Introduction
Avermectins such as ivermectin, doramectin and eprinomectin are now widely used in livestock anthelmintics to control internal and external parasites of sheep and cattle. Over the last 20 years, there has been growing concern and debate regarding the effects of these pesticides on the non-target insects that feed in livestock dung (e.g. Wall & Strong, Reference Wall and Strong1987). When avermectins are metabolized by the treated animal, residues of the drugs and their breakdown products are excreted in the faeces of avermectin-treated animals (Campbell, Reference Campbell1985; Halley et al., Reference Halley, Nessel, Lu and Campbell1989) where they retain their insecticidal properties. Thus, non-target insects that are coprophagous (for all or part of their life cycle) may consequently be exposed to insecticidal residues in dung. Any adverse effects associated with avermectin exposure, e.g. reduced diversity or abundance within dung insect assemblages, can have consequences for nutrient cycling through delayed dung degradation (e.g. Wall & Strong, Reference Wall and Strong1987; Madsen et al., Reference Madsen, Grønvold, Nansen and Holter1988) and for the parasites and foraging predators that are dependent upon dung-breeding invertebrates (e.g. McCracken, Reference McCracken1993; Floate & Fox, Reference Floate and Fox1999).
Previous research has shown that exposure to avermectin residues in dung can cause reduced hatching success, increased larval mortality and suppressed adult emergence in a variety of species of dung-breeding flies. This has been shown for the horn fly Haematobia irritans (Linnaeus) (Schmidt, Reference Schmidt1983; Fincher, Reference Fincher1992; Marley et al., Reference Marley, Hall and Corwin1993; Floate et al., Reference Floate, Spooner and Colwell2001), the face fly Musca autumnalis De Geer (Meyer et al., Reference Meyer, Simco and Lancaster1980), the dung fly Neomyia cornicina (Fabricius) (Gover & Strong, Reference Gover and Strong1995), the house fly Musca domestica Linnaeus, and the stable fly Stomoxys calcitrans (Linnaeus) (Floate et al., Reference Floate, Spooner and Colwell2001) (all Diptera: Muscidae) and the blowfly Lucilia cuprina (Wiedemann) (Mahon & Wardhaugh, Reference Mahon and Wardhaugh1991) (Diptera: Calliphoridae). Furthermore, exposure to avermectins can increase the asymmetry of certain bilateral characters in flies (e.g. lengths of wing veins, Clarke & Ridsdill-Smith, Reference Clarke and Ridsdill-Smith1990), which may indicate that individuals in the population have been subjected to environmental stress during development (Parsons, Reference Parsons1992; Clarke & McKenzie, Reference Clarke and McKenzie1992). While some studies have found no link between the level of fluctuating asymmetry in a population and fitness (e.g. Martin & Hosken, Reference Martin and Hosken2002), increased asymmetry has been associated with reduced survival of individuals in populations (Clarke, Reference Clarke1995; Møller & Swaddle, Reference Møller and Swaddle1997). Exposure to avermectins could potentially bring about lethal or sublethal effects in dung-breeding flies, which could have consequences for the abundance and/or the physiological quality of individuals in these insect populations.
Few studies have examined the population effects of avermectin exposure on dung insects in fields in real farm situations. Previous research has focused on effects on insects in the laboratory or within individual dung pats in the field where avermectin residue levels may not be representative of situations in which livestock are undergoing a typical treatment regime. In reality, the extent of dung insects' exposure to avermectins on farmland may vary both spatially and temporally where, for example, only a proportion of a herd or flock may be treated or where farms within one geographical area are dosing livestock at different times of the year. This study examined the effects on populations of yellow dung flies Scathophaga stercoraria Linnaeus (Diptera: Scathophagidae) on commercial farms, where the pastures forming the focus of the study were grazed with either untreated cattle or cattle receiving manufacturer-recommended treatment regimes of an avermectin product.
