Introduction
The oriental fruit moth, Grapholita molesta (Busck) (Lepidoptera: Tortricidae), is an important pest of many members of the Rosaceae family (including fruit trees such as apple, pear, peach, etc.). It is widely distributed throughout the fruit-growing regions of more than 45 countries on every continent except Antarctica (Rothschild & Vickers, Reference Rothschild and Vickers1991; Natale et al., Reference Natale, Mattiacci, Hern, Pasqualini and Dorn2003; Myers et al., Reference Myers, Hull and Krawczyk2007; Timm et al., Reference Timm, Geertsema and Warnich2008; Bisognin et al., Reference Bisognin, Zanardi, Nava, Aeioli, Botton, Garcia and Cabezas2012), and has caused great economic loss (Myers et al., Reference Myers, Hull and Krawczyk2007; Piňero & Dorn, Reference Piňero and Dorn2009) in most of these areas. The oriental fruit moth is a host-switching pest species (Li et al., Reference Li, Chen, Li, Zhang, Li and Wu2016). In China, the first generation larvae feed mainly on peach shoots, while the second generation prefers feeding on the fruit of apple, pear, peach, etc. trees. The larvae have caused considerable economic loss in almost every region throughout the country where apple and peach trees are grown (Fu et al., Reference Fu, Zhao and Lin2004). Surveys of regional abundance, effects of environments factors, and control of G. molesta have been and continue to be the primary focal points of research on the pest. Environment factors (such as temperature, photoperiod, humidity, host plants, etc.) can affect the duration and viability of various developmental stages, oviposition and fecundity of an insect species (Du et al., Reference Du, Guo, Zhang and Wu2009; He et al., Reference He, Meng, Hua and Chen2011; Yang et al., Reference Yang, Fan, Ma, Wang and Wei2016). Previous research has demonstrated that every life-history trait (reproduction, development, mortality) is temperature dependent (Bale et al., Reference Bale, Masters, Hodkinson, Awmack, Bezemer and Brown2002; Johnson et al., Reference Johnson, Coutinho, Berlin, Dolphin, Heyer, Kim, Leung, Sabellon and Amarasekare2016). It has been reported that a seasonal rise in temperature can advance the emergence time of the overwintering generation of adult G. molesta, as well as cause accelerated development in this and other species, resulting in greater damage being caused by the oriental fruit moth and other pest species (Bale et al., Reference Bale, Masters, Hodkinson, Awmack, Bezemer and Brown2002; Quarles, Reference Quarles2007; Jung et al., Reference Jung, Kim, Lee and Park2013). It is still inconclusive, however, whether this earlier or accelerated development of an insect pest is beneficial to the maintenance or future growth of its population. Determining the impact of temperature change on the development of G. molesta populations will be beneficial in analyzing and predicting the long-term response strategies of the pest populations to predicted global warming.
Life tables, which were first utilized to study an insect population by Pearl & Parker (Reference Pearl and Parker1922), have become increasingly sophisticated since their initial use (see Wu et al., Reference Wu, Chen and Li1980), and are now considered an important tool in the demographic analysis of insect populations (Han et al., Reference Han, Zhai and Zhang2003; Liu et al., Reference Liu, Luo, Zhou and Wei2010). Unfortunately, traditional life table research is extremely time and labor intensive, ignores stage differentiation in insect species, and excludes the contribution that male individuals make to the population (instead, using sex ratio to calculate the ‘female’ offspring). Applying these life table techniques to insect species has inevitably resulted in errors in their life table parameters and interpretations (Huang & Chi, Reference Huang and Chi2012; Yu et al., Reference Yu, Chen, Zheng, Shi, Guo, Yeh, Chi and Xu2013; Akca et al., Reference Akca, Ayvaz, Yazici, Smith and Chi2015). The age-stage, two-sex life table is ideal for studying the dynamics of insect populations (Chi & Liu, Reference Chi and Liu1985; Chi, Reference Chi1988), and has been widely used on a number of diverse species, including the sweet potato weevil (Reddy & Chi, Reference Reddy and Chi2015), black bean aphid (Akca et al., Reference Akca, Ayvaz, Yazici, Smith and Chi2015), green lacewing (Chen et al., Reference Chen, Liu, Liu, Cheng, Wang and Xu2017), etc.
Du et al. (Reference Du, Guo, Zhang and Wu2009) used an artificial diet and traditional life table to analyze the effects of temperature on the development of G. molesta and found that 25.1°C was the optimum temperature for culturing G. molesta, producing the highest survival rate and the highest intrinsic rate. Although these findings are useful in providing data on culturing G. molesta, using the traditional life table to analyze the data is inappropriate in this instance because it does not include the entire population. Female-based traditional life tables by their very nature are non-inclusive because they are limited to the female component of the population, excluding any contribution that male individuals may make to the population.
