Introduction
Diapause is the arrestment of development to increase an organism's survival potential in response to unfavorable environmental conditions (Danks, Reference Danks1987). In different insect species, it may occur at different stages of the life cycle from egg to adult. In adults, diapause is characterized by halted reproductive maturation and is thus often defined as reproductive diapause (Tauber et al., Reference Tauber, Tauber and Masaki1986). Adult diapause may occur in both male and female insects. In diapausing females, the ovarial development is suppressed, and ovaries are normally small without oogenesis (Brazzel & Newsom, Reference Brazzel and Newsom1959; Stoffolano, Reference Stoffolano and Barton Browne1974). Diapausing males have conspicuously reduced testes and atrophied accessory glands, and there is no spermatogenesis (Pfaender et al., Reference Pfaender, Rabb and Sperenkel1981). Resumed development occurs after diapause termination and when environmental conditions favoring post-diapause development (Koštál, Reference Koštál2006). Reproductive diapause has been found in 90% of the beetle species examined for diapause, of which most are in the families Coccinellidae, Chrysomelidae, and Curculionidae (Danks, Reference Danks1987; Hodek, Reference Hodek2012). In Curculionidae, hibernation via reproductive diapause has been reported in a number of species (Brazzel & Newsom, Reference Brazzel and Newsom1959; Spurgeon et al., Reference Spurgeon, Sappington and Suh2003; Bel-Venner et al., Reference Bel-Venner, Mondy, Arthaud, Marandet, Giron, Venner and Menu2009). This includes the currently studied species, the annual bluegrass weevil (ABW), Listronotus maculicollis Kirby (Rothwell, Reference Rothwell2003; Wu et al., Reference Wu, Kostromytska, Xue and Koppenhöfer2018), and its congenerics, the Argentine stem weevil, L. bonariensis (Kuschel) (Goldson, Reference Goldson1981) and the carrot weevil, L. oregonensis (LeConte) (Stevenson & Boivin, Reference Stevenson and Boivin1990).
The ABW is a major pest of short-mown turfgrass on golf courses and grass tennis courts, and is considered one of the most difficult-to-manage turfgrass insect pests in eastern North America (McGraw & Koppenhöfer, Reference McGraw, Koppenhöfer and Pessarakli2007; Vittum, Reference Vittum, Brandenburg and Freeman2012). Around the New York metropolitan area, ABW goes through 2–3 generations per year. In late summer to early fall, the adults move off the short-mown grass to overwinter under leaf litter, clumps of tall grass, and under the grass around the base of trees in golf course roughs (Diaz & Peck, Reference Diaz and Peck2007). From late March to late April, adults migrate back to the short-mown turf areas where they feed on grass leaf blades, mate, and start laying eggs around late April/early May. Female ABWs deposit eggs in the grass plant's stem or between the leaf sheaths. After hatching, the larvae initially tunnel the grass stem but as older larvae feed externally on the crown and other parts of the plant. High larval populations can destroy large areas of turfgrass.
Application of synthetic insecticides in spring when most of the adults have migrated from their overwintering sites onto the short-mown turf but before oviposition starts has been one of the most commonly used and effective strategy to control ABW (Koppenhöfer et al., Reference Koppenhöfer, Alm, Cowles, McGraw, Swier and Vittum2012; McGraw & Koppenhöfer, Reference McGraw and Koppenhöfer2017). This approach curbs the build-up of the ABW spring generation, which generally causes the most severe damage. Because adult migration occurs over the course of several weeks with sometimes several migration pushes and peaks in densities on the short-mown areas (Diaz et al., Reference Diaz, Seto and Peck2008; McGraw & Koppenhöfer, Reference McGraw and Koppenhöfer2009, Reference McGraw and Koppenhöfer2010), golf turf managers have a tendency to apply adulticides too early and with that often also more than once (Koppenhöfer AM, pers. obs.). However, excessive insecticide use has led to the widespread problems with insecticide resistance (McGraw & Koppenhöfer, Reference McGraw and Koppenhöfer2017; Kostromytska et al., Reference Kostromytska, Wu and Koppenhöfer2018).
Effective monitoring of ABW populations may largely reduce unnecessary applications and optimize timing and, with that, the efficacy of warranted applications. It is important for optimal monitoring of adult emergence and egg-laying activity in spring to understand the factors that affect diapause termination and post-diapause development. Currently, the timing of adulticide applications is based on scouting for adult populations, indicator plant phenology (i.e., Forsythia sp. bloom) and/or growing degree days (GDD; base temperature 10°C, starting 1 March) (Vittum, Reference Vittum1980; Rothwell, Reference Rothwell2003; McGraw & Koppenhöfer, Reference McGraw and Koppenhöfer2009). However, the scouting approaches are generally labor intensive, and the status of reproductive diapause cannot be externally determined other than through observations on mating behavior and oviposition post diapause. The phenology of indicator plants varies with locations, and plants are often not near the sites of infestation, and late frosts can damage the blossoms of the indicator plants. GDD tracking may be the most reliable approach for monitoring the pest population dynamics provided that monitors are placed at the site of infestation, but GDD accumulation may diverge from ABW phenology during short periods of unusually warm weather in March. Moreover, whether ABW adults initiate reproductive development at 10°C or at other temperatures in spring has never been investigated physiologically.
In previous studies, we found that low temperature played an important role in maintaining the diapause status and post-diapause quiescence of ABW, and a period of chilling resulted in more rapid and synchronized development of reproductive organs under warm conditions (Wu et al., Reference Wu, Kostromytska, Xue and Koppenhöfer2018). Under natural conditions, the weevils were regulated to terminate diapause as early as in January and to resume reproductive development when conditions permitted. However, how spring temperature affects reproductive growth after diapause termination has not been investigated. Yet, this information is particularly important as it may reflect the impact of temperature change on the timing of adult emergence and reproduction in spring, when implementation of control practices may be warranted. In the current study, we explored how temperature affected the post-diapause reproductive growth in this weevil and verified the threshold temperature triggering reproductive development to be used as the base temperature in the GDD model for ABW management. Our study related the pest physiological traits to the timing of applying management practices in response to temperature changes in spring. It provided a baseline to improve the current monitoring model to optimize pest control while avoiding unnecessary pesticide applications.
Materials and methods
Insect collection and maintenance
The weevils were collected in late October to early November from overwintering sites at Pine Brook Golf Course (Manalapan Township, NJ) using warm water extraction (Vittum et al., Reference Vittum, Villani and Tashiro1999). In the laboratory, they were placed in 840-ml plastic cups filled with 500 g of pasteurized (72°C for 3 h) moist (5% v/w) sand and maintained in a growth chamber (10 h light at 6°C:14 h dark at 4°C) for cold storage. For experiments, they were transferred to the same size plastic containers with moist sand at the bottom and kept at different conditions as described for each experiment. Fresh clippings of annual bluegrass, Poa annua L., and thin pieces of artificial diet (Black cutworm rearing diet, Bio-Serv, Frenchtown, NJ) were supplied as food and were changed as needed during the experiment.
Insect dissection and determination of post-diapause development
The weevils were sexed externally by their abdominal characteristics following the description of Cameron & Johnson (Reference Cameron and Johnson1971) before being dissected at 45 × magnification under a dissecting microscope in 0.01% saline solution, which was used to prevent desiccation of reproductive organs. To facilitate dissection, weevils were immobilized by freezing and then embedded into the wax of a dissecting petri dish (10 cm diameter) using a hot probe so that only the elytra protruded. Development in male weevils was measured as the width of accessory gland (average of the widest and narrowest measurements) and seminal vesicle (widest), and the diameter of prostate gland (widest) using an eyepiece micrometer; in females, the width of germaria (widest) and length of ovaries (from lateral oviduct to germarium tip) were measured (Goldson, Reference Goldson1981). For both males and females, the average measurements of left and right sides were used in data analysis.
In addition to the size measurements of organs above, the developmental status of male and female reproductive systems was recorded, i.e., whether development had resumed after diapause termination or not. In females, the presence of oocytes with vitellogenin in the ovarioles indicated that reproductive organs had resumed development. In males, resumed reproductive growth was determined as the point when the sizes of the reproductive organs increased from small to medium following the developmental stages described by Cameron & Johnson (Reference Cameron and Johnson1971). In the small stage, the seminal vesicle is small (≤0.11 mm width) and difficult to distinguish from the remainder of the vas deferens, the accessory glands are thin (≤0.11 mm width) and un-convoluted, and the prostate gland is small (≤0.22 mm diameter). In the medium stage, the seminal vesicle is slightly expanded (0.12–0.17 mm width), the accessory glands are longer, thicker (0.12–0.17 mm width) and slightly convoluted, and the prostate gland with lobes is partially inflated (0.23–0.33 mm diameter). The weevils were reproductively mature when they had large reproductive systems, filling a large portion of the abdominal cavity. Mature females had eggs in the calyx; in mature males, the testes had irregular lobes, the seminal vesicle was large (>0.17 mm width) and bulbous, the accessory gland was large (>0.17 mm width) and convoluted, and the prostate gland was large (>0.33 mm diameter) with inflated semi-transparent lobes (Cameron & Johnson, Reference Cameron and Johnson1971).
Temperature effect on post-diapause growth of reproductive organs
To elucidate the role of temperature in post-diapause reproductive development, adult ABWs were transferred from cold storage to different temperatures combined with a L:D 12:12 photoperiod, and males and females were dissected weekly to measure their reproductive organs as described above. In the first experiment, adults were transferred to 7, 14, or 21°C on 10 December 2014, and ten males and ten females per temperature were assessed at the start of the experiment and then weekly for 56 days. In the second experiment, adults were transferred to 7, 14, or 21°C on 26 January 2015, and 20 males and 20 females per temperature were dissected at the beginning and then weekly for 28 days. The major difference between the two experiments was that at the beginning of the first experiment weevils might still have been in reproductive diapause whereas at the start of the second experiment they should have had terminated diapause according to Wu et al. (Reference Wu, Kostromytska, Xue and Koppenhöfer2018). The first experiment was conducted without the knowledge of chilling effect on diapause termination, based on which the second experiment was modified. In the third experiment, adults were transferred to 7, 9, 11, 13, or 15°C on 26 January 2016, and ten males and ten females per temperature were dissected at the beginning of the experiment and then every week for 28 days.