We investigated avermectin effects on S. stercoraria by collecting abundance data in fields grazed by avermectin-treated or untreated cattle over two years. This species is a conspicuous north temperate dung-breeding fly that is common on and around livestock dung, especially in spring months. Females go to fresh dung to oviposit and males are attracted to the dung where they compete with one another for mating access to females (Otronen, Reference Otronen1995). While adult S. stercoraria predate on other dung invertebrates, their larvae are coprophagous and, therefore, exposure to avermectin residues is likely to be greatest in developing larvae. As cattle are commonly treated with an avermectin worming product when they are turned out to grazing at around the end of April and beginning of May (Webb, Reference Webb2004), the excretion of avermectin residues in dung from grazing cattle is likely to coincide with the spring peak in abundance of S. stercoraria. Thus, if avermectin exposure had a significant deleterious effect on fly populations, one might expect population differences to be apparent in flies emerging from dung over the summer season.
This research therefore tested the hypotheses that exposure to avermectins in pastures: (i) reduced the size of S. stercoraria populations during that season, and (ii) increased the asymmetry of bilateral characters in response to exposure to avermectins during development in naturally occurring dung in cattle-grazed fields.
Materials and methods
A questionnaire survey targeted at livestock farmers in the study region of south-west Scotland ascertained that doramectin then ivermectin (both administered as a pour-on) were the most commonly used avermectins in that region (Webb, Reference Webb2004). The wider study, from which the S. stercoraria abundance data were obtained, was carried out on seven dairy farms and one beef and sheep farm in Ayrshire, south-west Scotland between April and July in both 2002 and 2003. The study farms were selected according to their use of avermectin products so that they were representative of the treatment strategies in the wider area. Over the two years, sampling was focused on 14 treated fields grazed by cattle that were treated with either a doramectin or an ivermectin product and 12 untreated fields grazed by cattle that had received no worming treatment during the study period and for a minimum of six months prior to the study (fig. 1). All cattle were turned out to grazing in late April and early May. Of the cattle receiving standard, manufacturer-recommended treatment regimes of doramectin (Dectomax™) or ivermectin (Ivomec®, Noromectin®) pour-on, the majority (those in two-thirds of study fields) were treated at turnout (i.e. within four days before or after turnout) followed by a second dose some eight weeks later. Cattle in the remaining treated fields were dosed within 14 days following turnout, and given a second dose some eight weeks later. A subset of the fields (six ‘untreated’ and six ‘treated’) was sampled in both years while all of the other fields were sampled throughout only one of the two study years.

Fig. 1. Maps showing study location in south-west Scotland and spatial distribution of fields sampled during 2002 and 2003. Closed circles represent fields grazed by avermectin-treated cattle and open circles are fields grazed by untreated cattle.
Dung fly sampling
Adult dung flies (Scathophagidae) were sampled using dung-baited pitfall traps. Traps were 1 litre plastic containers (11.5 cm diameter) sunk flush with the ground and filled with approximately 3 cm depth of 70% monopropylene glycol (to act as a killing agent and preservative). Wire mesh was secured over the traps to serve as a support for dung baits. Baits were formed using a hemispherical mould of 6 cm diameter and 3 cm depth and were placed at the centre of the mesh. Dung for baits was collected on one occasion in both 2002 and 2003 from housed cattle at each study farm in April, prior to their receiving any avermectin treatment. Each year, dung from each farm was mixed thoroughly to homogenize it, and stored in a sealed container at 4°C. In order to maintain good biosecurity, dung from different farms was kept in separate containers and used as bait only on farms from where it had been collected. As the quality and consistency of dung varies naturally between farms, the number of farms and fields sampled was maximized in order to minimize that source of variation. Dung remains attractive to dung insects after being kept in cold storage for up to six months therefore bait attractiveness did not diminish significantly over the course of the study (Webb, Reference Webb2004).
A pilot study showed that four baited pitfall traps adequately represented the dung insect species present in a pasture in the geographical study area (Webb, Reference Webb2004). However, to insure against losing traps because of cattle trampling or disturbance, eight baited pitfall traps were set in two grids of four in each pasture, with traps spaced approximately 8 m apart within each grid. Traps were emptied and re-baited approximately every 7–10 days in 2002 and every 14 days in 2003 thus giving a maximum of nine and six collections per field over the sampling seasons in 2002 and 2003, respectively. The same two traps from each grid were selected from each sampling date in each pasture and S. stercoraria were counted and summed across those four traps. Scathophaga furcata (Say) was distinguished from S. stercoraria, but the former occurred in extremely low numbers, was not trapped in all study fields and hence was not considered any further in the analyses.