Previous research determined that apples are a preferred host of G. molesta (Yang et al., Reference Yang, Fan, Ma, Wang and Wei2016). To develop a comprehensive and thorough understanding of the ecological preferences of G. molesta, we used apples to rear G. molesta to compare the development of the larvae and adult moths under varying constant temperatures, and to check the development of G. molesta under outdoor natural condition from July to September to simulation the third generation of the G. molesta wild population. Our data were then used to analysis the adaptive significance of these natural environments on G. molesta, and to provide supportive data to enable predicting future population trends of G. molesta under varying temperature regimes.
Materials and methods
Insects
G. molesta adults were obtained from an established, laboratory colony maintained by the Shandong Institute of Pomology and subsequently reared on Red Fuji apple (Malus pumila Mill.) in a growth chamber at 25 ± 1°C, with a photoperiod of 15L: 9D and relative humidity (RH) of 60 ± 10%. The populations of G. molesta were reared on Red Fuji apple for more than two generations to establish a stable experimental population.
Effects of different constant temperatures on growth and development of G. molesta
To maintain genetic diversity in the G. molesta population, at least 100 pairs of adults were placed into a 2000 ml beaker, sealed with plastic film, and allowed to oviposit on the film. Approximately 300 fresh eggs oviposited within a 24 h period on the film were collected for each of the temperature treatments. To improve the survival of G. molesta larvae, the 300 fresh eggs were subdivided into 15 equal parts with each card containing 20 eggs, and each of those subsets of 20 used to inoculate the calyx of a single apple rather than one egg one apple. The five constant temperatures used were 19, 22, 25, 28 and 31°C. After hatching, larvae bore into the fruit at the calyx depression of the apple. The survival status of individual G. molesta larvae was recorded daily and the apple replaced when warrented. Larvae were removed from the apple when they developed into the fifth instar (mature larvae) and placed into a finger tube (1.8 cm diameter, 7 cm height) containing shredded paper as the pupation medium. Pupal weights were obtained at the 3rd day after pupation via an electronic scale. After the emergence of adults, five pairs of newly eclosion adults were placed in a plastic cup (200 ml) and sealed with a plastic film, which also acted as an oviposition base. A bottle cap (3.0 cm diameter, 1.2 cm height) containing 5% sugar water served as a nutrient source for the adults. All eggs oviposited in each 24 h period were counted until the death of all individuals.
Effects of natural fluctuating temperature environments on growth and development of G. molesta
In total, 620 eggs collected on 18 July 2016 from the G. molesta laboratory population were used for this experiment. Fresh eggs from the plastic film were cut into egg ‘cards’ with each card containing 20 eggs. A single egg card was placed into the calyx of each Red Fuji apple. Apples with G. molesta eggs were placed into a plastic box under two layers of gauze, and the upper layer sealed with a separate layer of gauze to prevent larval escape. The plastic box was then moved to an orchard and placed on the ground below the foliage of an apple tree to avoid exposure to direct sunlight. After the larvae hatched, all apples were checked daily and the development of G. molesta was observed and recorded as above.
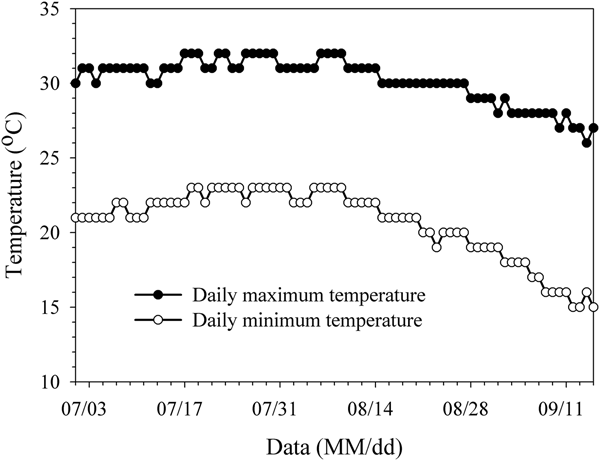
To compare the experimental results, the trends of nearby wild populations were investigated form 1 July to 15 September 2016. During this time period, apples were in the fruit expansion stage to the mature stage. According to data obtained from the Chinese weather network, the average atmospheric temperature in Tai'an during the experimental period was 24.98°C with the highest daily maximum temperature and the lowest daily minimum temperature of 32 and 15°C, respectively (fig. 1). In the apple orchard, randomly selected five sampling points set pear moth sex pheromone (Pherobio Technology Co., Ltd, Beijing) to monitors the occurrence of the oriental fruit. The survey method used was identical of that used by Ling et al. (Reference Ling, Wang, Xu, Yu and Li2010).

