprinomectin on adult survival varies by species even in the same genus (Onthophagus). Furthermore, dung beetles belonging to the genus Copris may have a higher drug sensitivity to eprinomectin than other genera same as ivermectin.
It has been reported that there were no effects of ivermectin on production of brood balls of Euoniticellus intermedius Reiche (Fincher, Reference Fincher1992), Onthophagus gazella Fabricius (Fincher, Reference Fincher1992; Sommer & Nielsen, Reference Sommer and Nielsen1992), Diastellopalpus quinquedens Bates (Sommer et al., Reference Sommer, Grønvold, Holter, Madsen and Nansen1993), C. jessoensis, and L. minutus (Iwasa et al., Reference Iwasa, Maruo, Ueda and Yamashita2007). However, the numbers of brood balls of C. hispanus were reduced in dung from ivermectin-treated cattle at 3 days post-treatment (Wardhaugh & Rodriguez-Menendez, Reference Wardhaugh and Rodriguez-Menendez1988), and those of Onthophagus landolti Harold decreased at concentrations of 0.1, 1.0, and 10.0 ppm in dung spiked with ivermectin (Pérez-Cogollo et al., Reference Pérez-Cogollo, Rodríguez-Vivas, Delfín-González, Reyes-Novelo and Ojeda-Chi2015). Moreover, there were no brood balls and/or oviposition of C. acutidens and C. ochus in dung from ivermectin-treated cattle at 1 day after treatment (Iwasa et al., Reference Iwasa, Maruo, Ueda and Yamashita2007). In dung from eprinomectin-treated cattle, there was no effect on the number of brood balls of C. jessoensis and L. minutus (Iwasa & Sugitani, Reference Iwasa and Sugitani2014). In newly emerged adults of O. taurus, however, brood balls in dung from eprinomectin-treated cattle decreased at 3 days post-treatment (Wardhaugh et al., Reference Wardhaugh, Longstaff and Morton2001). The present results suggest that a high concentration of eprinomectin residues in dung could cause a significant reduction in the numbers of brood balls of O. lenzii and C. ochus, and its effect may persist longer in C. ochus.
In most cases, larval survivals or adult emergence rates of dung beetles (Aphodius, Caccobius, Copris, Diastellopalpus, Digitonthophagus, Euoniticellus, Liatongus, and Onthophagus) decreased in dung from ivermectin-treated cattle (Wardhaugh & Rodriguez-Menendez, Reference Wardhaugh and Rodriguez-Menendez1988; Fincher, Reference Fincher1992; Lumaret et al., Reference Lumaret, Galante, Lumbreras, Mena, Bertrand, Bernal, Cooper, Kadiri and Crowe1993; Sommer et al., Reference Sommer, Grønvold, Holter, Madsen and Nansen1993; Krüger & Scholtz, Reference Krüger and Scholtz1997; Errouissi et al., Reference Errouissi, Alvinerieb, Galtierb, Kerboeufc and Lumaret2001; Wardhaugh et al., Reference Wardhaugh, Longstaff and Morton2001; Iwasa et al., Reference Iwasa, Maruo, Ueda and Yamashita2007). However, there are few reports on the effects of eprinomectin on larvae of dung beetles. Wardhaugh et al. (Reference Wardhaugh, Longstaff and Morton2001) showed that eprinomectin residues in dung at 3–7 days post-treatment reduced the larval survival of O. taurus. Adult emergence rates of C. jessoensis and L. minutus were reduced in dung from eprinomectin-treated cattle at 1–3 days post-treatment (Iwasa & Sugitani, Reference Iwasa and Sugitani2014). The present results show that adult emergence rates of O. lenzii and C. ochus were reduced at 1, 3, 7 days and 3, 7, 14 days post-treatment, respectively (tables 1 and 2). The period during which eprinomectin-treated cattle produce dung toxic to larvae of O. lenzii is consistent with that of O. taurus, and both periods are longer than those for C. jessoensis and L. minutus. Iwasa et al. (Reference Iwasa, Maruo, Ueda and Yamashita2007) suggested that the effect of ivermectin on the larval survival of small dung beetles such as C. jessoensis is short-term in comparison with medium-sized dung beetles. The same phenomenon may be present in dung from cattle treated with eprinomectin.