Selection of data for asymmetry assessments
Fluctuating asymmetry methods were used to examine the asymmetry of wing and hind tibiae length in S. stercoraria individuals in pastures using data from a subset of four fields. These bilateral characters have been frequently used in fluctuating asymmetry studies for S. stercoraria (e.g. Liggett et al., Reference Liggett, Harvey and Manning1993; Hosken et al., Reference Hosken, Blanckenhorn and Ward2000; Martin & Hosken, Reference Martin and Hosken2002) and other dipteran species (Møller, Reference Møller1996). Adult S. stercoraria were collected using dung-baited pitfall traps in 2002 from two fields grazed by doramectin-treated and two fields grazed by untreated cattle. The cattle in the treated fields had been dosed with a doramectin pour-on (Dectomax™) at the beginning of May 2002 when they were turned out for grazing. The fields were situated on two dairy farms with one ‘treated’ and one ‘untreated’ field on each farm. Traps were set 4–5 weeks after the dosing of cattle in ‘treated’ fields and collected 10 days later in mid-June to ensure that most flies trapped in those fields would have developed from eggs oviposited in dung that contained doramectin residues. Doramectin residues can be excreted from an animal for at least 14 days following treatment (Toutain et al., Reference Toutain, Upson, Terhune and McKenzie1997) and the development time of S. stercoraria from egg to adult has been estimated at 24–42 days (Gibbons, Reference Gibbons1987; Strong & James, Reference Strong and James1993). Therefore, there was a sufficient temporal overlap to expect that a high proportion of the adults trapped at that time would have been exposed to doramectin residues during their development in dung. Flies trapped in a field may not all have emerged from dung within that field. Thus, to increase the chance that flies trapped in the same field experienced similar developmental conditions, the four fields from where flies were collected were selected on the basis that they were surrounded by fields undergoing the same grazing regime, i.e. treated study fields were adjacent to fields that were also grazed by doramectin-treated cattle. A mark–recapture study of the sheep blowfly Lucilia sericata (Meigen) (Diptera: Calliphoridae) estimated the dispersal distances of the flies to be between 100–200 m (Smith & Wall, Reference Smith and Wall1998). Therefore, it was assumed that most scathophagids were unlikely to disperse further than either their pasture of emergence or to adjacent fields provided that there was a dung resource available. Thus, the flies trapped were likely to have emerged either in the study field or in an adjacent field containing the same ‘type’ of dung, i.e. dung from either untreated or avermectin-treated cattle.
Asymmetry measurements
Measurements were made for 113 male flies that were trapped during the 10-day period; 54 flies (30 from one field and 24 from the other) were collected in fields grazed by doramectin-treated cattle and 59 flies (30 from one field and 29 from the other) in fields grazed by untreated cattle. All males, with intact wings and hind legs, collected during the trapping period were measured. Males were trapped in larger numbers than females and hence only males were measured to exclude any bias associated with sex-specific differences. Wings and hind legs were dissected from each fly, placed under a coverslip, and measured under a microscope to the nearest 0.1 mm. Wing length measurements were made in a straight line from point A to point B (fig. 2), and hind tibia measurements were taken as the full length from the point where the tibia joined the femur to where it met the first tarsal segment.

Fig. 2. Wing of Scathophaga stercoraria showing wing length measurements taken in a straight line from the proximal end of the costal vein (A) to the tip of the wing (B). Photo reprinted from Strong & James (Reference Strong and James1993), with permission from Elsevier.
