Introduction
The Sunn pest, Eurygaster integriceps Puton (Heteroptera: Scutelleridae), is one of the most serious insect pests of wheat and barley in the wide area of the Near and Middle East, west Asia, and many of the new independent states of central Asia. It also is found in eastern and southern Europe and North Africa (Paulin & Popov, Reference Paulian and Popov1980). This insect has a monovoltine life cycle (one generation per year) with two different phases. The first phase (growth and development) occurs in wheat fields during the spring, whereas the second phase (diapause as an adult) usually occurs in mountain areas during the summer and winter. During feeding, they enter their stylets into the host plant, inject their watery saliva containing digestive enzymes, and suck up the liquefied cells' contents (Boyd et al., Reference Boyd, Cohen and Alverson2002). Feeding punctures appear as minute darkish spots on the seeds. Sunn pest feeding on different stages of developing seeds causes quantitative and qualitative damage because they inject enzymes into the grain that degrade gluten protein and cause the rapid relaxation of dough, which results in the production of bread with poor volume and texture (Popov et al., Reference Popov, Barbulescu and Vonica1996). Most of the economic loss attributed to this species is caused by nymphal and adult injury to the wheat kernels, so that yield loss due to Sunn pest outbreaks in some area is 100%.
In recent years, the use of synthetic insecticides in crop pest control programs around the world has resulted in damage to the environment, pest resurgence, pest resistance to insecticides and lethal effects on non-target organisms (Abudulai et al., Reference Abudulai, Shepard and Mitchell2001). Problems associated with the use of synthetic insecticides led researchers to look for natural plant protection compounds, such as botanical insecticides and antifeedants. Among the plant families studied, the Meliaceae, Asteraceae, Labiateae, Piperaceae and Annonaceae are the most promising (Isman, Reference Isman2006). In this context, Artemisia annua extract may be one of the best extracts for control of the Sunn pest population. The genus Artemisia is a member of a large plant family Asteracea (Compositae), encompassing more than 300 different species of this diverse genus. The species A. annua, known as sweet worm wood, grows wild in Europe and America and is planted widely in China, Turkey, Vietnam, Afghanistan and Australia (Bhakuni et al., Reference Bhakuni, Sharma and Kumar2001; Shekari et al., Reference Shekari, Jalali Sendi, Etenbari, Zibaee and Shadparvar2008). Several isolated compounds from this species have shown anti-malarial, antibacterial, anti-inflammatory, plant growth regulatory and cytotoxicity (antitumor) activities (Akhtar & Isman, Reference Akhtar and Isman2004). Botanical products are useful tools in many pest management programs because they are effective against pests and, specifically, may be the target plants for natural enemies as their suitable habitats. Also, they are highly effective, safe and ecologically acceptable (Weinzierl & Henn, Reference Weinzierl and Henn1991; Senthil Nathan & Kalaivani, Reference Senthil Nathan and Kalaivani2005).
As well as the emphasis on botanical insecticides, the common trend in the past two decades towards reducing reliance on synthetic insecticides has renewed worldwide interest in microorganisms, including some mycetes that could represent a proper source of molecules for safe pest control practice without the environmental risk connected with synthetic pesticides. Well-known entomopathogenic fungi commonly used for pest control include: Beauveria bassiana, Metarhizium anisopliae and Paecilomyces fumosoroseus (Feng et al., Reference Feng, Johnson and Kish1990; Wraight et al., Reference Wraight, Carruthers, Bradleg, Jaronski, Lacey, Wood and Galaini-Wroigh1998). B. bassiana was used for many sucking pests and showed satisfactory results. Wraight et al. (Reference Wraight, Carruthers, Bradleg, Jaronski, Lacey, Wood and Galaini-Wroigh1998), reviewing entomopathogenic effects of P. fumosoroseus, P. farinosous and B. bassiana isolated from silverleaf whitefly, showed that all of them have pathogenic effect on this pest. Hatting et al. (Reference Hatting, Wraight and Miller2004) showed that B. bassiana could control up to 65% of Duraphis noxia Mordvilko (Hemiptera: Aphididae) in field conditions. Talaei (Reference Talaei and Kharazzi Pakdel2002) tested B. bassiana on E. integriceps and showed that they were highly effective, using spraying methods, especially on the nymphal instars and adults, and demonstrated that B. bassiana was effective on E. integriceps in hibernating sites.
It is clear that botanical insecticides affect insect physiology in many different ways, such as enzyme activities and immune reactions that are still to be discovered. Hence, in this study, we studied (i) the determination of an effective dose of A. annua extract, (ii) the effect of plant extracts on some major digestive enzymes, and (iii) the effect of plant extracts on cellular immune reactions of E. integriceps.