Fig. 1. The daily maximum temperature and the daily minimum temperature from July to September in Tai'an city.
Demographic analysis
Raw data of all individuals were analyzed by using the age-stage, two-sex life table method (Chi & Liu, Reference Chi and Liu1985; Chi, Reference Chi1988; Huang & Chi, Reference Huang and Chi2012). The developmental time, the age-stage survival rate (S xj) (where S xj is the probability that a newly laid egg can survive to age x and develop to stage j), and fecundity (f xj) (the number of eggs laid by female adult at age x), intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R 0) and mean generation time (T) were calculated using the program, TWOSEX-MS Chart (available at http://140.120.197.173/Ecology) (Chi, Reference Chi2016) as described by Chen et al. (Reference Chen, Liu, Liu, Cheng, Wang and Xu2017) and Yu et al. (Reference Yu, Chen, Zheng, Shi, Guo, Yeh, Chi and Xu2013). The bootstrap method (with 200,000 bootstraps) was used to achieve precise estimates of the variance and standard errors of the developmental time and pupal weight. Differences between treatments were examined by using the paired bootstrap test based on the confidence interval of differences (Efron & Tibshirani, Reference Efron and Tibshirani1993; Yu et al., Reference Yu, Chen, Zheng, Shi, Guo, Yeh, Chi and Xu2013; Akca et al., Reference Akca, Ayvaz, Yazici, Smith and Chi2015; Akköprü et al., Reference Akköprü, Atlihan, Okut and Chi2015).
Because of reared G. molesta in groups, we could get the matrices N and F total, and we can determine s xj and f xj from matrices N and F total, to calculate the population parameters R 0, r, and λ. The net reproductive rate (R 0) was calculated as:
 $$R_0 = \sum\limits_{x = 0}^\infty {\sum\limits_{\,j = 1}^m {s_{xj}f_{xj}}}. $$
$$R_0 = \sum\limits_{x = 0}^\infty {\sum\limits_{\,j = 1}^m {s_{xj}f_{xj}}}. $$The intrinsic rate of increase was estimated using the iterative bisection method from the Euler–Lotka formula:
 $$\sum\limits_{x = 0}^\infty {\left( {e^{ - r(x + 1)}\sum\limits_{\,j = 1}^m {\,f_{xj}s_{xj}}} \right)} = 1.$$
$$\sum\limits_{x = 0}^\infty {\left( {e^{ - r(x + 1)}\sum\limits_{\,j = 1}^m {\,f_{xj}s_{xj}}} \right)} = 1.$$But the standard errors of the population parameters cannot be estimated based on matrices N and F total, because we cannot get the variability of development rate and fecundity among individuals by group reared. Because the larvae and the adults were bred as a group, the paired bootstrap test that was included in the age-stage, two-sex life table software could not be used to analyze the difference of population parameters and survival rates between treatments. Instead, all treatments were replicated three times to compare the population parameters, survival rates and pupal weights by using one-way ANOVA implemented in SPSS 16.0, with the significant differences being observed at the P < 0.05 level (Duncan's new multiple range test).
Hatchability, boring rate, exiting rate, pupation rate and emergence rate were calculated. The computation methods used were as follows:
Hatchability = the number of hatched larvae/the number of eggs;
Boring rate = the number of decay holes/the number of hatched larvae;
Exiting rate = the number of larvae taken off apples/the number of decay holes;
Pupation rate = the number of pupae/the number of larvae taken off apples;
Emergence rate = the number of adults/the number of pupae.
Results
Effects of different constant temperatures on growth and development of G. molesta
The development duration of G. molesta egg, larval, prepupal, pupal and adult stages significantly decreased with increases in laboratory temperatures (P < 0.05) (table 1). Life table parameters of G. molesta populations at different temperatures are shown in table 2. As the treatment temperature increased, the net reproductive rate (R 0), intrinsic rate of increase (r), and finite rate of increase (λ) initially increased and then decreased, with the maximum value occurring during the 25°C treatment. The 25°C treatment showed significant differences with all other treatments (R 0: F (12; 5) = 793.48, P < 0.0001; r: F (12; 5) = 167.70, P < 0.0001; λ: F (12; 5) = 209.54, P < 0.0001). The mean generation time (T) gradually shortened with increases in treatment temperature, with significant differences occurring between treatments (F (12; 5) = 176.50, P < 0.0001) except when T was at 28 and 31°C (P = 0.087). The values for R 0 > 1, r > 0, λ > 1 at 19, 22, 25 and 28°C; while at 31°C, corresponding values were R 0 < 1, r < 0, λ < 1, implying that a constant high temperature of 31°C is unsuitable for development of the oriental fruit moth population.