Threshold temperature for overwintered reproductive development
To determine the lower threshold temperature for post-diapause development, adults were transferred from cold storage to 7, 9, 11, 13, or 15°C (L:D 12:12), and ten males and ten females per temperature were dissected at the beginning of the experiment and then weekly for 56 days. The developmental status of the reproductive system in each weevil was evaluated based on the criterion described above. The experiment was conducted three times with adults transferred to the treatment temperatures on 26, 27, and 29 January 2016. Repetition in such a short time was to minimize the influence of the chilling period on the experiment. Prior to the start of the experiment, the status of reproductive development was evaluated for each replicate using ten males and ten females. The threshold temperature triggering post-diapause reproductive development, and the accumulated degree-days (based on the threshold temperature) required for 50% of individuals developing, were determined in male and female weevils separately.
Reproductive status under field conditions
Adults were collected from overwintering sites every 2 weeks from late October 2015 to late March 2016 to determine their reproductive status. For each collection, 15 males and 15 females were dissected, and the percentage of maturity and the insemination rate were evaluated. Insemination was determined by examining the presence of sperms in the spermatheca under 450 × magnification using a compound microscope.
Data analysis
The measurement data were log or square-root transformed as necessary to fit normality before analysis. Two-way analysis of variance (ANOVA) (Proc GLM) was used to determine the effect of temperature and warm-up duration (excluding 0 day) on sizes of weevil reproductive organs (males and females) and the percentage of weevils showing resumed development of reproductive systems (SAS Institute, 2012). Since some growth might have already occurred between 0 days and the 7-day observation, treatments (all temperature × warm-up duration combinations) were analyzed including the 0-day observations using one-way ANOVA. Multiple comparisons between treatments were made using Tukey's HSD. The percentage of full maturity and the insemination rate under field observations were analyzed using Proc Logistics model. The difference was considered significant when P < 0.05.
The threshold temperature and degree-days were calculated according to Arnold (Reference Arnold1959), Campbell et al. (Reference Campbell, Frazer, Gilbert, Gutierrez and Mackauer1974), and Broufas & Koveos (Reference Broufas and Koveos2000). For this method, first the estimated number of days required for 50% of weevils to resume development (t 50%) was calculated for each temperature. The reciprocal, 1/t 50%, represented the daily rate of post-diapause reproductive development. The threshold temperature for post-diapause development was estimated using the X-intercept method (Arnold, Reference Arnold1959). Based on linear regression of temperature (X-axis) and the post-diapause developmental rate (1/t 50%, Y-axis) (Proc Reg), the X-intercept value was determined as the threshold temperature for post-diapause development in each sex. The reciprocal of the slope(s) of regression represented the accumulated degree-days based on the calculated threshold temperature for 50% development in each sex. The standard error (S.E.) of degree-days was calculated as (S.E. of s)/s2 (Campbell et al., Reference Campbell, Frazer, Gilbert, Gutierrez and Mackauer1974).
Results
Temperature effect on post-diapause growth of reproductive organs
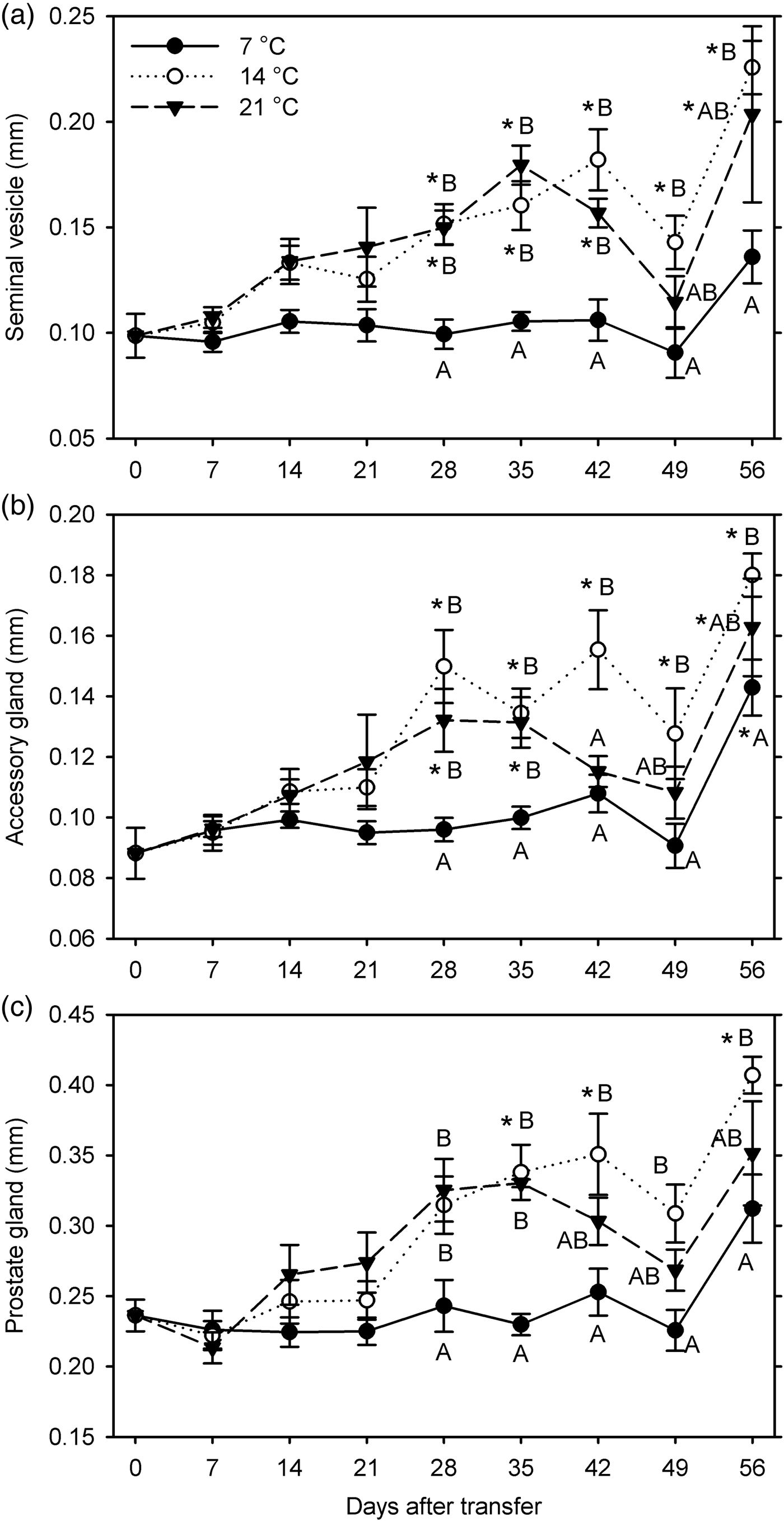
In the first experiment (7, 14 and 21°C for 56 days starting 10 December 2014), a few weevils died during warm-up before dissection, or reproductive organs of some weevils were damaged during dissection and could not be measured. In males, significant growth compared with the 0-day measurements was observed at 14°C (seminal vesicle and accessory gland at 28–56 days after transfer [DAT], prostate gland at 35, 42, and 56 DAT) and at 21°C (seminal vesicle at 28, 35, 42, and 56 DAT, accessory gland at 28, 35, and 56 DAT) but not at 7°C (except for accessory gland at 56 DAT) (F 24, 209 ≥8.43; P < 0.001) (fig. 1). The measurements of all three organs were significantly affected by temperature (F 2, 201 ≥ 24.13; P < 0.001) and warm-up duration (F 7, 201 ≥ 13.16; P < 0.001). These factors significantly interacted for seminal vesicle (F14, 201 = 1.89; P = 0.03) and prostate gland measurements (F 14, 201 = 2.03; P = 0.018) but not for accessory gland width (P = 0.072) (fig. 1). Compared with the sizes at 7°C, all measurements were greater at 28–56 DAT at 14°C but only at 28–42 DAT (seminal vesicle) or 28–35 DAT (accessory and prostate glands) at 21°C. Measurements did not differ significantly between 14 and 21°C except for the size of accessory gland at 42 DAT.

Fig. 1. Measurements of male reproductive organs (a: width of seminal vesicle; b: width of accessory gland; c: diameter of prostate gland) for Listronotus maculicollis transferred from laboratory cold conditions (6°C/4°C; L:D 10:14) to various temperatures (7, 14, 21°C) and L:D 12:12 on 10 December 2014 and dissected at 0–56 days after transfer (n = 10 for each point). In each measurement, means with the same letter do not differ significantly between temperatures on the same sampling day; *indicates significant difference from 0 DAT (Tukey's HSD, α = 0.05).