Environmental and management variables
Climate data were obtained from the nearest weather station, located at SAC Auchincruive, which was situated 1–16 km to the west of any one of the study fields. Total rainfall (mm), sunshine hours and the mean daily maximum and minimum temperatures were calculated for each individual trapping period. The aspect, altitude, size of each field, field boundary type (fence, gappy hedge, well-established hedge, woodland), surrounding land use and avermectin use were recorded. Sward height was measured ten times in the area around the traps, using the direct method (Stewart et al., Reference Stewart, Bourn and Thomas2001), and mean sward height was calculated for each trapping period.
The availability of fresh dung within a study field in each trapping period was gauged using a ‘dung index’. The index was the product of number of days that cattle were grazing a pasture and the number of cattle, corrected for pasture size, to give an index of the number of dung pats that would be deposited per unit area per trapping period. An ‘avermectin index’ was devised for each trapping period to estimate the proportion of land within a 400 m radius, from the centre of the study field, that was grazed by avermectin-treated livestock. The 400 m radius was selected because it encompasses the maximum area travelled by many dung-breeding insects (Smith & Wall, Reference Smith and Wall1998; Roslin, Reference Roslin2000). Similarly, the ‘pasture index’ estimated the percentage area of grazed pasture within a 400 m radius around the study field to reflect the potential availability of livestock dung in surrounding fields.
Scathophaga stercoraria often pupate in soil below the dung pats. In addition, soil type could potentially influence the quality of pasture as a grazing resource (and thus the suitability of herbivore dung for colonization by insects). Hence, information on soil characteristics of the pastures was collected for consideration in the analyses. Six soil cores (10 cm length×6.5 cm diameter) were taken from the area around the traps in all study fields and sent for laboratory analysis to determine soil pH, soil moisture and soil organic matter. Cores were taken once in January 2003 and once in August 2003, with the former taken to reflect 2002 study fields and the latter taken to reflect 2003 study fields. The temporal difference in soil sampling meant that comparisons of soil characteristics between years were treated with caution, however, comparisons could be made between fields for each year.
An index of management intensity was calculated for each study pasture. Management information was collected through interview of farmers who were asked about management practices in the fields: sward type and age, soil disturbance, cutting regimes, grazing intensity, fertilizer input and herbicide use. A score between 0 and 4 was assigned to each factor and summed to give an overall management intensity score (MIS) between 0–24, with 24 being the most intensively managed (Downie et al., Reference Downie, Ribera, McCracken, Wilson, Foster, Waterhouse, Abernethy and Murphy2000).
Data analyses
Abundance
The effect of avermectins on S. stercoraria population size was investigated using mixed models to analyse data from all fields. Abundance was corrected to a standard period of 10 days to allow comparisons between trapping periods of different duration. General linear modelling of abundance data was carried out using mixed models with repeated measures in SAS (SAS Institute, 2001) using the GLIMMIX macro with a Poisson error, log link function and a Satterthwaite correction. Field and farm were included as random factors and sampling date was included as a repeated measure. The autoregression of order 1 covariance structure was used because it assumes that measures further apart in time are less correlated than measures closer together (Littell et al., Reference Littell, Milliken, Stroup and Wolfinger1996), e.g. pitfall catches taken one week apart in May are likely to be more similar than catches from May and July. The Akaike's information criterion was used to support selection of the best covariance structure and model (Littell et al., Reference Littell, Milliken, Stroup and Wolfinger1996). All environmental and management variables (table 1) were considered independently and the quadratic relationships of all continuous variables and interactions of potential interest were tested. Ordination was used to simplify and reduce the number of habitat parameters that were introduced into the model (Fox, Reference Fox2004; Rushton et al., Reference Rushton, Ormerod and Kerby2004). Altitude, aspect, field boundary type and ‘pasture index’ data were ordinated using detrended correspondence analysis to generate a general ‘habitat’ score. Models were built using a step-up procedure, whereby variables were retained in the model if they were significant and any variables that were no longer significant were removed. Only the final models selected are presented in this paper.
Table 1. List of environmental and management variables included in mixed model analyses of Scathophaga stercoraria abundance data. The recorded ranges of continuous variables across ‘treated’ and ‘untreated’ fields are given in parentheses.