Table 1. Developmental duration of various stages of G rapholita molesta at different constant and fluctuating temperatures.

Data are means ± SE. The data in the same column followed by different letters are significantly different at P < 0.05. Standard errors were estimated by using 200,000 resampling. Differences between two treatments were compared by using a paired bootstrap test.
Table 2. Life table parameters of Grapholita molesta population at different constant and fluctuating temperatures.

Values followed by the same letters are not significantly different (Duncan's new multiple range test, P > 0.05).
Pupal weights observed under different temperatures are shown in table 3. Male pupal weights initially increased and then decreased with increased temperatures, with the maximum values for pupal weight occurring at 25°C, with significant differences between the 19 and 25°C treatments (F (175; 5) = 2.43, P = 0.037). There also had the maximum value for female pupal weight at 25°C, though there were no significant differences among the female pupal weights at any of the treatment temperatures (F (186; 5) = 1.93, P = 0.091). The female pupal weights were heavier than the corresponding male weights in all treatments.
Table 3. Effect of different constant and fluctuating temperatures on pupal weight of Grapholita molesta.

Values followed by the same letters are not significantly different (Duncan's new multiple range test, P > 0.05).
Five key indices that are closely associated with the survival rate of a G. molesta population; hatchability, and boring, exiting, pupation, and emergence rates are shown in table 4. The hatchability rate was not significantly different in G. molesta at 22, 25 and 28°C (F (12; 5) = 175.80, P = 0.751, 0.744, 0.141), but was significantly lower at 19°C and higher at 31°C (P < 0.0001). As laboratory temperatures increased, the boring and exiting rates initially increased and then declined. The highest boring rate was at 25°C, which was significantly higher than in other treatments (F (12; 5) = 135.70, P < 0.0001), with the lowest boring rate occurring at 31°C. The highest exiting rate occurred at 28°C and the second highest at 25°C. Both were significantly higher than in the other treatments (F (12; 5) = 272.83, P < 0.0001). No significantly differences in the pupation rates were observed in G. molesta at the 22, 25, 28 and 31°C treatments (F (12; 5) = 328.00, P > 0.05), but all higher than that was at 19 and 31°C (P < 0.0001). The emergence rate at 25°C was shorter than in the 19 and 22°C treatments, but also above 80%, and was significantly higher than that at 28 and 31°C (F (12; 5) = 951.33, P < 0.0001). The survival rates from egg to adult, again increased and then decreased with temperature increase, with the highest survival rate occurring during the 25°C treatment, which was significantly higher than in other treatments (F (12; 5) = 150.80, P < 0.0001).
Table 4. Effects of different constant and fluctuating temperatures on the survival rate of Grapholita molesta.

Values followed by the same letters are not significantly different (Duncan's new multiple range test, P > 0.05).
Effects of natural fluctuating temperature environments on growth and development of G. molesta
The development duration was calculated for G. molesta moths reared under outdoor natural condition, with temperature varying from 22 to 30°C, and the average temperature of 24°C throughout the experimental period. Of the 620 eggs used in this experiment, only 112 eggs fully developed into adults. The average durations of eggs and larvae reared under fluctuating ambient temperature were significantly shorter than those reared under constant 19, 22, 25 and 28°C temperatures (P < 0.05); the prepupal durations were shorter than those reared under constant 19 and 22°C temperatures; and the pupal durations were shorter than those reared under all constant temperature treatments except under 31°C (table 1). The adult lifespan under fluctuating temperature were shorter than those reared under constant 19, 22, and 25°C temperatures. The population parameters are shown in table 2. The R 0 value under fluctuating temperature was significantly shorter than the corresponding value for those reared under constant 22 and 25°C temperatures, but was higher than those reared at a constant 19, 28 and 31°C temperature (F (12; 5) = 793.48, P < 0.0001). The r value for those reared outdoors was significantly higher than all the constant laboratory temperatures populations (F (12; 5) = 167.70, P < 0.0001). The λ value for the outdoor reared treatments was not significantly different from those reared at the constant 25°C temperature (F (12; 5) = 209.54, P = 0.154), but was significantly higher than in other treatments (P < 0.0001). The T value under fluctuating temperature was significantly shorter than all of the constant temperature laboratory treatments (table 2).