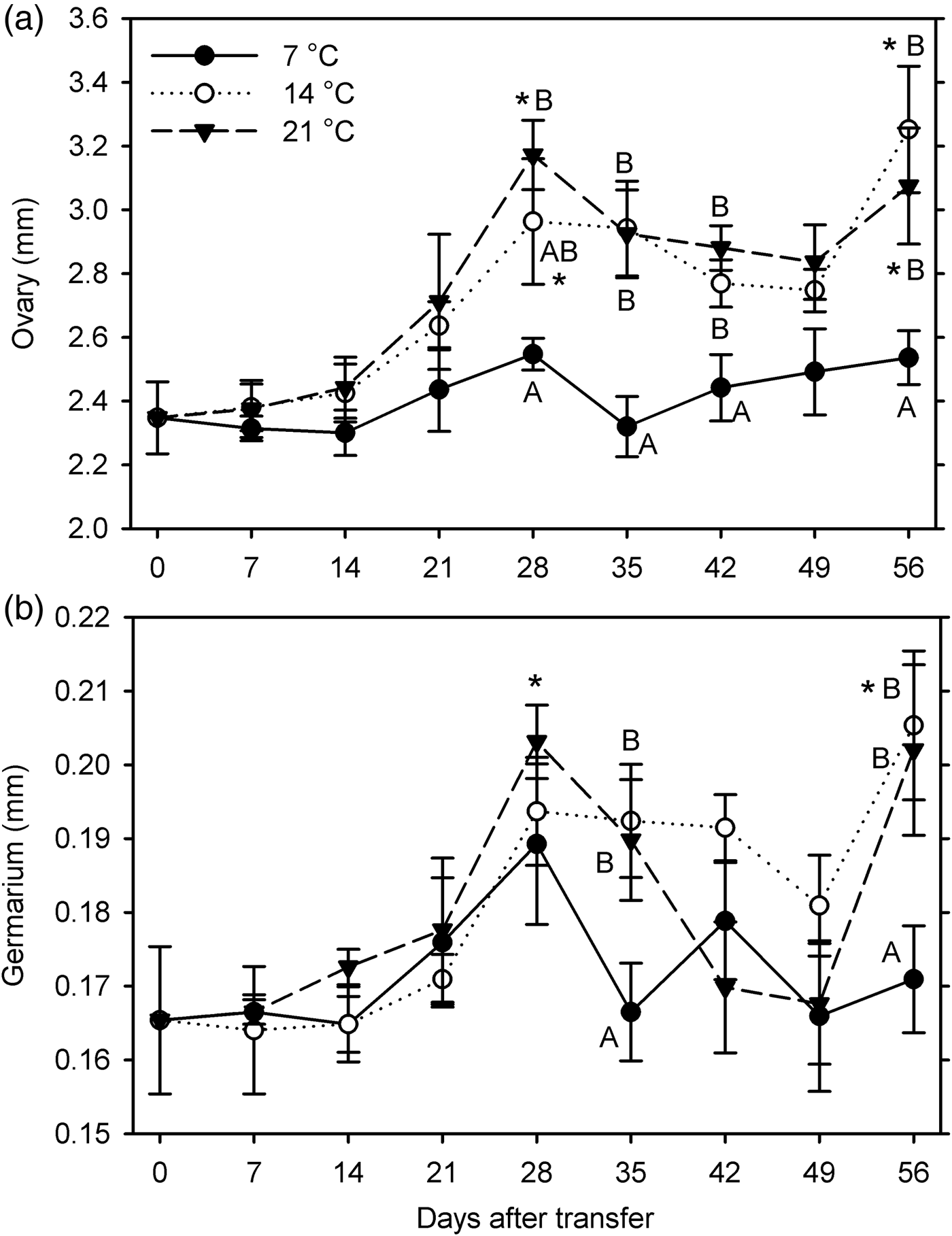
In females, significant growth compared with the 0-day measurements was observed at 14°C (ovary at 28 and 56 DAT, germarium at 56 DAT) and 21°C (ovary at 28 and 56 DAT, germarium at 28 DAT) but not at 7°C (F 24, 243 ≥ 3.29; P < 0.001) (fig. 2). Both organ measurements were significantly affected by temperature (F 2, 233 ≥ 4.96; P ≤ 0.008) and warm-up duration (F 7, 233 ≥ 6.64; P < 0.001); these factors did not interact (P ≥ 0.096) (fig. 2). Combined across all exposure times, ovary length was significantly shorter and germarium width was smaller at 7°C than at 14 and 21°C; sizes did not differ significantly between 14 and 21°C.
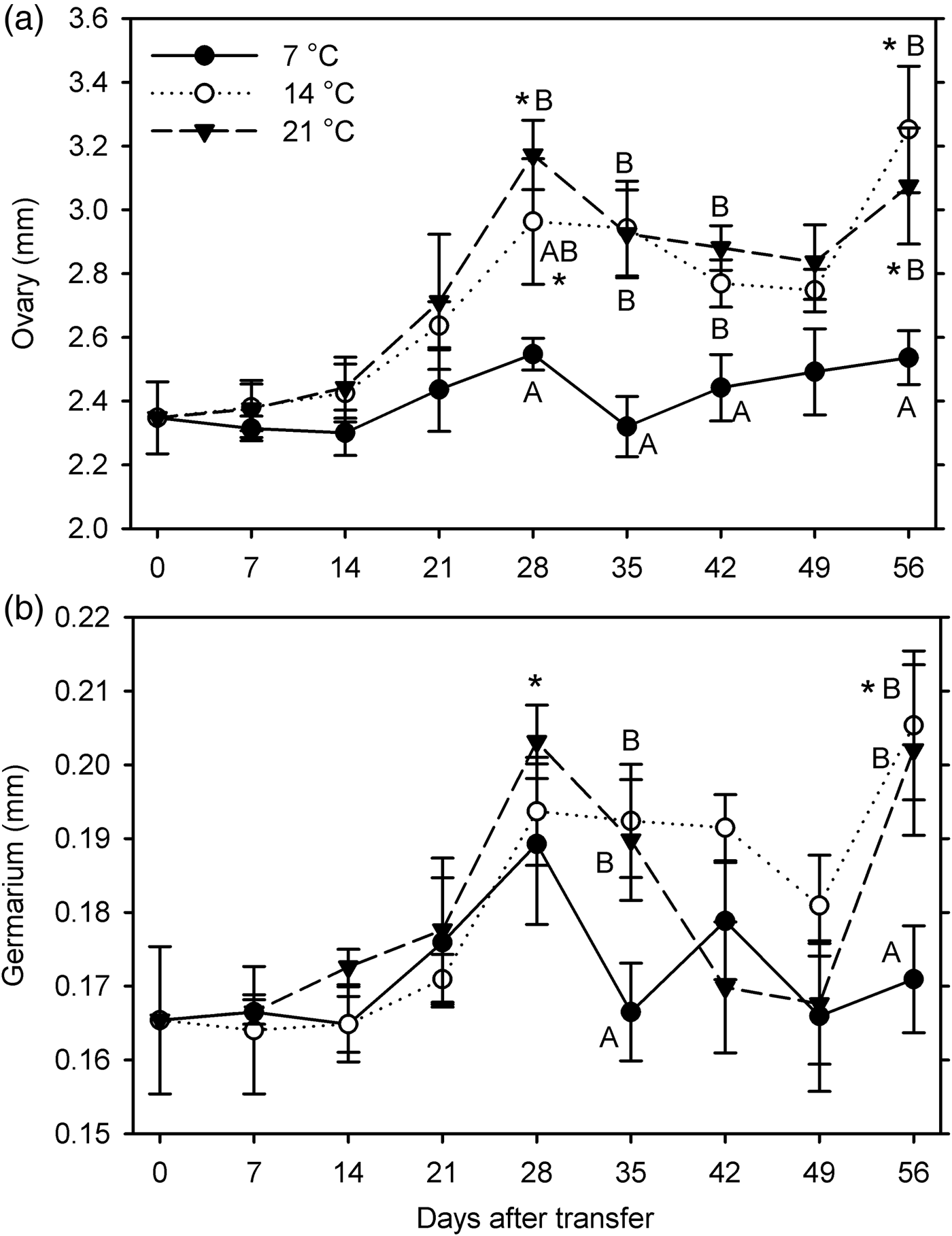
Fig. 2. Measurements of female reproductive organs (a: length of ovaries; b: width of germarium) for Listronotus maculicollis transferred from laboratory cold conditions (6°C/4°C; L:D 10:14) to various temperatures (7, 14, 21°C) and L:D 12:12 on 10 December 2014 and dissected at 0–56 days after transfer (n = 10 for each point). In each measurement, means with the same letter do not differ significantly between temperatures on the same sampling day; *indicates significant difference from 0 DAT (Tukey's HSD, α = 0.05).
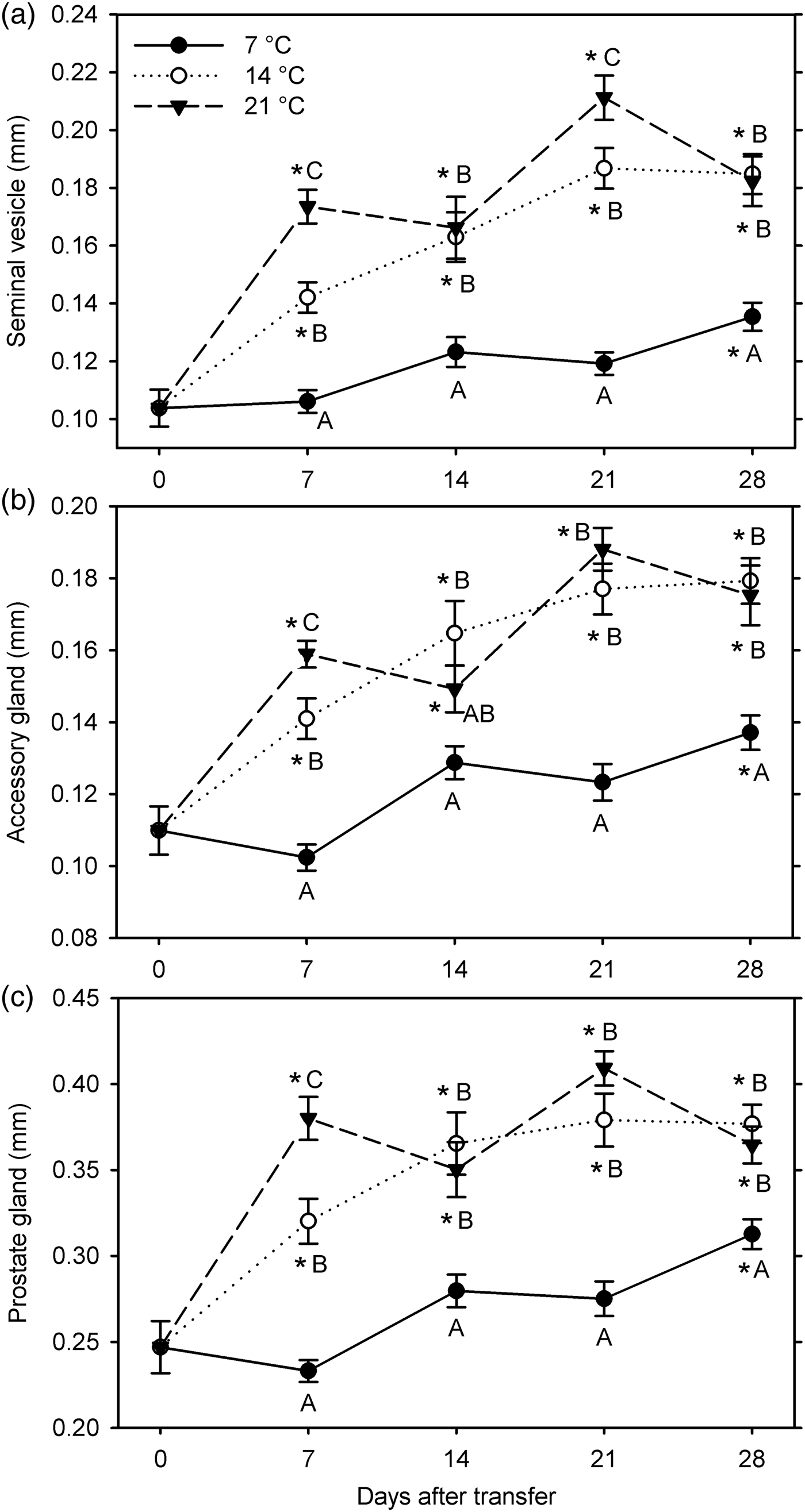
In the second experiment (7, 14 and 21°C for 28 days starting 26 January 2015), all male reproductive organs showed significant growth compared with the 0-day observations at 14°C and 21°C (at 7–28 DAT) and at 7°C at 28 DAT (F 12, 249 ≥ 22.65; P < 0.001) (fig. 3). The measurements of all the three organs were significantly affected by temperature (F 2, 229 ≥ 73.90; P < 0.001) and warm-up duration (F 3, 229 ≥8.98; P < 0.001); these factors significantly interacted for all measurements (F 6, 229 ≥ 3.23; P < 0.005) (fig. 3). All measurements were greater at 14 and 21°C than at 7°C at 7–28 DAT, except for the accessory gland width at 21°C at 14 DAT. In addition, measurements were greater at 21°C than at 14°C at 7 DAT for all organs and at 21 DAT for the seminal vesicle.