Materials and methods
Insects
The insects were collected from the Karaj wheat farm and reared on wheat seeds, variety Fallat, in the laboratory at 27±2°C under a 14 h light:10 h dark photoperiod (Kazzazi et al., Reference Kazzazi, Bandani and Hoszeinkhani2005). Insects were fed on seeds and water-soaked pieces of cotton were used as water sources for them.
Beauvaria bassiana culture
B. bassiana isolate B1 was cultured at 25±1°C on Sabouraud Dextrose Agar (SDA) (pH 5.6) amended with 1% yeast extract. After 14 days, conidia of B. bassiana were washed off with a 0.01% aqueous solution of Tween 20 and different concentrations of spores were prepared as required.
Methanolic extract from leaves of A. annua
In June 2008, A. annua leaves were collected around paddy fields and were washed with distilled water and dried at room temperature in the shade. Methanolic extraction was carried out according to the procedure described by Shekari et al. (Reference Shekari, Jalali Sendi, Etenbari, Zibaee and Shadparvar2008). In brief, 30 g of dried leaves were stirred with 300 ml of 85% methanol in a flask for one hour. The methanolic solution was incubated for 48 h at 4°C, stirred for an additional hour, and then filtered through Whatman no. 4 filter paper. The solvent was removed by vacuum in a rotary evaporator, and the dark green residue was dissolved in 10 ml acetone and used as a starting stock solution. Further dilutions with either acetone or distilled water were used to prepare different concentrations.
Bioassay and treatments
Five concentrations of A. annua extract were used for the evaluation of toxicity and LD values along with a control treated with acetone alone. In each experiment, 30 insects were tested with five replicates for each concentration. For the determination of lethal and sublethal concentration effects on mortality, digestive enzymatic profiles and cellular immune responses, adults were held at starvation status for 12 h then allowed to feed on food and water containing A. annua extract, while the control group was fed on natural food and distilled water (without plant extracts). After a period of 5–8 days, control and treated bugs were divided into two groups; bugs of the first group underwent midgut extraction (see below) while insects of the second group were injected laterally into the thorax with 1 μl B. bassiana spore suspension at a concentration of 107 spore ml−1 using a 10-μl Hamilton syringe (Figueiredo et al., Reference Figueiredo, Castroa, Nogueirab, Garciaa and Azambuja2006).
Sample preparation for enzymatic assay
Enzyme samples from the midguts of adults were prepared by the method of Cohen (Reference Cohen1993), with slight modifications. Briefly, adults were randomly selected and their midguts were removed by dissection under a stereo microscope in ice-cold saline buffer (6 μmol l−1 NaCl). The midgut was separated from the insect body, rinsed in ice-cold saline buffer, placed in a pre-cooled homogenizer and ground in 1 ml of universal buffer containing succinate (5 mM), glycine (2 mM) and 2-morpholinoethanensulfonic acid (pH=7.2). The homogenates from both preparations were separately transferred to 1.5 ml centrifuge tubes and centrifuged at 15,000 rpm for 20 min at 4°C. The supernatants were pooled and stored at −20°C for subsequent analyses (Kazzazi et al., Reference Kazzazi, Bandani and Hoszeinkhani2005).
Digestive enzyme assays
α-Amylase activity
α-Amylase activity was assayed by the dinitrosalicylic acid (DNS) procedure (Bernfeld, Reference Bernfeld1955), using 1% soluble starch (Merck, Darmstadt, Germany) as substrate. Twenty microliters of the enzyme were incubated for 30 min at 35°C with 500 μl universal buffer and 40 μl soluble starch. The reaction was stopped by the addition of 100 μl DNS and heating in boiling water for 10 min. DNS is a color reagent; hence, the reducing groups released from starch by α-amylase action were measured by the reduction of DNS. The boiling water stops the α-amylase activity and catalyzes the reaction between DNS and the reducing groups of starch. Then, absorbance was read at 540 nm. One unit of α-amylase activity was defined as the amount of enzyme required to produce 1 mg maltose in 30 min at 35°C. A blank sample without substrate with α-amylase extract and a negative control containing no α-amylase extract with substrate were run simultaneously. All assays were performed in duplicate and each assay was repeated at least three times.