The female pupal weights for individuals reared under field condition were heavier than corresponding male pupal weights (table 3). There were no significantly differences in weights between the female pupae reared under field conditions and females reared under constant temperatures (F (186; 5) = 1.93, P = 0.091), although the weights of male pupae reared under field condition were significantly lighter than those reared under a constant 25°C (F (175; 5) = 2.43, P = 0.037). The survival rates of G. molesta reared under field conditions are shown in table 4. The hatchability values of eggs exposed to fluctuating field temperatures were not significantly different from those reared under constant 22, 25 and 28°C temperatures (F (12; 5) = 175.80, P = 0.775, 0.155, 0.999), but were significantly shorter than those reared at a constant 19°C temperature and significantly longer than those from the constant 31°C temperature (P < 0.001). The boring rate of larvae exposed to fluctuating temperature was barely significantly lower than those reared at the 25°C temperature (F (12; 5) = 135.70, P < 0.0001). The exiting rates of larvae under fluctuating temperatures were significantly lower than for those reared under constant 22, 25, 28 and 31°C temperatures (F (12; 5) = 272.83, P < 0.0001). Their pupation rate also was significantly lower than in all constant temperature treatments (F (12; 5) = 328.00, P < 0.0001), although nearly all the adult moths developing under fluctuating temperature condition successfully emerged from the pupal stage. The survival rates from egg to adult under fluctuating temperatures were significantly lower than for those reared under constant 22 and 25°C temperatures (F (12; 5) = 150.80, P < 0.0001), but significantly higher than that reared under constant 19, 28 and 31°C temperature (P < 0.0001).
The fecundities of G. molesta females developing from the various treatments are shown in fig. 2. With increases in treatment temperature, the early spawning time of G. molesta gradually advanced, although all times were later than in G. molesta moths reared under the fluctuating temperature conditions. G. molesta moths reared under the 19°C treatment had the longest oviposition period (42 days), followed by the 22 and 25°C treatments (36 and 38 days, respectively). The length of the oviposition period under fluctuating temperature condition was 26 days, which was shorter than those reared at 19, 22 and 25°C, but longer than those from the 28 and 31°C populations (22 and 19 days, respectively). The fecundity of females reared under fluctuating temperatures (104.53 eggs) was less than in those reared under the 25°C treatment (115.92 eggs), but higher than other treatments. The fecundity of G. molesta under the 19, 22 and 28°C treatments was 69.95, 76.33 and 51.29 eggs, respectively; while fecundity at 31°C was only 13.42 eggs per female.
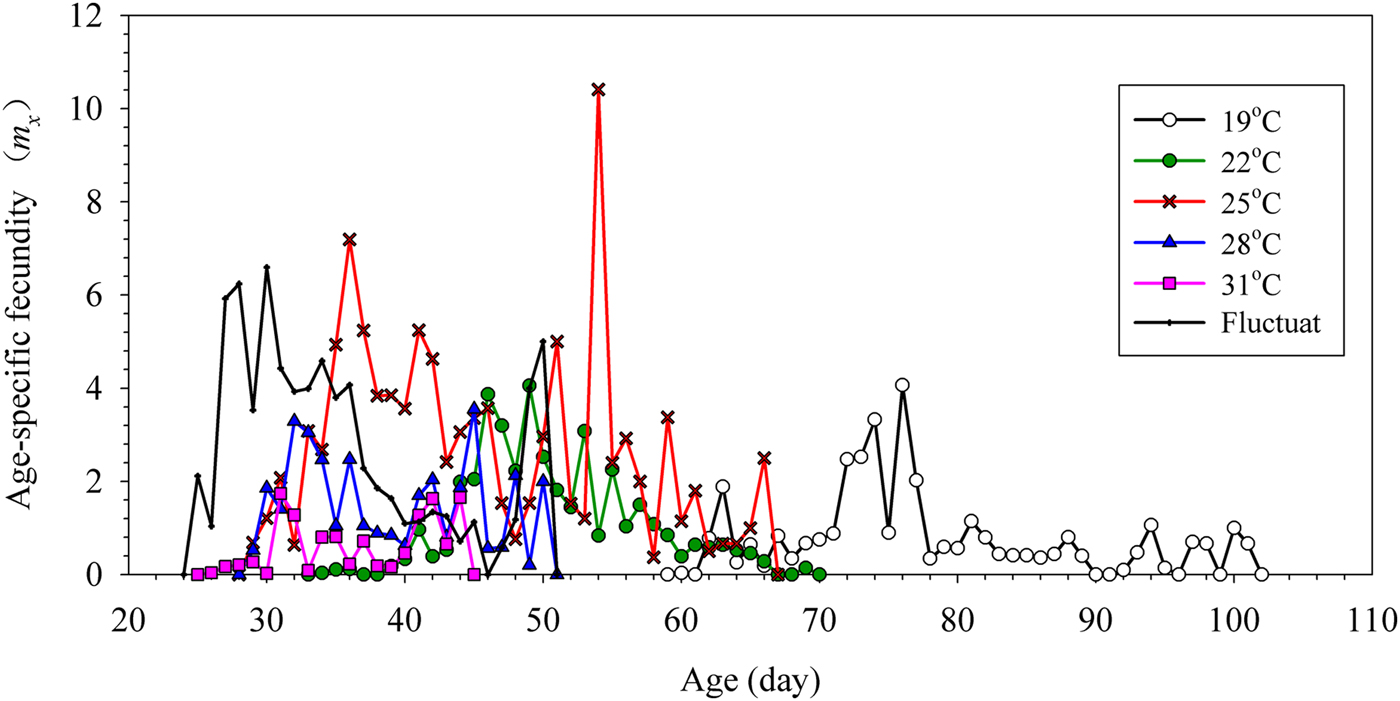
Fig. 2. The age-specific fecundity mx of Grapholita molesta under different constant and fluctuating temperatures.