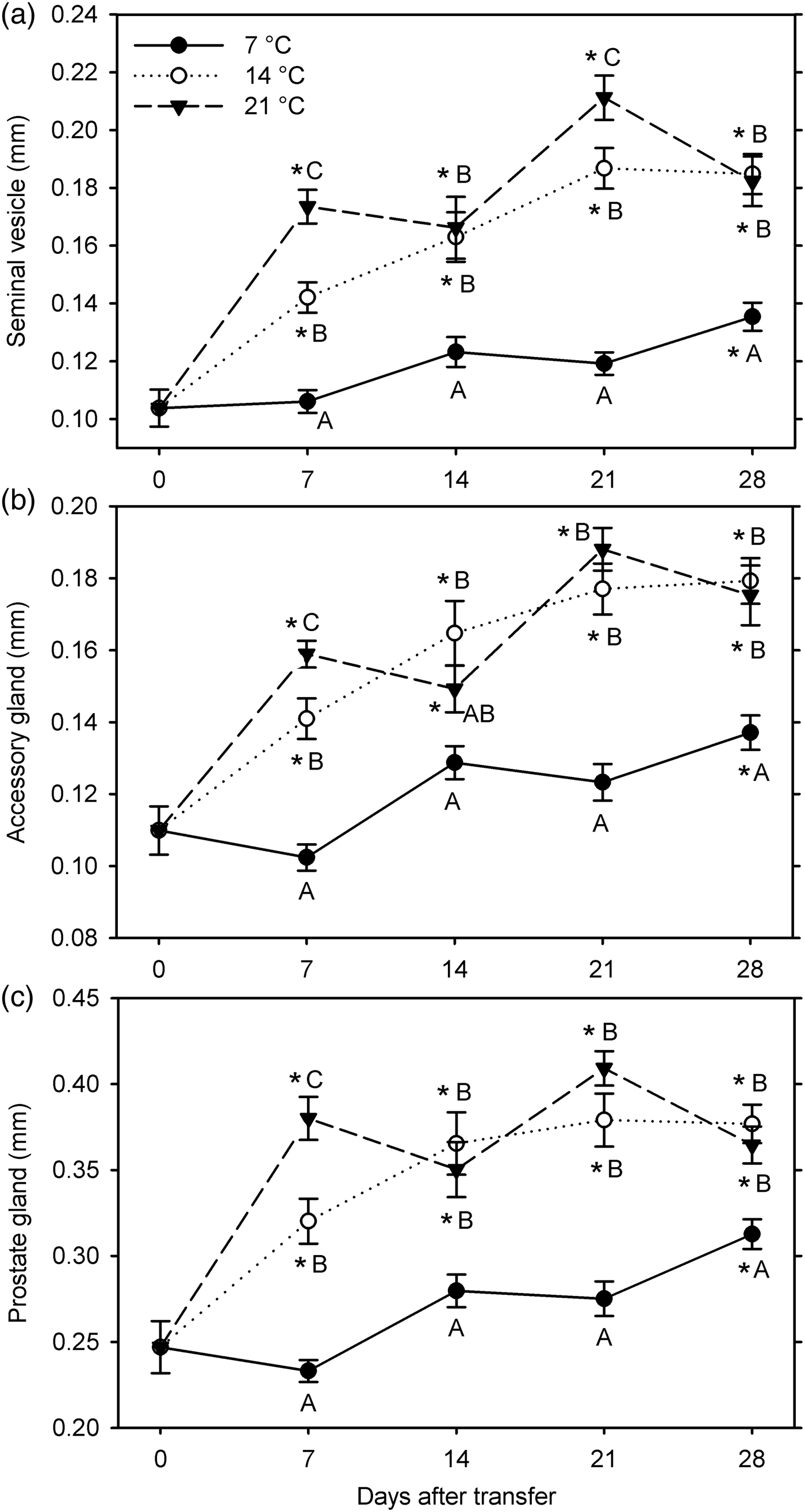
Fig. 3. Measurements of male reproductive organs (a: width of seminal vesicle; b: width of accessory gland; c: diameter of prostate gland) for Listronotus maculicollis transferred from laboratory cold conditions (6°C/4°C; L:D 10:14) to various temperatures (7, 14, 21°C) and L:D 12:12 on 26 January 2015 and dissected at 0–28 days after transfer (n = 20 for each point). In each measurement, means with the same letter do not differ significantly between temperatures on the same sampling day; *indicates significant difference from 0 DAT (Tukey's HSD, α = 0.05).
Females in the second experiment showed significant growth compared with the 0-day observations in the ovary at 14°C (at 28 DAT) and at 21°C (at 7, 21, and 28 DAT) and in germarium width at 21°C (at 7 DAT); no growth occurred at 7°C in either organ (F 12, 254 ≥ 4.17; P < 0.001). Both organ measurements were significantly affected by temperature (F 2, 234 ≥ 15.23; P < 0.001). They were affected by warm-up duration for germarium width (F 3, 234 = 3.17; P = 0.025) but not significantly for ovary length (P = 0.054). There was no significant interaction between temperature and warm-up duration for measurements of either organ (P ≥ 0.134) (fig. 4). Compared with measurements at 7°C, ovary length was significantly greater at 14°C at 14–28 DAT and at 21°C at 7–28 DAT, and germarium width was significantly greater at 14°C at 14 and 28 DAT and at 21°C at 7–14 DAT. Measurements did not differ significantly between 14 and 21°C except for ovary length being greater at 21°C at 7 DAT.

Fig. 4. Measurements of female reproductive organs (a: length of ovaries; b: width of germarium) for Listronotus maculicollis transferred from laboratory cold conditions (6°C/4°C; L:D 10:14) to various temperatures (7, 14, 21°C) and L:D 12:12 on 26 January 2015 and dissected at 0–28 days after transfer (n = 20 for each point). In each measurement, means with the same letter do not differ significantly between temperatures on the same sampling day; *indicates significant difference from 0 DAT (Tukey's HSD, α = 0.05).
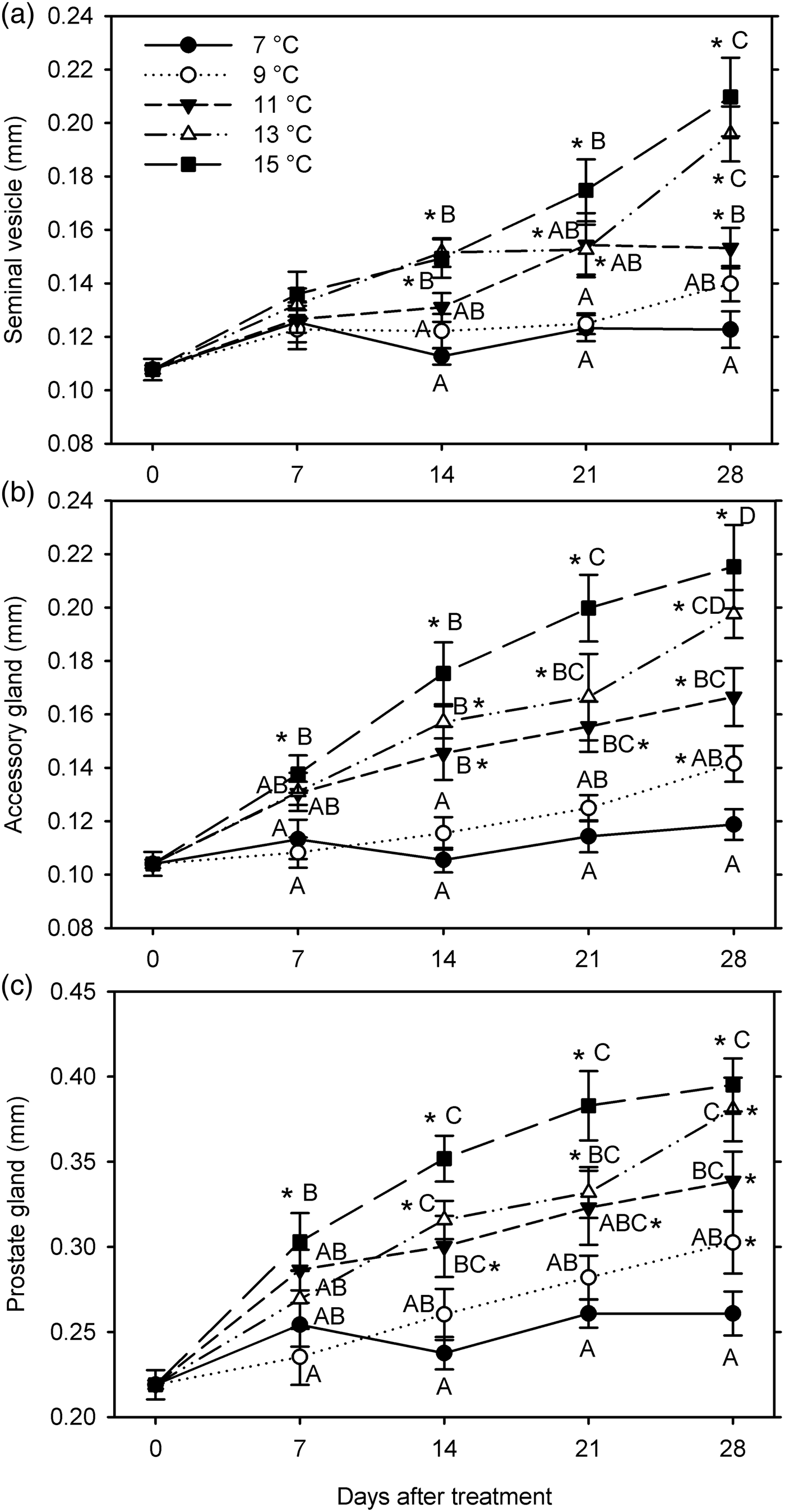
In the third experiment (7, 9, 11, 13, and 15°C for 28 days starting 26 January 2016), none of the male reproductive organs grew at 7°C; significant size increases in organ measurements (except seminal vesicle) occurred at 9°C (at 28 DAT), at 11–13°C (at 14–28 DAT), and at 15°C (at 7–28 DAT), and initiated growth of seminal vesicle was 7 days delayed at 11 and 15°C (F20, 209 ≥ 10.38; P < 0.001) (fig. 5). All male measurements were significantly affected by temperature (F 4, 199 ≥25.73; P < 0.001) and warm-up duration (F 3, 199 ≥ 16.65; P < 0.001); temperature and warm-up duration significantly interacted for the seminal vesicle (F 12, 199 = 2.59; P = 0.003) but not for other measurements (P ≥ 0.111) (fig. 5). In general, all measurements increased with increasing temperature. However, there was no significant difference between 7 and 9°C, between 11 and 13°C (except for the seminal vesicle at 28 DAT), and between 13 and 15°C. The sizes of seminal vesicle and prostate gland did not differ significantly between 9 and 11°C, and accessory gland width only differed at 14 DAT.