Asymmetry
For fluctuating asymmetry analyses, the error of measuring a trait should be estimated to ensure that it does not exceed the actual measured asymmetry of that trait (Palmer & Strobeck, Reference Palmer and Strobeck1986). To estimate measurement error, the left and right wing and hind tibiae lengths of each of 10 flies from untreated fields were measured on three separate occasions on consecutive days, without reference to the previous measurements of the individuals being measured at the time. These individuals were all drawn from untreated fields to avoid differences in measurement error arising between flies from treated versus untreated fields. Following Swaddle et al. (Reference Swaddle, Witter and Cuthill1994), a mixed model ANOVA was used to test whether the variance of estimated asymmetry between individuals was greater than the variance due to measurement error. Variance was significantly greater between individuals than between repeated measurements for wing length asymmetry (F9, 36=2.31, P=0.04) but not for hind tibia length asymmetry (F9, 36=0.86, P=0.57). Therefore, only wing length data were analysed and presented here, since one could not be certain whether or not differences in hind tibia length asymmetry were due to measurement error.
Visual inspections of scatter plots were used to check for outliers, e.g. aberrant individuals and bad raw measurements (Palmer & Strobeck, Reference Palmer, Strobeck and Polak2003). The measurements of the bilateral character should meet the assumptions of fluctuating asymmetry, which are a normal distribution around a mean of zero (Palmer & Strobeck, Reference Palmer and Strobeck1986). To check that this was the case with the data available, the signed asymmetry scores of wing length were calculated by subtracting the length of the right wing from that of the left wing. The signed asymmetry scores of wings fitted a normal distribution (Kolmogorov-Smirnov test: D=0.047, n=113, P>0.15) and therefore there was no evidence for antisymmetry (e.g. Møller, Reference Møller1996; Ahtiainen et al., Reference Ahtiainen, Alatalo, Mappes and Vertainen2003). The mean signed asymmetry score did not differ significantly from zero (one sample t-test: t=0.27, n=113, P=0.79). This excluded the possibility that the data exhibited directional asymmetry (e.g. Møller, Reference Møller1996; Sneddon & Swaddle, Reference Sneddon and Swaddle1999). As the assumptions of fluctuating asymmetry were met, the absolute (unsigned) asymmetry scores for wing length were calculated by subtracting the smaller side of the bilateral character from the larger side. To examine fluctuating asymmetry between samples, non-parametric tests should be used on absolute asymmetry data (Palmer & Strobeck, Reference Palmer and Strobeck1986), thus wing length asymmetries of flies were compared with a Mann-Whitney U-test.
As wing length and body mass are highly positively correlated (r2=0.995) in S. stercoraria (Borgia, Reference Borgia1982), we used wing length as an indicator of body size in this study. The mean wing lengths of the flies used in the fluctuating asymmetry analysis, i.e. 54 flies from treated fields and 59 flies from untreated fields, were compared between fields grazed either by untreated or doramectin-treated cattle.
Results
Abundance
A total of 27,464 S. stercoraria were trapped over the two sample years. Their abundance changed non-linearly through the season and this pattern differed between years (table 2). In 2002, numbers were highest in April and then declined through May and levelled off in June and July (fig. 3). In 2003, abundance was lower than in 2002 but the pattern was similar in that numbers were highest in April and then declined before levelling off in late June. There was no effect of avermectin treatment on the abundance of S. stercoraria (F1, 18.9<0.01, P=0.97). All other variables from table 1 were not significant (F<2.42, P>0.12).

Fig. 3. Mean (±1 se) number of Scathophaga stercoraria trapped in treated and untreated fields in 2002 and 2003. Abundance data are grouped into months for presentation only (□, untreated 2002;

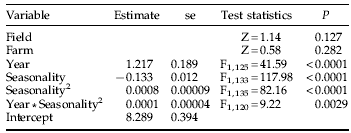
Table 2. The final model for Scathophaga stercoraria abundance in fields grazed by avermectin-treated and untreated cattle from April to July in 2002 and 2003.