α- and β-glucosidase activity
For solubilization of membrane hydrolyses (α- and β-glocusidases) in Triton X-100, membrane preparations were exposed to Triton X-100 for 20 h at 40°C, in a ratio of 10 mg of Triton X-100 mg−1 of protein, before being centrifuged at 15,000 rpm for 30 min. No sediment was visible after the centrifugation of this supernatant at 10,000 rpm for 60 min. The activity of the enzymes remains unchanged, at −20°C, for periods of at least a month (Ferreira & Terra, Reference Ferreira and Terra1983). The α- and β-glucosidases activity was assayed by incubating 50 μl of enzyme solution with 75 μl of p-nitrophenyl-α-D-glucopyranoside (pNαG) (5 mM), p-nitrophenyl-β-D-glucopyranoside (pNβG) (5 mM) and 125 μl of 100 mM universal buffer (pH 5.0) at 37°C for 10 min. The reactions were stopped by adding 2 ml of sodium carbonate (1 M) and read at 450 nm (Ferreira & Terra, Reference Ferreira and Terra1983).
Lipase activity
The enzyme assays were carried out as described by Tsujita et al. (Reference Tsujita, Ninomiya and Okuda1989). Hence, 30 μl of midgut tissue extracts, 0.5 ml of universal buffer solution (1 M) (pH 7.2) and 100 μl of p-nitrophenyl butyrate (50 mM), as substrate, were incorporated, mixed thoroughly and incubated at 37°C. After 1 min, 100 μl distilled water was added to each tube (control and experimental samples) and absorbance was read at 405 nm. One unit of enzyme releases 1.0 nanomole (10−9 mole) of p-nitrophenol per minute at pH 7.2 at 37°C using p-nitrophenyl butyrate as substrate. The negative control tube was placed in a boiling water bath for 15 min to destroy the enzyme activity and then cooled prior to being added to the substrate.
Protease activity
General protease activity of adult midguts was determined using azocasein as substrate (Elpidina et al., Reference Elpidina, Vinokurov, Gromenko, Rudenskaya, Dunaevsky and Zhuzhikov2001). The reaction mixture was 80 μl of 2% azocasein solution in 40 mM universal buffer of specified pH and 30 μl enzyme. The reaction mixture was incubated at 37°C for 60 min. Proteolysis was stopped by the addition of 300 μl of 10% trichloroacetic acid (TCA). Appropriate blanks, in which TCA was added first to the substrate, were prepared for each assay. Precipitation was achieved by cooling at 4°C for 120 min, and the reaction mixture was centrifuged at 16,000 rpm for 10 min. An equal volume of 1 N NaOH was added to the supernatant and the absorbance was recorded at 440 nm.
Effect of A. annua extract on kinetic parameters (V max and K m) of digestive enzymes
For this experiment, 20 μl of appropriately diluted enzyme preparations from control and treated adults were used in each assay. Final concentrations for substrate were 0.2, 0.4, 0.6, 0.8, 1 and 1.2% for α-amylase; 1, 2, 3, 4, 5 and 6 mM for α- and β-glocusidases; 0.5, 1, 1.25, 1.5, 2 and 2.25% for protease; and 10, 20, 30, 40, 50 and 60 mM for lipase. The Michaelis constant (K m) and the maximum velocity (V m) were estimated by Sigmaplot software version 11 and the results of K m and V max were the means±SE of three replicates (n=3) for each concentration.
Hemolymph collection and cell counting: in vivo experiments
E. integriceps hemolymph samples were collected carefully from a severed front leg with 50 μl sterile glass capillary tube (Sigma). The product was immediately diluted in anticoagulant solution (0.01 M ethylenediamine tetraacetic acid, 0.1 M glucose, 0.062 M NaCl, 0.026 M citric acid, pH 4.6) as described by Azambuja et al. (1991a). At different times after inoculation, total and differentiate (Plasmatocytes and Granulocytes) hemocyte count, free circulating hemocytes and spore associated hemocytes were determined by direct observation in a hemocytometer chamber by phase-contrast optics microscopy. Differential hemocyte counts were performed prior to inoculation according to Azambuja et al. (1991a).
Phagocytosis assay (in vivo)
The phagocytic activity of hemocytes was quantified using the in vivo microscopic procedure assay described by Rohloff et al. (Reference Rohloff, Wiesner and Gotz1994) with some modification. As targets for phagocytosis, the B. bassiana spores were used. Ten micolitres of B. bassiana spores at a concentration of 107 spores ml−1 were injected between the 2nd and 3rd leg of each adult with an Hamilton syringe. After intervals of 30 and 60 min, 30 μl of hemolymph was taken from each injected adult and gathered in a capillary tube. Each sample was put on a hemocytometer slide and incubated in a moist dark chamber at room temperature for 5 min with 20 μl of Giemsa solution added, and the mixture was incubated for another 5 min. Cells in 20 fields (objective 100) were counted, and the relative percentage of each cell type was then estimated according to morphological parameters previously described (Zibaee, A., Bandani, A.R. & Malagoli, D. unpublished dataFootnote 1). The counting of phagocytosis numbers was performed in three replicates and was shown as mean±SE.