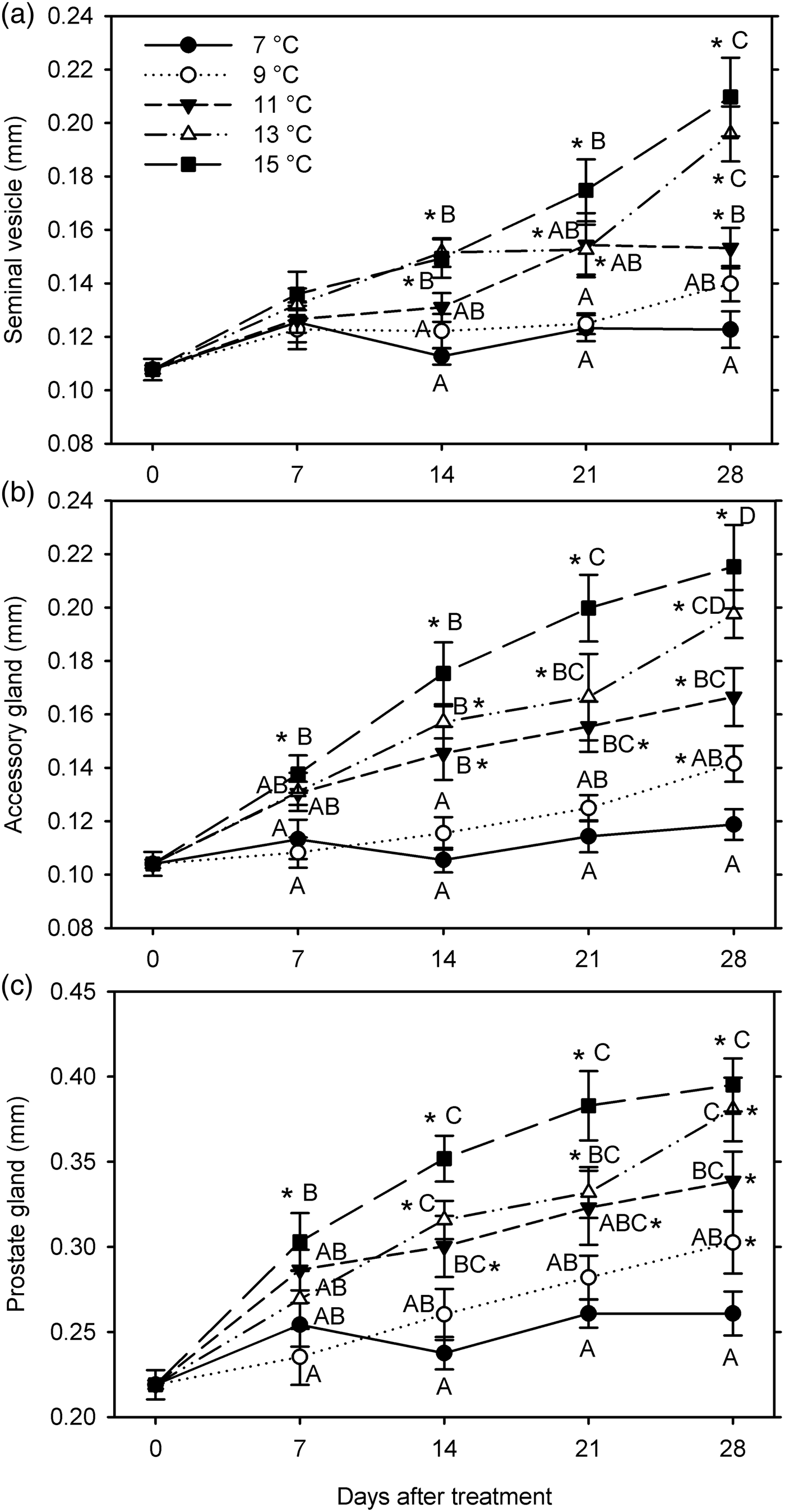
Fig. 5. Measurements (mm, mean ± SEM) of male reproductive organs for Listronotus maculicollis exposed to different temperatures, LD 12:12 and dissected at 0–28 days after transfer (DAT) (n = 10 for each point). In each measurement, means with the same letter do not differ significantly between temperatures on the same sampling day; *indicates significant difference from 0 DAT (Tukey's HSD, α = 0.05).
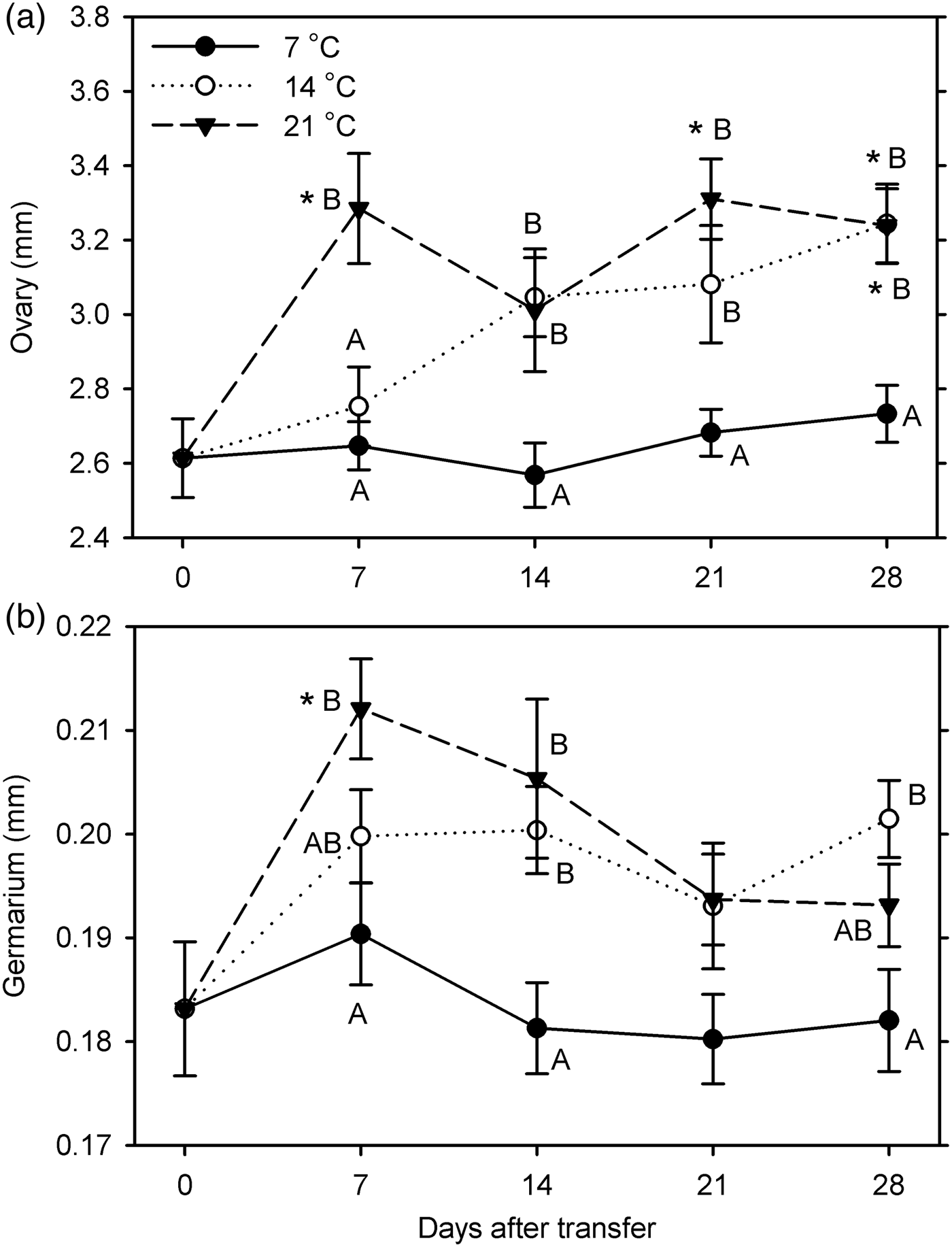
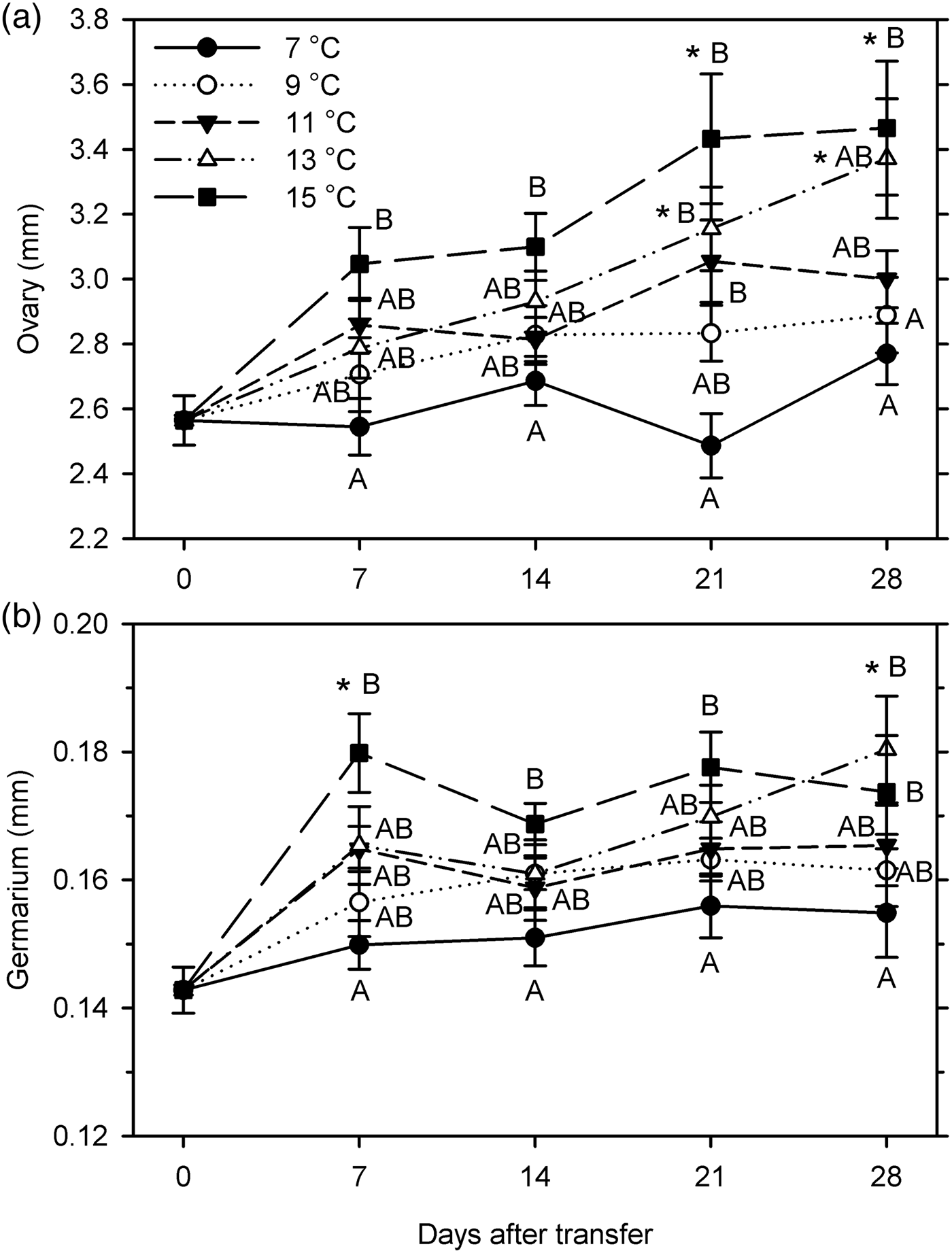
Female organ measurements increased significantly only at 13–15°C (ovary length at 21–28 DAT; germarium width at 28 DAT at 13°C and at 7 DAT at 15°C) (F 20, 209 ≥ 2.83; P < 0.001) (fig. 6). Female measurements were significantly affected by temperature (F 4, 199 ≥ 9.62; P < 0.001); ovary length was also affected by warm-up time (F 3, 199 = 5.60; P = 0.001) but germarium width was not (P = 0.204). There was no significant interaction between temperature and warm-up time for either measurement (P ≥ 0.345) (fig. 6). The female organs were larger at higher temperatures, especially comparing 7 and 15°C. However, for both ovary length and germarium width, there were no significant differences between 7 and 9°C, between 9 and 11°C, between 11 and 13°C, and between 13 and 15°C.
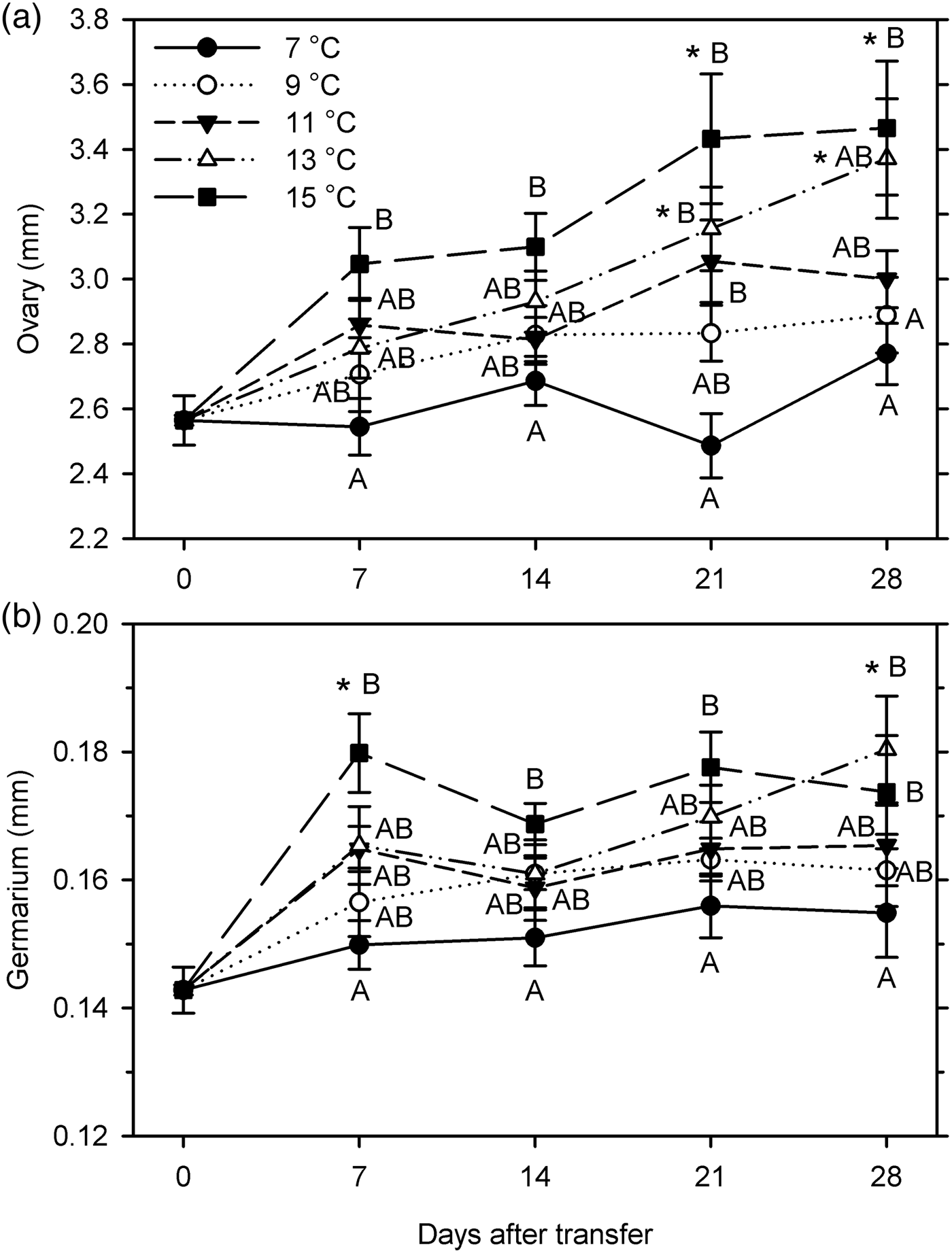
Fig. 6. Measurements (mm, mean ± SEM) of female reproductive organs for Listronotus maculicollis exposed to different temperatures, LD 12:12 and dissected at 0–28 days after transfer (DAT) (n = 10 for each point). In each measurement, means with the same letter do not differ significantly between temperatures on the same sampling day; *indicates significant difference from 0 DAT (Tukey's HSD, α = 0.05).