Asymmetry
The wing lengths of individuals measured in the study ranged from 6.5 mm to 10.1 mm with a mean (±se) length of 8.34 (±0.05) mm. The mean wing lengths of male flies measured for fluctuating asymmetry did not differ significantly between treated and untreated fields (fig. 4a). However, the absolute asymmetry of wings was significantly higher in yellow dung flies caught in treated fields than in flies from untreated fields (fig. 4b). There was no correlation between wing length and wing asymmetry (Spearman rank rs=−0.02, n=113, P=0.84). Scathophaga stercoraria abundance data from the four fields that were used for asymmetry data were analysed in order to check that these fitted the pattern of the results from the wider sampling study. As with data from all fields, there was no significant difference in abundance between the fields grazed by doramectin-treated and untreated cattle (F1, 2.04=0.03, P=0.87).

Fig. 4. The effect of avermectin treatment on Scathophaga stercoraria wing lengths and asymmetry scores: (a) Mean (±1 se) wing lengths of S. stercoraria trapped in 2002 in pastures grazed by untreated cattle and pastures grazed by doramectin-treated cattle. There is no significant difference between treated and untreated fields (t=1.7, df=224, P=0.09); (b) Mean (±1 se) absolute asymmetry scores for wings of S. stercoraria trapped in fields containing doramectin-treated cattle (0.135±0.014) or untreated cattle (0.08±0.01). Asymmetry is significantly higher in fields with treated cattle (Mann-Whitney, n=113, P=0.002).
Discussion
There was no difference in the abundance of adult S. stercoraria trapped in fields grazed either by untreated or by avermectin-treated cattle. However, wing length asymmetry was significantly higher in flies trapped in fields grazed by doramectin-treated cattle. Thus, although there was a possible sublethal effect of doramectin treatment on S. stercoraria, as suggested by greater wing length asymmetry of individuals in treated fields compared to untreated fields, there was no apparent effect of avermectin treatment of cattle on yellow dung fly abundance at the field scale.
Abundance of S. stercoraria varied significantly between the two study years, with abundance higher throughout the 2002 sampling season compared to 2003. Such variation between years has been observed elsewhere for this species (Gibbons, Reference Gibbons1987; Ward & Simmons, Reference Ward and Simmons1990). While weather variables were not significant in the final model, it cannot be discounted that the difference in yellow dung fly abundance between the two study years may have been due to some indirect or unmeasured response to weather conditions. Higher numbers of flies occurred during the cooler, wetter summer of 2002, possibly because mortality of adults due to high summer temperatures (Ward & Simmons, Reference Ward and Simmons1990) would have been lower. It has also been proposed that adult S. stercoraria become inactive during hot weather (Blanckenhorn et al., Reference Blanckenhorn, Henseler, Burkhard and Briegel2001). Therefore, the higher temperatures in the 2003 sampling season may have induced adult quiescence and thus the relatively lower numbers in baited pitfall traps may have reflected a period of inactivity of yellow dung flies. While winter weather conditions were not included in these analyses, it is possible that the variation in abundance between years was partly dependent on the survival of S. stercoraria during the previous winter, as extremely cold weather and hard frosts can increase the mortality of adult flies (Gibbons, Reference Gibbons1987) and overwintering larvae and pupae (e.g. Pitts & Wall, Reference Pitts and Wall2006). There was a significant effect of season on the number of S. stercoraria trapped. The recorded abundance of flies generally followed the typical seasonal pattern of this species with high numbers in spring and a subsequent decline throughout the summer months (Parker, Reference Parker1970; Gibbons, Reference Gibbons1987).
Wing length asymmetry was higher in fields where the majority of trapped S. stercoraria would have developed in dung containing doramectin residues. This potentially indicates that doramectin exposure during development had a sublethal effect on yellow dung flies in pastures grazed by treated cattle, which supports findings from earlier laboratory studies that ivermectin exposure increased the asymmetry of wing characters in yellow dung flies (Strong & James, Reference Strong and James1993). Higher levels of asymmetry could cause lower reproductive success (Liggett et al., Reference Liggett, Harvey and Manning1993; Allen & Simmons, Reference Allen and Simmons1996; Møller, Reference Møller1996), which in turn could lead to a reduced population size. However, as stated above, there was no observable effect on S. stercoraria abundance at any time throughout the April to July sampling season.