Nodulation assay (in vivo)
Injections were carried out according to the method described above. Nodulation was assessed at 1, 3, 6, 12 and 24 h intervals. Adults were chilled on ice, and hemolymph was gathered in a capillary tube. Then, 200 μl samples, in three replicates, were poured into a hemocytometer and nodules were counted (Franssens et al., Reference Franssens, Smagghe, Simonet, Claeys, Breugelmans, De Loof and Broeck2006).
Phenoloxidase activity
In order to test the effect of B. bassiana spores on the PO (phenoloxidase) system in the treated adults by A. annua, a hemocyte lysate supernatant (HLS) was prepared. Briefly, hemolymph from adults was mixed with anticoagulant buffer and centrifuged at 10,000 rpm for 5 min, the supernatant was discarded, and the pellet was washed gently twice with a phosphate buffer (pH=6.5: Leonard et al., Reference Leonard, Kenneth and Ratcliffe1985). Cells were homogenised in 500 ml of phosphate buffer centrifuged at 12,000 rpm for 15 min, and the hemocyte lysate supernatant (HLS) was used in PO assays. Samples were pre-incubated with buffer at 30°C for 30 min before the addition of 50 ml of 10 mM aqueous solution of L-dihydroxyphenylalanin (L-DOPA). The mixture was incubated for a further 5 min at 30°C, and PO activity was measured in a spectrophotometer at 490 nm. One unit of PO activity represents the amount of enzyme required to produce an increase in absorbance of 0.01 min−1 (Dularay & Lackie, Reference Dularay and Lackie1985). Activity in the treated assays was compared with that of controls. Assays were done in triplicate and the whole experiment was repeated twice. For measurement of phenoloxidase kinetic parameters, different concentrations of L-Dopa; 3, 3.5, 4, 5, 6, 7, 8, 9 and 10 mM were mixed with 20 μl of enzyme solution and read at 490 nm. The Michaelis constant (K m) and the maximum velocity (V m) were estimated by Sigmaplot software version 11, and the results of K m and V max were the means±SE of three replicates (n=3) for each concentration.
Protein determination
Protein concentrations were measured according to the method of Bradford (Reference Bradford1976), using bovine serum albumin (Bio-Rad, München, Germany) as a standard.
Statistical analysis
The mortality and lethal concentration were obtained by using Probit analysis (Robertson et al., Reference Robertson, Preisler and Russell2007) and POLO-PC software (LeOra Software, 1987). In this case, significant differences among the concentrations were recorded when 95% confidence intervals (CI) did not overlap. Other data were compared by one-way analysis of variance (ANOVA) followed by Tukey's Studentisized test when significant differences were found at P=0.05 (SAS Institute, 1997). Differences among samples were considered statistically significant (P<0.05).
Results
Effect of A. annua extracts on mortality and digestive enzyme profiles
The LD values, confidence limit (95%) and regression slope at 24 h and 48 h exposure to plant extract are shown in table 1. The LD values at 24 h after exposure were higher than those at 48 h, and the LD50 for adults was 25% and 11.67% for 24 h and 48 h after treatment, respectively. The mortality of adults, due to using different concentrations of plant extract, varied between 4% and 100%, which showed a dose and time dependent relationship (fig. 1).

Fig. 1. Dose-response line of A. annua extract effect on mortality of E. integriceps adults. X-axis shows the concentration of plant extract and concentration zero means control or treatment without plant extract.
Table 1. Statistical comparison of A. annua extract effect on E. integriceps adults by oral ingestion method.

1 Concentration in percent; 2 means followed by different letters are significantly different (Robertson et al., Reference Robertson, Preisler and Russell2007); letters following numbers show statistical differences.
After bracketing tests, some possible concentrations were prepared, added to food and water; then, adults were fed on these materials.
The LD values calculated by POLO-PC software 24 h and 48 h after ingestion of plant extract by adults.
Results showed that botanical insecticides affected the digestive enzymatic profiles of E. integriceps at several concentrations by using oral ingestion treatment. Figure 2 demonstrates the efficacy and insecticidal activity of botanical insecticides against digestive enzyme activities of the Sunn pest. The activity of digestive enzymes was considerably decreased when the insects were fed on food and water treated with botanical extract in comparison with control ones. There were highly significant differences in reduction of enzyme activities due to using different concentrations of plant extract, except for α- and β-glucosidase activities. The maximum and minimum suppression of digestive enzyme activities were shown in protease and α-glucosidase activities, respectively.

Fig. 2. Digestive enzyme activities (μmol min−1 mg−1 protein) in adults of E. integriceps after treatment with different concentrations of A. annua extract. Means (±SEM) followed by the same letters above bars indicate no significant difference (P<0.05) according to the Tukey test.