Threshold temperature for overwintered reproductive development
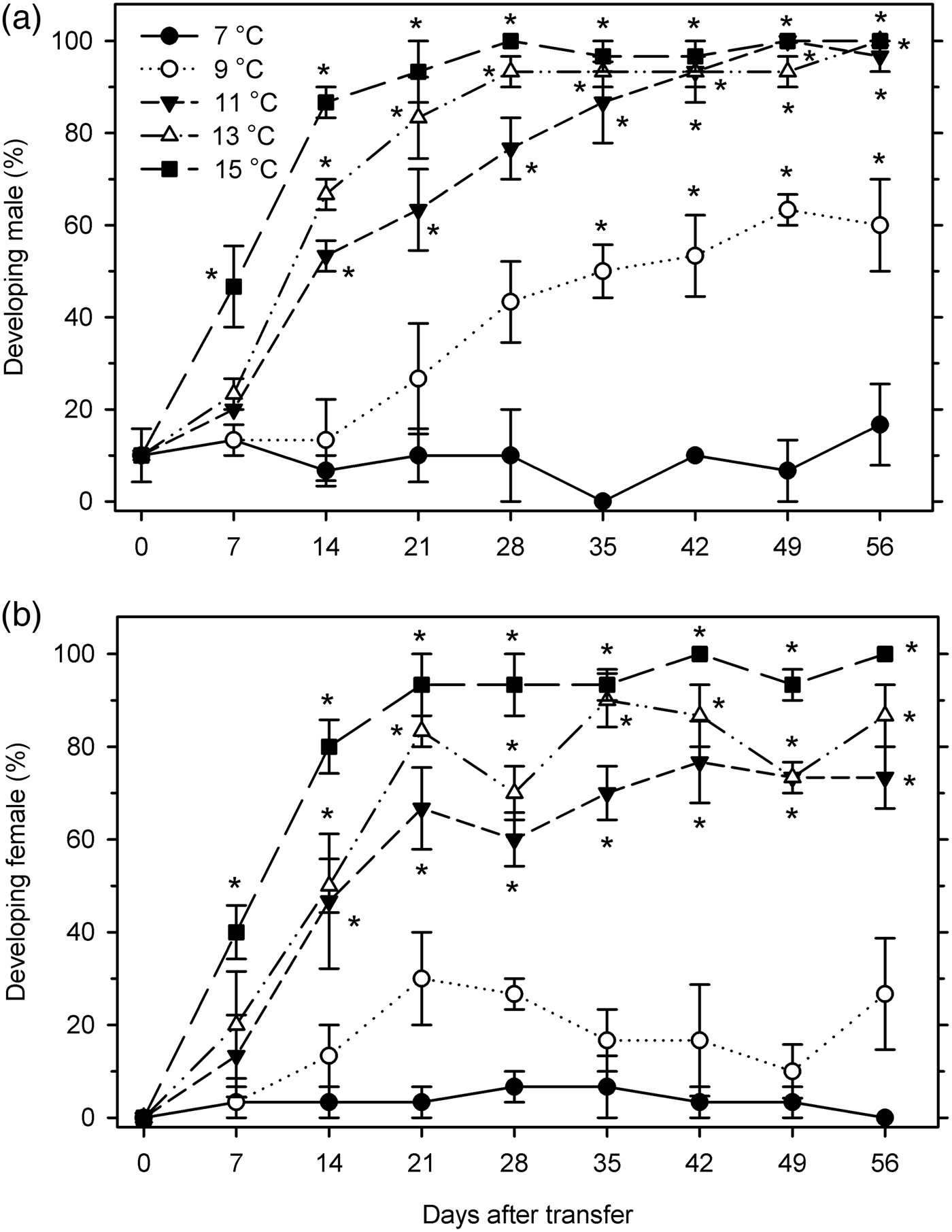
The developmental status of each sex was assessed at 7–15°C to determine the threshold temperature for overwintered reproductive development. Males did not develop at 7°C, and started significant development beginning at 35 DAT at 9°C, at 14 DAT at both 11 and 13°C, and at 7 DAT at 15°C (F 40, 122 = 38.63; P < 0.001) (fig. 7a). It required 44.4, 18.1, 12.7, and 8.2 days for 50% males to resume development at 9, 11, 13, and 15°C, respectively. Female post-diapause development followed a similar, albeit slightly delayed pattern. Females did not develop significantly at 7 or 9°C, and initiated development at 14 DAT at 11–13°C and at 7 DAT at 15°C (F 40, 122 = 31.14; P < 0.001) (fig. 7b). At 11, 13, and 15°C, the percentage of post-diapause development was estimated to reach 50% after 18.7, 13.5, and 7.7 days, respectively.
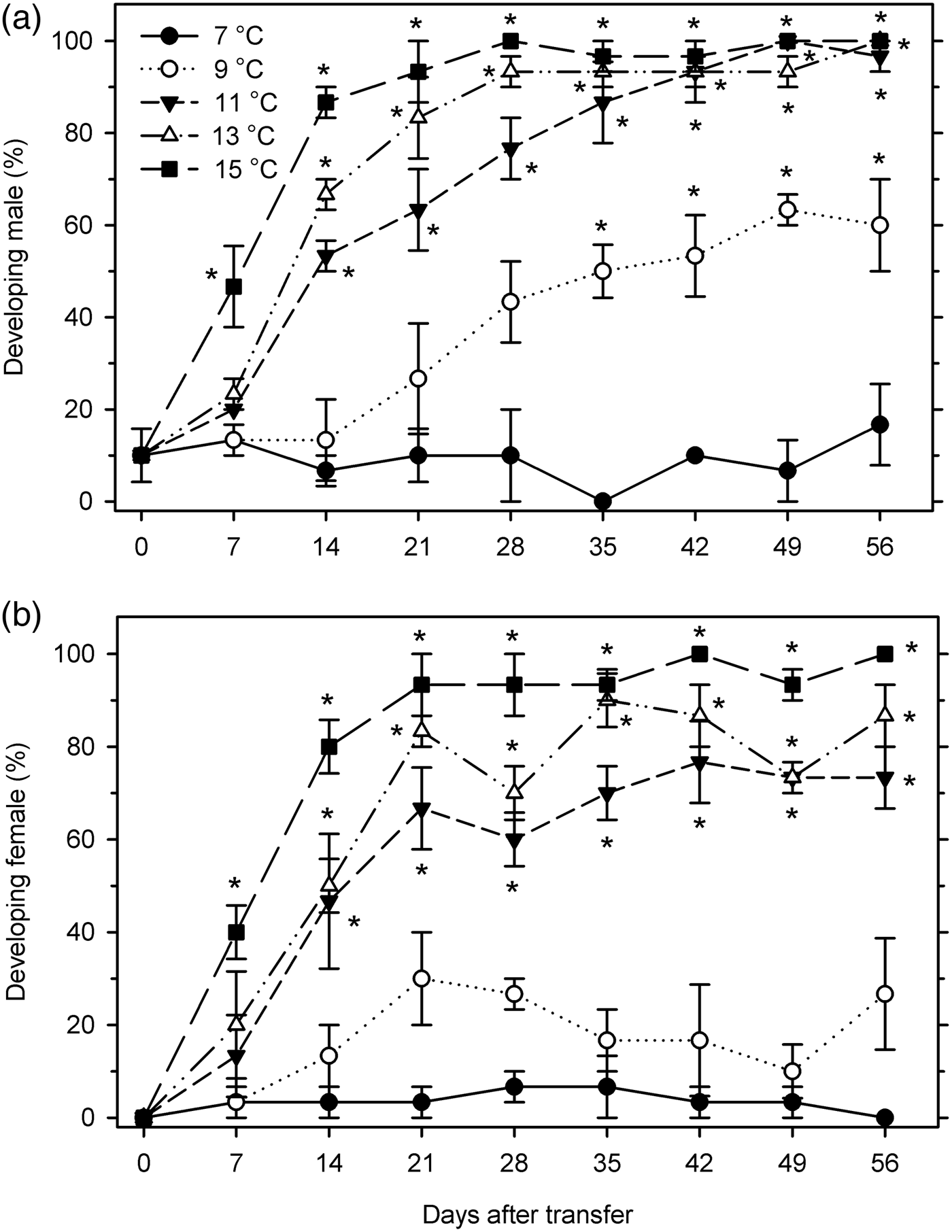
Fig. 7. Percentage of developing individuals (mean ± SEM) in Listronotus maculicollis (a: male; b: female) exposed to different temperatures, LD 12:12 and dissected at 0–56 days after transfer (DAT) (n = 30 for each point). *indicates significant development compared with 0 DAT.
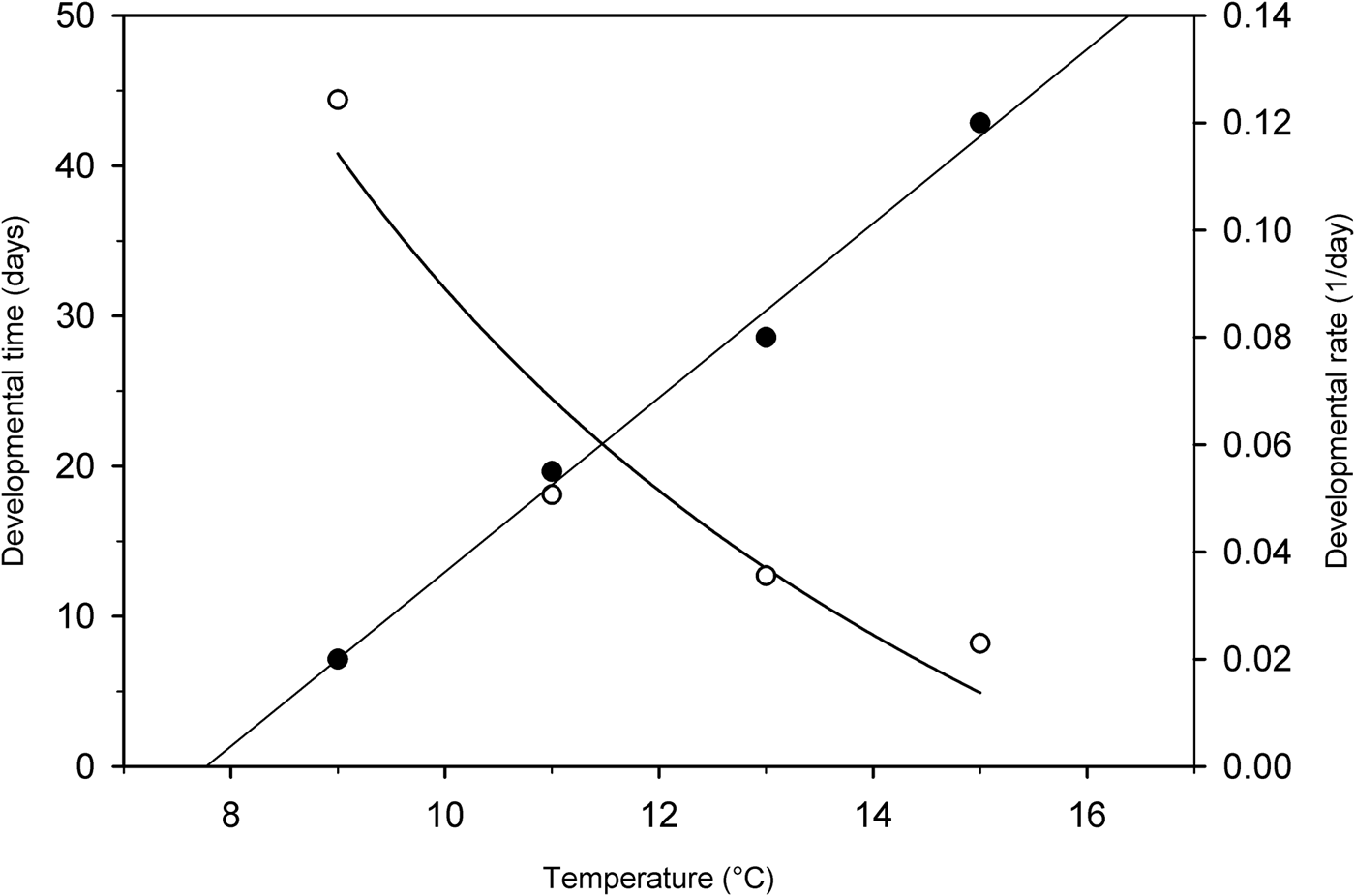
A simple linear regression demonstrated that temperature was a strong predictor in male reproductive development (F 1, 3 = 281.67; P = 0.004). A significant regression equation was determined (y = 0.0162x–0.126, r 2 = 0.993) (fig. 8). The threshold temperature estimated from the regression models was 7.8°C, below which no male development occurred. The accumulated degree-days required for 50% of males resuming development, represented by the reciprocal of the slope of regression line, was 61.7 (SE = 3.7). The regression of the rate of post-diapause reproductive development on temperature was not significant in females (y = 0.0193x–0.165, r 2 = 0.936; P = 0.163), probably because there was no significant growth of females under low temperatures; thus the threshold temperature was not effectively calculated.