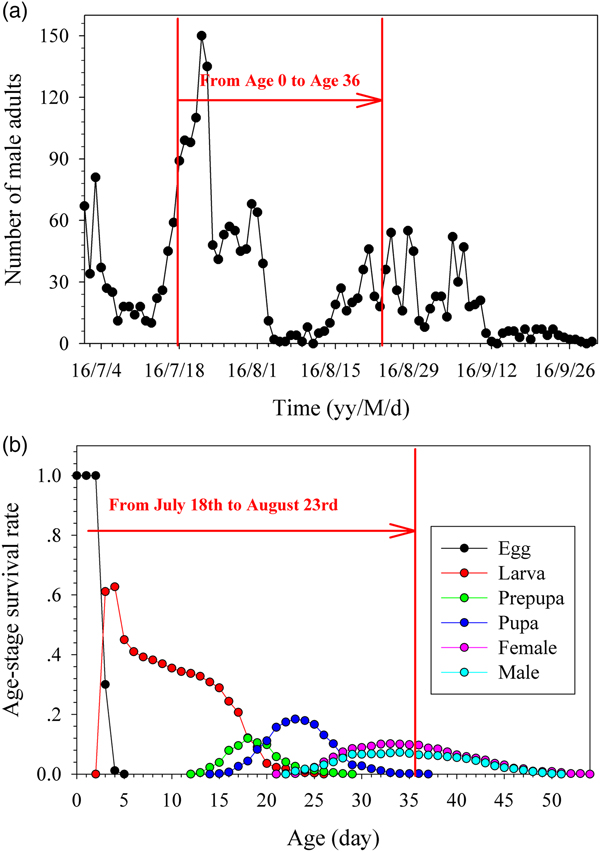
The seasonal dynamics of a naturally occurring population of G. molesta is shown in fig. 3. There are five peaks per year of the moth in Tai'an city as detected by synthetic sex pheromone traps, with the third and fourth peaks occurring from July to September (fig. 3a). The experiment was set up at the beginning of the third peak on 18 July, when the egg card was placed into the calyx of each Red Fuji apple, and the container placed in a shaded outdoor location. The age-stage survival rate was used to determine the development of each stage of G. molesta reared under fluctuating temperatures as shown in fig. 3b. Age 36 d in fig. 3b was the peak of adult emergence in the laboratory, which corresponded to 23 August in the natural population as seen in fig. 3a. This was the peak of the fourth generation in the field.

Fig. 3. Dynamics of natural population (a) and the age-stage survival rate of fluctuating temperature population (b); the red arrow indicates that test B began on 18 July (fig. 2a) corresponded to the beginning of the third generation of wild population A; the male adult emergence peak of test B corresponded to the beginning of the fourth generation of wild population A on 23 August. The result demonstrates that our fluctuating temperature simulation was in conformity with the natural development.