Table 2 demonstrated the kinetic parameters (V max and K m) of digestive enzymes extracted from control and treated adults of E. integriceps by A. annua extract. Results showed that the maximum velocity (V max) in the group of insects fed on different concentrations of plant extract decreased and had significant differences except for protease, which had an increase in this parameter. K m values increased in α-amylase, α-glucosidase, protease and lipase enzymes and decreased in β-glucosidase and showed significant differences in different concentrations of A. annua extract.
Table 2. Kinetic parameters (V max and K m) of digestive enzymes extracted from control and treated adults of E. integriceps.

Means (±SEM) followed by the asterisks indicate significant difference (P<0.05) according to the Tukey test;
* shows the statistical differences between treatment and control values.
Effects of A. annua extract on cellular immuno reactions
In order to determine whether or not the treatment of adults by different concentrations of A. annua extract would affect the cellular immune reactions, we compared the total number of hemocytes and measured the percentage of plasmatocytes and granular cells, as the most involved hemocytes in cellular immune reactions, circulating in the hemolymph prior to B. bassiana spores inoculation. No significant differences were encountered between the total hemocyte numbers obtained from adults fed on distilled water only (control) and the ones fed on distilled water containing different concentrations of plant extract except for 25% concentration (fig. 3a). We also observed that the percentages of plasmatocytes and granular cells varied from 20% to 83% with significant differences among groups (fig. 3b), so that, by increasing the concentrations, the percentage of the afore-mentioned hemocytes, especially plasmatocytes, sharply decreased and showed significant differences (fig. 3b).

Fig. 3. Effects of A. annua on (a) the total hemocyte counts (THC) and (b) the differentiate hemocyte count in the hemolymph of E. integriceps adults. Number of hemocytes ×104. *, P<0.05 vs control (□, granulocytes; ![]() , plasmatocytes).
, plasmatocytes).
In order to investigate the effects of A. annua extract on hemocyte phagocytosis, groups of treated adults and controls were challenged with 1 μl of a suspension of 1×107 spores ml−1 on days 5–8 after ingestion of food and water containing different concentrations of plant extract. Control and A. annua treated adults at 30 and 90 min after spores' inoculation exhibited an increase in the percentage of insects with free spores circulating in hemolymph than did control insects (table 3). In addition, insects treated with plant extract presented a number of spores in hemolymph ranging from 8 to 28% at 180 min, while in control groups no cells were detected at this time (table 3).
Table 3. Effects of A. annua on the percentage of adults with spores circulating in the hemolymph of E. integriceps adults inoculated with B. bassiana.

1 n=30 insects in three replicates;
2 minutes after inoculation.
Counting of hemocytes were made by a handling hemocytometer. Means (±SEM) followed by the asterisks indicate significant difference (P<0.05) according to the Tukey test.
* shows the statistical differences between treatment and control values.
The number of hemocytes not associated with B. bassiana spores in the groups treated with different concentrations of A. annua extract was higher than in untreated control insects and showed no significant differences except for 25% concentration (fig. 4). However, the number of hemocytes associated with B. bassiana spores was significantly different among all groups (fig. 4).

Fig. 4. Effects of A. annua on the number of hemocytes binding to spores in the hemolymph of E. integriceps adults, 30 min after inoculation with B. bassiana. Values demonstrated mean±SE, n=30 insects with three replicates. Number of hemocytes ×104. *, P<0.05 vs control (□, hemocyte with no spores bound;![]() , hemocytes binding to spores).
, hemocytes binding to spores).
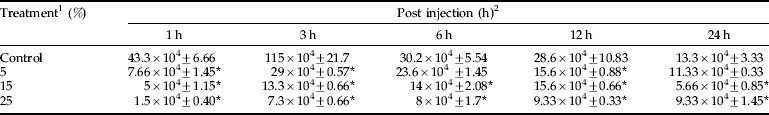
A. annua extract significantly (P<0.05) affected nodule formation in E. integriceps adults 6 h after injection with B. bassiana spores. In other intervals, no significant differences were observed among control and treatments (table 4).
Table 4. Effects of A. annua on the nodule formation of E. integriceps adults inoculated with B. bassiana spores.
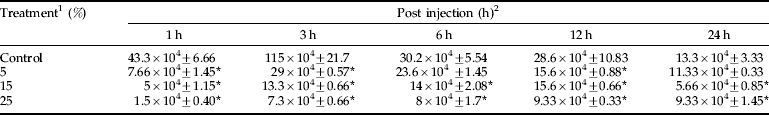
1 concentrations in percentage; 2, amount of nodules (nodule×104 ml−1).
Means (±SEM) followed by the asterisks indicate significant difference (P<0.05) according to the Tukey test;
* shows the statistical differences between treatment and control values.