As shown in the present results, all offspring of C. ochus died at an early stage (egg or first instar of larva) in dung from 14 days post-treatment (table 2), although epriomectin was undetected in it (fig. 1). Doherty et al. (Reference Doherty, Stewart, Cobb and Keiran1994) reported that the adult emergence rates of O. gazella were severely reduced at a very low concentration (0.008 ppm) in dung directly spiked with abamectin. It is possible that dung from 14-days post-treatment with undetectable residues of eprinomectin (<0.05 ppm) could cause a high toxicity against the early stage of C. ochus. Copris species are listed as ‘rare’ or ‘endangered’ species in many prefectures of Japan (Hori, Reference Hori2005). Iwasa et al. (Reference Iwasa, Maruo, Ueda and Yamashita2007) suggested that the decrease of C. ochus in Japan is partly attributed to the contamination of dung with anthelmintic drugs such as ivermectin. For evaluation of ecotoxicity of drugs used in pastures, attention should be paid to impact on the population of dung beetles such as Copris species, which show a high drug sensitivity.
Wardhaugh & Rodriguez-Menendez (Reference Wardhaugh and Rodriguez-Menendez1988) showed that the feeding activity of C. hispanus was greatly suppressed at 1–8 days after treatment by ivermectin injection. In their field experiment, Dadour et al. (Reference Dadour, Cook and Neesam1999) reported that dung dispersal by O. taurus decreased at 7 and 10 days after treatment in dung from cattle treated with ivermectin by injection. Dung removal by O. landolti was inhibited in dung which was spiked with ivermectin at 0.1, 1.0 and 10.0 ppm (Pérez-Cogollo et al., Reference Pérez-Cogollo, Rodríguez-Vivas, Delfín-González, Reyes-Novelo and Ojeda-Chi2015). The present results indicate that dung from cattle treated by pour-on formulation at the recommended dose of eprinomectin causes a high concentration of eprinomectin residues, associated with the suppression of feeding of O. lenzii and C. ochus. This concentration occurred in dung produced at 3 days post-treatment when adult survival was also affected (fig. 1 and table 2).
It is known that avermectin acts on both glutamate-gated and GABA-gated chloride channels in nerve and muscle cells of invertebrates, resulting in somatic paralysis and death (Cully et al., Reference Cully, Vassilatis, Liu, Paress, Van-Der-Ploeg, Schaeffer and Arena1994; Arena et al., Reference Arena, Lui, Paress, Frazier, Cully, Mrozik and Schaeffer1995). Verdú et al. (Reference Verdú, Cortez, Ortiz, González-Rodríguez, Martinez-Pinna, Lumaret, Lobo, Numa and Sánchez-Piñero2015) reported that ivermectin in dung decreased the olfactory and locomotor capacities of Scarabaeus cicatricosus Lucas, and it caused a significant negative effect on foraging success. Eprinomectin may have an effect on the feeding activity of dung beetles in the same mode of action as ivermectin, causing reduction of adult survival.
Our results show a significant effect of eprinomectin on survival, reproduction, and feeding activity of two species of dung beetles which have an important function in dung dispersal and decomposition in Japan. Further study is necessary to clarify the effect of eprinomectin on other dung beetles inhabiting dung pats and to evaluate the impact of this drug on dung degradation in the pasture.
Acknowledgements
We wish to thank Drs T. Oshida and N. Kumano of Obihiro University of Agriculture and Veterinary Medicine, and Dr T. Nakamura of Hirosaki University for their valuable suggestions. We are also grateful to Mr. S. Yamanaka and K. Mihara of Obihiro Yachiyo Farm, Mr. K. Hasegawa of Ôno Farm, Mr. T. Arai of Ôtawara city office, and Mr. T. Nakamura of Nakamura Farm in Mukawa town for their kind help and arrangements during the survey in pastures. Our thanks are also due to Assistant Professor G. A. Hill of Obihiro University of Agriculture and Veterinary Medicine for checking the English in this manuscript.