Discussion
Despite the fact that insects do not develop at a constant temperature in natural circumstances, life table studies obtained under constant temperatures provide useful information on the biology and ecology of insects and help in the studying and understanding of the dynamics within and between populations. Constant temperature experiments in the laboratory indicate that, the duration of G. molesta egg, larval, prepupal, pupal and adult stages significantly decreased with increasing temperatures. Because shorter development times are often more strongly selected for relative to other life history traits (Kingsolver & Pfennig, Reference Kingsolver and Pfennig2004; Kingsolver & Huey, Reference Kingsolver and Huey2008), developmental time can be an important criterion for demonstrating how a phenotype may vary under different environmental conditions (Couret et al., Reference Couret, Dotson and Benedict2014). Higher temperatures are often associated with faster developmental rates and have been shown to have variable impacts on the survival of immature insects (Kingsolver & Huey, Reference Kingsolver and Huey2008; Kirby & Lindsay, Reference Kirby and Lindsay2009; Arrese & Soulages, Reference Arrese and Soulages2010). Though all populations of G. molesta reared under constant 19–31°C temperatures were able to complete their life cycle, it was determined by two factors: the immature survival rates and the ability of the adult females to successfully oviposit accurately reflected the fitness of the population. Survival rate was closely related with the quality and the dynamic of G. molesta population. With the exception at 31°C, more than 90% of G. molesta eggs in each of the constant temperature treatments were able to complete the hatching stage and emerge as first instar larvae. Host resistance and the capacity of a boring insect to infest its host can be reflected by quantifying the boring rate. During our study, the majority of mortality in the G. molesta populations occurred during the boring stage, where larval boring rates were found to be below 50% in all constant temperature treatments other than the 25°C population. The exiting rate, which reflects the ability of larvae to complete their growth and development within the host, was the highest at 28°C. The pupation rates of emerged larvae exceeded 90% in all treatments, while emergence rates decreased at higher temperatures. According to the above results, the survival rates from egg to adult reached their maximum value at 25°C. Although the adult duration at 25°C was shorter than that at 19 and 22°C, it was longer than it was at 28 and 31°C. G. molesta had their highest fecundity at 25°C. G. molesta individuals reared at a constant 31°C had the shortest developmental time but also had the lowest survival rate. Their minimum fecundity resulted in the population parameter r < 0, λ < 1, leading to negative population growth. G. molesta individuals reared at a constant 25°C had the highest finite rate of increase (table 2), the heaviest pupal weights (table 3) the highest fecundity (fig. 2), and the highest survival rate from egg to adult (table 4). Based on these figures, 25°C was considered as the optimum developmental temperature, which is the same conclusion drawn by Du et al. (Reference Du, Guo, Zhang and Wu2009) after using the traditional life table.
Although insect developmental studies are frequently conducted at constant temperatures, ambient temperatures do not remain constant, and in the natural environment, temperatures do not fluctuate in a predictable manner, making it difficult to simulate these conditions for experimental purposes (Warren & Anderson, Reference Warren and Anderson2013). To overcome this we subjected the test insects to the outdoor fluctuating temperature environment to simulate natural environmental conditions. Various traps and baits have commonly been used to lure G. molesta adults in apple orchards (Padilha et al., Reference Padilha, Arioli, Boff, Rose and Botton2017). In China, traps containing synthetic sex pheromones are widely used in orchards to attract males as a direct means of control or for mating disruption, as well as a forecasting tool (Zhou et al., Reference Zhou, Li and Yu2011; Tu et al., Reference Tu, Zhang, Chen and Guo2012; Du et al., Reference Du, Liu, Tan and Wu2013). In our study, we used sex pheromones to monitor population dynamics of the moth in the field. The beginning of the outdoor variable temperature study corresponded to the peak of the third generation of the wild population, while the male adult emergence peak of the outdoor variable temperature population occurred during the beginning of the fourth generation of the wild population (fig. 3). The results indicated that our experiments closely approximated the seasonal cycle of naturally occurring populations in the field.
The effects of fluctuating temperatures and constant temperatures on insect developmental rates have received extensive attention. Some researchers believe that temperature change has a stimulating effect on the growth and development of insects compared with development at a constant temperature, and the other one is that in the optimum temperature range of temperature did not affect the growth and development of insects (Pan et al., Reference Pan, Chen, Xiao, Ji and Xie2014). Humpesch (Reference Humpesch1982) concluded that the egg development rate in the mayflies, Ecdyonurus spp. and Rhithrogena cf. hybrida, were the same under fluctuating temperatures as they were at constant temperature, while another study conducted by Radmacher & Strohm (Reference Radmacher and Strohm2011) on the solitary bee, Osmia bicornis (L.), found that fluctuating (versus constant) temperatures accelerated development of most stages at different temperature regimes. In our study, the duration of the larval stage reared under fluctuating temperatures was shorter than in individuals reared under constant 19, 22, 25 and 28°C temperatures. The adult lifespan was shorter under fluctuating temperature than it was in moths reared under constant 19, 22, 25°C temperatures (table 1). The population parameters r and λ were higher in this group than in all treatments reared under constant laboratory temperatures, but the mean generation times (T) were shorter, implying that the outdoor G. molesta population has a higher population growth potential and faster growth rate than the indoor constant temperature population, including those reared at the optimum constant growth temperature (25°C) (table 2).