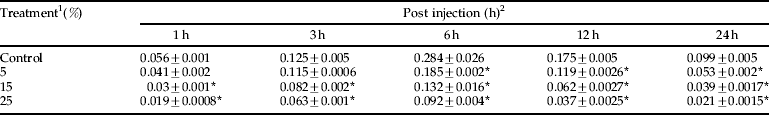
The ability of plant extract to interfere with the activity of the PO system in a hemocyte lysate was investigated. Table 5 shows the effect of three different concentrations of A. annua extract on PO activity. The activity of PO in the presence of fungus spores had significant differences at intervals 1, 3, 6, 12 and 24 after injection, so that the activity of PO decreased due to increasing extract concentrations (table 5; P<0.05). Kinetic parameters of phenoloxidase activity were shown in table 6 and fig. 5, which demonstrated a reduction in V max and an elevation of K m due to using different concentrations of A.annua extract.

Fig. 5. Double reciprocal plot to show the effect of different concentration of A. annua extract on the phenoloxidase acyivity of E. integriceps adults (1/Vmax=intercept on the 1/V0 ordinate, −1/Km=intercept on the negative side of the 1/[S] abscissa).
Table 5. Effects of A. annua on phenoloxidase activity (μmol/min/mg protein) of E. integriceps adults inoculated with B. bassiana spores.
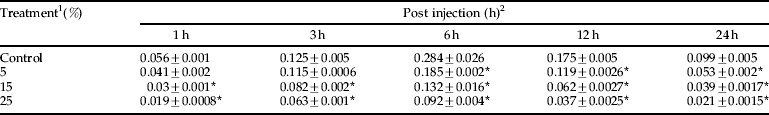
1 concentrations in ppm; 2, means (±SEM) followed by the asterisks indicate significant difference (P<0.05) according to the Tukey test.
* shows the statistical differences between treatment and control values.
Table 6. Kinetic parameters (V max and K m) of phenoloxidase extracted from the hemolymph of control and treated adults of E. integriceps.

Means (±SEM) followed by the asterisks indicate significant difference (P<0.05) according to the Tukey test;
* shows the statistical differences between treatment and control values.
Discussion
Crude botanical insecticides have been used for centuries and are well known in tribal and traditional cultures (Schmutterer, Reference Schmutterer1990), hence naturally occurring biopesticides are seen to be a logical choice for investigation. This study shows that A. annua extracts affect the digestive enzymatic profiles and immune system of E. integriceps and cause different disruptions in their functions. Several studies have shown that feeding is necessary for the stimulation of digestive enzyme activities (Sibley, Reference Sibley, Townsend and Calew1981; Broadway & Duffey, Reference Broadway and Duffey1988). In this study, exposure of E. integriceps adults to sublethal doses of A. annua extract in the laboratory reduced digestive enzyme activities, such as α-amylase, α- and β-glucosidase, lipase and protease. Higher enzyme activities in the midgut of control insects are most probably due to the consumption and utilization of large quantities of food. Imbalance in enzyme-substrate complex and inhibition of peristaltic movement of the gut (Hori, Reference Hori1969) might have inhibited the enzyme activities in the treated insects. Chapman (Reference Chapman, Kerkut and Gilbert1985) reported that enzyme production is clearly related to the feeding behavior (amount of food that passes through the alimentary canal). The activity of these enzymes is related to the physiological conditions of the Sunn pest and reflects the absorption, digestion and positive transport of nutrients in the midgut.