Potential underlying mechanisms
There are several possibilities that could explain the lack of effect of avermectin treatment on yellow dung fly abundance. It is possible that the dispersal of flies and mixing of populations could have masked any variation in abundance caused by exposure to avermectin residues in cattle-grazed fields. If there was significant dispersal by independent movement and by wind, then local declines of flies in treated fields may not have become apparent. Indeed, it is possible that there were population sinks in fields grazed by treated cattle, which were buffered by an influx of adult flies from neighbouring ‘untreated’ fields. However, the likelihood of this was reduced as fields grazed by avermectin-treated cattle had a high probability of being surrounded with fields also grazed by treated cattle (Webb, Reference Webb2004), as was taken into account by inclusion of the avermectin index in the statistical modelling analyses. Thus, if a population sink occurred in treated fields, immigration from adjacent fields would be low because populations in those fields would also experience a decline.
It cannot be discounted that the lack of effect on population size occurred because there was no actual effect on the number of larvae developing into adults. For example, Floate (Reference Floate1998) found that the emergence rate of S. stercoraria from ivermectin-treated dung was unaffected. In addition, even if larval development in dung from treated animals is inhibited (McCracken & Foster, Reference McCracken and Foster1993; Strong & James, Reference Strong and James1993), the number of flies actually emerging from treated dung may not be any lower than from untreated dung once intra-specific competition and other density-dependent processes in untreated dung have been taken into account. It is also possible that natural fluctuations in S. stercoraria populations could potentially conceal an avermectin effect on the number of flies trapped if variance was too high to detect differences in mean abundance. However, there was little difference in the mean values of S. stercoraria abundance between treated and untreated fields (as shown in fig. 3) and therefore, the lack of significance of treatment cannot be attributed to a high variance.
While there was no significant effect of avermectin treatment on S. stercoraria abundance in fields, avermectin exposure could potentially cause sublethal effects in an individual or population's fitness. In the present study, increased wing length asymmetry was detected on two sites in one year and it is possible, therefore, that these effects were specific to those sites or to that particular year. However, the data were drawn from fields on two farms which were approximately 6 km apart, thus suggesting that the finding was not simply a chance event at one location. However, this work could be expanded to consider effects on other farms in other geographical locations and should be repeated over a number of years to determine how consistent this finding is.
Increased asymmetry in a population may be associated with sublethal effects, such as reduced competitive ability or mating success. For example, male S. stercoraria with higher wing length asymmetry were less likely, than symmetrical males, to be paired with a female on dung (Liggett et al., Reference Liggett, Harvey and Manning1993). It was suggested that this was due to symmetrical males having superior competitive ability, not necessarily in terms of direct male–male competition but perhaps some other competitive advantage, e.g. locating the dung resource and females. However, we do not have information about the relative reproductive behaviour or fecundity of the asymmetric males in this study. A significant reduction in fecundity might be expected to result in a population decline. However, such a decline was not observed for S. stercoraria in this study. Furthermore, the abundance of adult dung flies did not differ between the two treated and two untreated fields used to provide fluctuating asymmetry data, which discounted the possibility that a significant effect on population size in those four fields was being masked in the wider data analyses of all fields. The levels of absolute asymmetry as a proportion of S. stercoraria wing length in this study (range: 0.96–1.61%) was similar in magnitude to that found by Møller (Reference Møller1996) for M. domestica in the field (range: 0.76–2.12%). However, Møller (Reference Møller1996) did not estimate fly population size in those study sites and indeed there are few studies that document the potential effect of fluctuating asymmetry on population size. Therefore, it is not possible to compare the extent of asymmetry in S. stercoraria populations in this study to others in terms of potential effects on fecundity or population size.