Historically, temperature and nutrition have been thought to be linked via an animal's metabolic rate and the consequent effect on energy requirements; that is, with increasing temperature, relatively more energy is required to fuel growth and development (Kingsolver & Huey, Reference Kingsolver and Huey2008; Miller et al., Reference Miller, Clissold, Mayntz and Simpson2009). This may explain why the fecundity of G. molesta reared at 28 and 31°C is lower than it is in moths reared at 25°C (fig. 2). Maintenance of metabolic rate in low-temperature conditions is thought to provide a fitness advantage because it enables growth and the completion of development (Sømme & Block, Reference Sømme, Block, Lee and Denlinger1991). The fact that maintenance metabolism represents a cost to organisms (Chown et al., Reference Chown, Haupt and Sinclair2016), may explain why the fecundity of G. molesta moths reared at 21 and 19°C is lower than those reared at 25°C (fig. 2). The pre-oviposition stage was significantly shortened with increasing temperatures, while the length of the pre-oviposition stage under fluctuating temperatures was shorter than it was in all constant temperature treatments. G. molesta was found to have a higher fertility when reared under fluctuating temperature than under all constant temperatures, except 25°C.
Previous studies have shown that variable temperature could expand the range of temperatures tolerated by insects and increase their survival rate. For example, adults of the bug Pyrrhocoris apterus (L.) and the darkling beetle Alphitobius diaperinus (Panzer) survived longer when they were exposed to fluctuating thermal regimes rather than to constant low temperatures (Koštál et al., Reference Koštál, Renault, Mehrabianova and Bastl2007). The survival rate was only 10% in Myzus persicae (Sulzer) exposed to a constant temperature of 32°C, but increased to 22% under natural fluctuating temperatures (30–39°C) (Davis et al., Reference Davis, Radcliffe and Ragsdale2006). In G. molesta, the survival rate under variable temperature conditions (22–31°C) was higher than those at constant temperatures of 19 and 31°C, but shorter than those at 22 and 25°C (table 4).
Pupal weight is an indirect indicator of insect adaptability in Lepidoptera, and the healthier (heavier) the pupa, the stronger the adaptability (Leuck & Perkins, Reference Leuck and Perkins1972; Storer et al., Reference Storer, van Duyn and Kennedy2001). Ryzhkova & Lopatina (Reference Ryzhkova and Lopatina2015) and Bryant et al. (Reference Bryant, Bale and Thomas1999) found that the pupal weight increased as the temperature rose in the European peacock butterfly, Inachis io (L.), but a study on Helicoverpa armigera found a positive correlation between the pupal weight and temperature under a short photoperiod (Chen et al., Reference Chen, Duan, Chen and Xue2012). Their results indicate that pupal weight may be affected by other factors in addition to temperature. In our study, there were no significantly differences in weights between the female pupae of G. molesta reared under different temperatures, and only slight differences in the male pupae. This may be due to the larvae being sheltered within the apples, protecting them from energy loss caused by external temperature stress. Another study, by Zhang et al. (Reference Zhang, Rudolf and Ma2015), found that rearing larvae of Plutella xylostella under high temperature did not affect their pupal weight, but adults exposed to heat generally had reduced fecundity. Diet was also found to influence the pupal weight of G. molesta (Yokoyama et al., Reference Yokoyama, Miller and Harvey1987; Yu et al., Reference Yu, Wang, Zhang, Guo, Fan and Hao2016), but there has not as yet been any research on the effect of temperature on pupal weight of G. molesta.
In our research, G. molesta reared under outdoor fluctuating temperatures had a higher population growth potential and faster growth rate than those reared under constant temperatures. They also had a higher fertility than moths reared at constant temperatures, and a relatively high survival rate. The results indicated that the population raised under outdoor fluctuating temperature condition had an elevated environment adaptiveness. Because the development of G. molesta in fluctuating temperature was consistent with a natural population (fig. 3), it is hoped that our results may contribute to further studies on simulation and accuracy forecast of natural populations.
Acknowledgements
We are grateful to Professor Hsin Chi from National Chung Hsing University, Taichung, Taiwan for his help in life table analysis, and Dr Cecil L. Smith from University of Georgia for Paper modification. This research was supported by the National Key Research and Development Program of China on Peach and plum, the Major technology innovation project of Shandong Province (grant no. 2017CXGC0214) and the Funds of Shandong ‘Double Tops’ Program (grant no. SYL2017XTTD11).
Author contribution
ZC, LX and YX conceived and designed the research. ZC, LX, LL and HW conducted the experiments. LX compiled the data. ZC and YX wrote the manuscript. All authors read and approved the manuscript.