Decreased levels of digestive enzymes at higher concentration of A. annua extract suggested the reduced phosphorous liberation for energy metabolism and decreased rate of metabolism and rate of metabolite transportation and may be due to the direct effects of plant extract on enzyme regulation. It is evident that exposure to botanical insecticides in the diet of adults has significant effects on several enzyme activities found in E. integriceps. Researches have shown a significant correlation between the activity level of α-amylase in the hemolymph and the midgut (Etebari et al., Reference Etebari, Mirhoseini and Matindoost2005; Zibaee et al., Reference Zibaee, Jalali Sendi, Etebari, Alinia and Ghadamyari2008a,Reference Zibaee, Sendi, Alinia and Etebarib). α-Amylase is an endo-digestive enzyme that catalyzes the breakdown of 1:4-α-glucosidase bonds in polysaccharides. It converts starches into maltose (disaccharide) and glycogen into glucose. Saleem & Shakoori (Reference Saleem and Shakoori1987) showed that sublethal concentrations of pyrethroids decreased the α-amylase activity in larval gut of the beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Lee et al. (Reference Lee, Kim and Lee1994) showed that some IGRs decreased the activity level of α-amylase and esterase in the treated larvae. Ascher & Ishaaya (Reference Ascher and Ishaaya2004) showed that the activity level of this enzyme increased 30% in S. littoralis Boisd (Lepidoptera: Noctuidae) treated with phentine acetate compared with control. Zibaee et al. (Reference Zibaee, Jalali Sendi, Etebari, Alinia and Ghadamyari2008a) showed that, along with elevation of spraying times, the activity level of α-amylase would sharply decrease in Chilo suppressalis Walker (Lepidoptera: Crambidae) larvae. Shekari et al. (Reference Shekari, Jalali Sendi, Etenbari, Zibaee and Shadparvar2008) demonstrated that α-amylase activity level in elm leaf beetle decreased 24 h after treatment and sharply increased at 48 h after treating with A. annua extract. Our results showed that A. annua extract caused the reduction of α-amylase activity in E. integriceps, and this reduction increased by higher concentrations of plant extract which coincided with previous reports (see above). The glycosidases catalyze the hydrolysis of terminal, non-reducing 1, 4-linked alpha-D-glucose residues with releasing of alpha-D-glucose. In our study, treatment of E. integriceps adults with three sublethal concentrations of A. annua extract showed the reduction in the activity level of α- and β-glucosidases in response to the increasing of plant extract concentrations, but the significant differences only were observed at 25%. This may be due to a drop in the consumption rates and leveling off or decline in food conversion efficiencies. This decrease coincided with other reports in insects. For example, Hemmingi & Lindroth (Reference Hemmingi and Lindroth1999, Reference Hemmingi and Lindroth2000), studying the effect of phenolic components in gypsy moth (Lepidoptera, Lymantriidae) and forest tent caterpillar (Lepidoptera, Lasiocampidae), demonstrated that glucosidase activities, in addition to growth and the length of time of each stadium period, declined for both insect species when reared on diets containing phenolic glycosides. Similar results were observed in the effect of A. annua extract on lipase activity of the Sunn pest. Senthil Nathan et al. (Reference Senthil Nathan, Chunga and Muruganb2006) showed that treating Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae), the rice leaffolder, with Btk, NSKE and VNLE (azadirachtin and neem components) sharply decreased the activity level of lipase in the midgut. Botanical insecticides may interfere with the production of certain types of proteins. Active principles present in the A. annua extract are responsible for such effects. However, Johnson et al. (Reference Johnson, Brookhart, Kramer, Barnett and McGaughey1990) made an exhaustive study on protease activity in the midguts of susceptible and resistant strains of Plodia interpunctella (Hubner) (Lepidoptera: Pyralidae), and the results indicated that resistance was not due to obvious changes in larval midgut protease activity. Different proteases can be produced in the insect gut depending on the plant material ingested (Broadway, Reference Broadway1989). Such differences could influence susceptibility through slower activation or faster metabolism of the toxins (Senthil Nathan et al., Reference Senthil Nathan, Choia, Seoa, Paika, Kalaivania and Kim2008).
Analysis of Lineweaver-Burk plots (tables 2, 6 and fig. 5) provides information regarding the mode of action of A. annua extract against E. integriceps different enzymes. In the majority of enzymes, the presence of the plant extract decreased the value of V max and increased K m. Since K m has an inverse relationship with the substrate concentration required to saturate the active sites of the enzyme, this indicates decreased enzyme affinity for the substrate (Wilson & Goulding, Reference Wilson and Goulding1986). In other words, K m is the measurement of the stability of the enzyme-substrate complex and a high K m would indicate weak binding while a low K m would indicate strong binding (Stryer, Reference Stryer1995). The effect of A. annua extract on V max shows that it interferes with the rate of break down of the enzyme-substrate complex. Thus, the plant extracts inhibit the enzymes by increasing K m and decreasing affinity of the enzyme to substrate. Plant extracts also diminished the V max value, which further indicates that they interfered with the rate of breakdown of the enzyme-substrate complex (Morris, Reference Morris1978). These results showed a mixed inhibition of plant extract on the enzyme activities of the Sunn pest. In this type of inhibition, plant extracts can bind to the enzyme at the same time as the enzyme binds to the substrate, and this binding affects the binding of the substrate and vice versa (Stryer, Reference Stryer1995). Although it is possible for mixed-type inhibitors to bind in the active site, this type of inhibition generally results from an allosteric effect, where the inhibitor binds to a different site on an enzyme. Inhibitor binding to this allosteric site changes the conformation (i.e. tertiary structure e or three-dimensional shape) of the enzyme so that the affinity of the substrate for the active site is reduced (Morris, Reference Morris1978; Stryer, Reference Stryer1995).