The observed increase in wing length asymmetry may not have compromised fitness of individuals to an extent that negatively affected the size of the population. In a study of captive S. stercoraria, Martin & Hosken (Reference Martin and Hosken2002) found no evidence of a relationship between fluctuating asymmetry and fitness. Although increased asymmetry of wing length is not necessarily an indication that larval quality or development of S. stercoraria was compromised, previous research has indicated that larval development is impaired in pats containing avermectin residues. Therefore, one would expect some degree of effect on larvae within pats from avermectin-treated cattle. It could be that larval survival or development was impaired, but at a level that had no subsequent effect on the abundance of adults trapped. Negative effects may have been mitigated by the overall high fecundity of this species whereby any localized declines may have failed to cause a significant reduction in the numbers trapped. As already discussed above, the possibility of significant immigration of flies from untreated to treated fields would have been limited given the choice of study fields.
It has also been suggested that the degree of fluctuating asymmetry in a character is negatively correlated to the size of that character (Møller, Reference Møller1992). If this were true for S. stercoraria, it could be expected that individuals with relatively higher asymmetry would have shorter wings and therefore lower body mass (as wing length is positively correlated to body size in yellow dung flies). However, asymmetry was not correlated to wing size and wing length did not differ between treated and untreated fields. Therefore, there are no apparent implications for the predators of S. stercoraria in terms of the profitability of individual prey items.
When carrying out studies of fluctuating asymmetry in the field, it cannot be discounted that unmeasured or unconsidered factors may be affecting asymmetry. While many environmental variables were measured here, the possibility that something else was increasing the asymmetry of S. stercoraria populations cannot be ruled out. As mentioned above, it was not certain whether all of the flies trapped in those fields had emerged from dung containing doramectin residues. However, as discussed above, this was mitigated by selecting fields that were surrounded by cattle undergoing the same treatment.
Higher asymmetry in S. stercoraria in fields with doramectin-treated cattle may have been a result of reduced predation pressure in those fields. Previous research has shown that the risk of being predated is higher in asymmetric individuals probably because they are less able to evade predators than their symmetric conspecifics (Møller, Reference Møller1996; Swaddle, Reference Swaddle1997). Foraging activity of barn swallows was greater in fields grazed by untreated cattle than in those grazed by treated cattle, possibly because the latter were located farther from farm buildings (and nest sites) than untreated fields (Webb, Reference Webb2004). Thus, selection pressure against asymmetric S. stercoraria individuals (from swallow predation) could be lower in fields grazed by treated cattle than in those grazed by untreated cattle and could consequently result in relatively higher asymmetry in yellow dung fly populations in treated fields.
In any study of fluctuating asymmetry, it is extremely important to identify whether differences in asymmetry could be due to variation arising from measurement error (e.g. Palmer & Strobeck, Reference Palmer and Strobeck1986). Hind tibia lengths were not considered for fluctuating asymmetry analysis in this study because preliminary analyses showed that any differences in hind tibia length asymmetry could have been due to measurement error. Thus, while fluctuating asymmetry of hind tibiae length may be indicative of developmental stress in some populations, hind tibia length asymmetry was not a suitable measure of doramectin exposure in this study. Indeed, others have shown that fluctuating asymmetry in S. stercoraria can be stress and/or trait specific (Hosken et al., Reference Hosken, Blanckenhorn and Ward2000).
Conclusions
This study is the first to present evidence of an increase in wing length asymmetry in S. stercoraria populations in fields grazed by doramectin-treated cattle yet no effect on the number of flies trapped between fields grazed by treated or untreated cattle. It indicates that there are likely to be complex interactions between avermectin effects and environmental variables in agricultural landscapes, which must be understood more thoroughly in order to consider the effects of avermectin use on dung insects and their predators. In addition, this study suggests that caution must be shown when extrapolating from small-scale experiments, at the level of individual dung pats, to the potential effects that may occur within pastures at a population level.
Acknowledgements
This research was carried out as part of a PhD studentship funded by the Royal Society for the Protection of Birds and the Scottish Agricultural College Dr D. MacLagan Trust Fund. The Scottish Agricultural College receives financial support from the Scottish Executive Environment and Rural Affairs Department. The authors are grateful to two anonymous referees for helpful comments on an earlier version of this manuscript.