A. annua extract treatment affected the total number of hemocytes circulating in the hemolymph, indicating that the responses could be due to a toxic effect on the E. integriceps immune cells. With regard to the cell types involved in spore-hemocyte association and phagocytosis, our experiments indicated that plasmatocytes were the main cell type implicated in these processes. In vivo experiments, performed with hemolymph from E. integriceps treated with plant extract, revealed a reduced number of hemocytes attached to fungal spores. As a consequence, an extremely low phagocytic activity was observed in these bioassay experimental groups. Since the attachment of fungal spores to the hemocyte surface is an essential prerequisite to the triggering of phagocytic responses, we suggest that the cellular activity or recognition of spore by hemocyte receptors may be compromised in the hemocytes of insects treated with A. annua. Most of the published articles on phagocytosis in insects have concentrated on microorganism/hemocyte interactions, emphasizing the complexities and sophistication of the process that consists of two steps, binding and internalization (Rabinovitch, Reference Rabinovitch1967). Besides the interaction between receptors and ligands triggering the phagocytic responses (Greenberg & Silverstein, Reference Greenberg, Silverstein and Paul1993), several authors have demonstrated that plasmatocytes are the predominant cell type involved in phagocytosis (Ratcliffe et al., Reference Ratcliffe, Leonard and Rowley1984; Anggraeni & Ratcliffe, Reference Anggraeni and Ratcliffe1991; Rohloff et al., Reference Rohloff, Wiesner and Gotz1994). Azambuja et al. (1991b) indicated that azadirachtin adversely affected the R. prolixus immune reaction when the insects were challenged with Enterobacter cloacae by decreasing nodule formation, antibacterial activity and lysozyme in the hemolymph, as well as by increasing the population of microorganisms in the hemolymph. Phagocytosis of microbial cells may involve interactions between lections on phagocytic cells and sugars on microbial surfaces (Nayar & Knight, Reference Nayar and Knight1997). Since A. annua extracts suppress phagocytosis (and also nodule formation and PO activity) at different concentrations, this suggests that it may interfere with the legend-receptor interactions that are likely to occur at the plasma membrane of specific hemocytes because the majority of interactions between cellular and humeral components of the insect immune system are receptor-mediated (Ratcliffe & Rowley, Reference Ratcliffe, Rowley and Soulsby1987). Therefore, plant extracts, which are produced inside the insect host at the sublethal level, either might be enough to interfere with the function of specific receptors, e.g. β-1,3-glucan-specific protein of many insect-species hemocytes, or cause ultrastructural alteration which interfere with normal hemocyte function (Vey et al., Reference Vey, Matha and Dumas2002).
Nodule formation, which is primarily a mechanism for sequestering particulate materials that enter the hemocoel, is also induced by injection of soluble molecules such as β-1,3-glucan from fungal cell wall, bacterial polysaccharides (Ratcliffe et al., Reference Ratcliffe, Leonard and Rowley1984; Smith et al., Reference Smith, Soderall and Hamilton1984; Gunnarsson & Lackie, Reference Gunnarsson and Lackie1985) and certain glycoproteins (Lackie & Vasta, Reference Lackie and Vasta1988). In this study, treatment of insects with plant extracts decreases the amount of nodule formation and PO activity at different intervals so that higher concentrations caused the lower nodule formations and PO activity. The suppression of glucan-induced nodule formation by fungal secondary metabolites makes sense in terms of the fungal strategy of immune suppression (Huxham et al., Reference Huxham, Lackie and McCorkindale1989). Hemocyte ability to recognize intruders is mediated by endogenous molecules that bind to particular sites on the foreign surface. Such mediators are either dissolved in the hemolymph or are exposed on the cell membrane of hemocytes.
In conclusion, plant allelochemicals may be quite useful in increasing the efficacy of biological control agents because plants produce a large variety of compounds that increase their resistance to insect attack. Biopesticides with plant origins are given new importance in recent years for their use against several insect species, including the Sunn pest. One of the reasons for their increased usage could be that compounds of plant origin are safer for humans and the environment. Our results indicate that A. annua extract has toxic effects on E. integriceps adults. Moreover, xenobiotic materials present in A. annua extract affect the activity of digestive enzymes and immune responses in this insect. An understanding of fungal-induced immune responses would identify the insect defenses; hence, the identification of fungal virulence factors could be manipulated to accelerate host death in a biological control scenario. In fact, the combination of biopesticides and microbes to control insect pest populations would be a safe and possibly rapid method to decrease their damage and environmental risk.
Acknowledgements
The author would like to thank Drs Jalal Jalali Sendi and R. Talaei and M. Tork for their useful comments and technical assistances. Also, special thanks to Dr Davide Malagoli from the University of Modena and Reggio Emilia for his help and useful comments on the insect immune system and editing the text. Additional thanks to two anonymous reviewers for their comments to improve the manuscript.