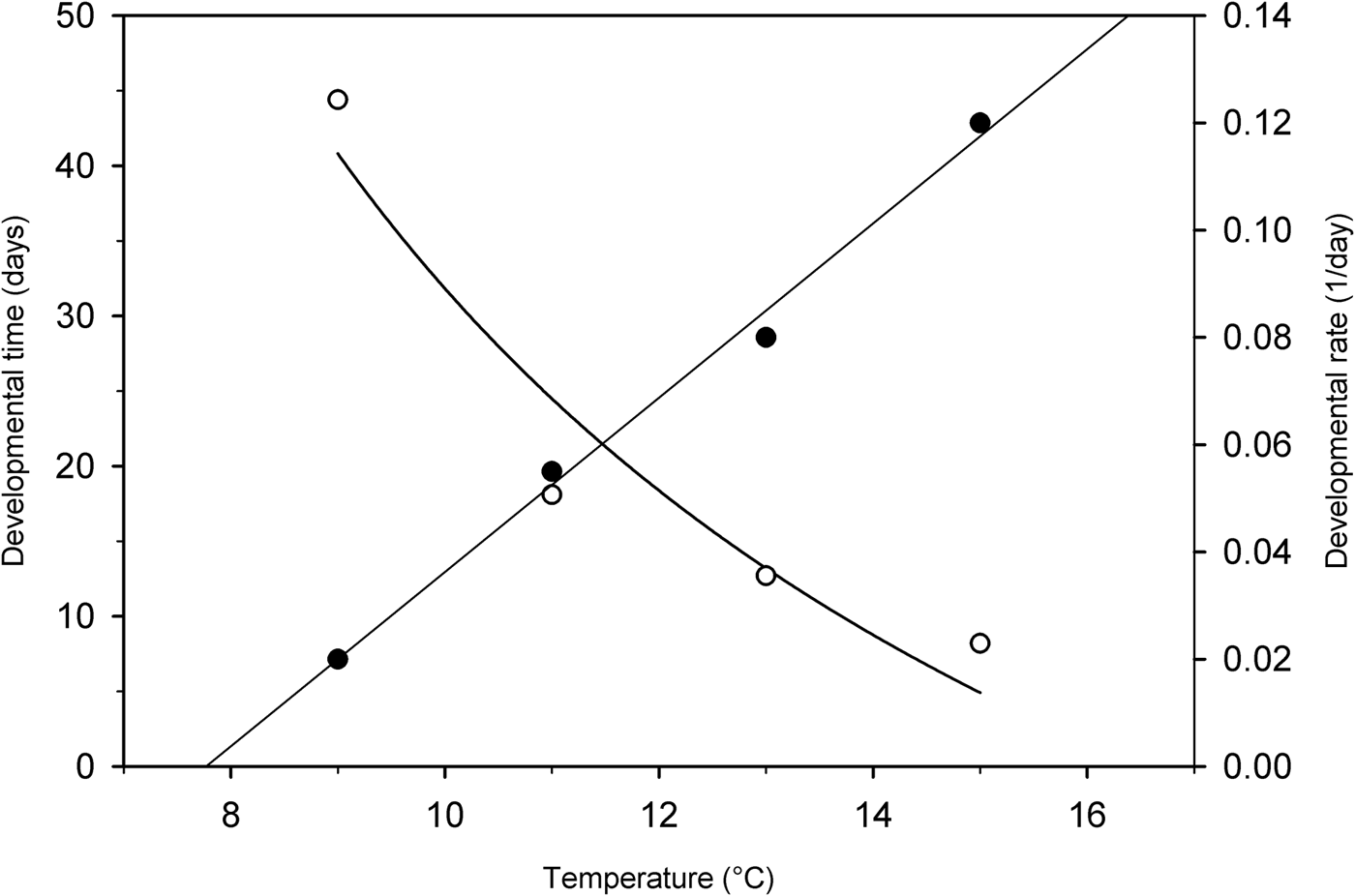
Fig. 8. Regression between temperature and developmental time (○) (t 50%) (y = 807.44/x–48.92; r 2 = 0.918) and developmental rate (●) (1/t 50%) (y = 0.0162x–0.126; r 2 = 0.993) for post-diapause reproductive growth of male Listronotus maculicollis. t 50% = the estimated number of days required for 50% of weevils to develop post diapause.
Reproductive status under field conditions
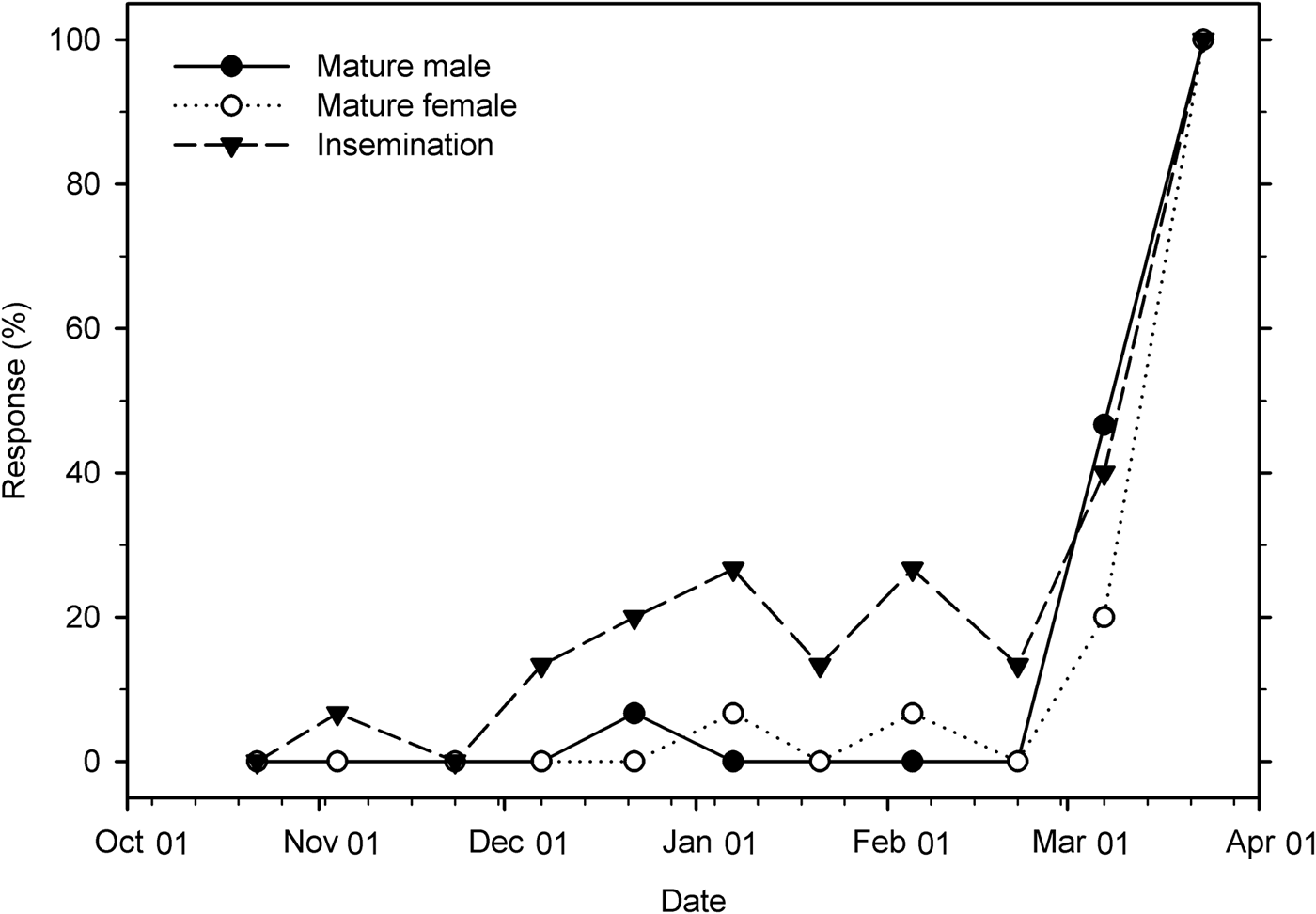
The percentage of mature male and female weevils changed significantly over time under field conditions (male: χ2 = 18.82; df = 1; P < 0.001; female: χ2 = 17.54; df = 1; P < 0.001). In both males and females, very few individuals (0–7%) were reproductively mature through 22 February, but on 7 March the rate increased to 47 and 20%, respectively, and reached 100% maturity on 23 March. The insemination rate was 0–7% through 23 November, fluctuated between 13% and 27% from 7 December to 22 February, then increased to 40% on 7 March, and reached 100% on 23 March (χ2 = 27.56; df = 1; P < 0.001) (fig. 9).
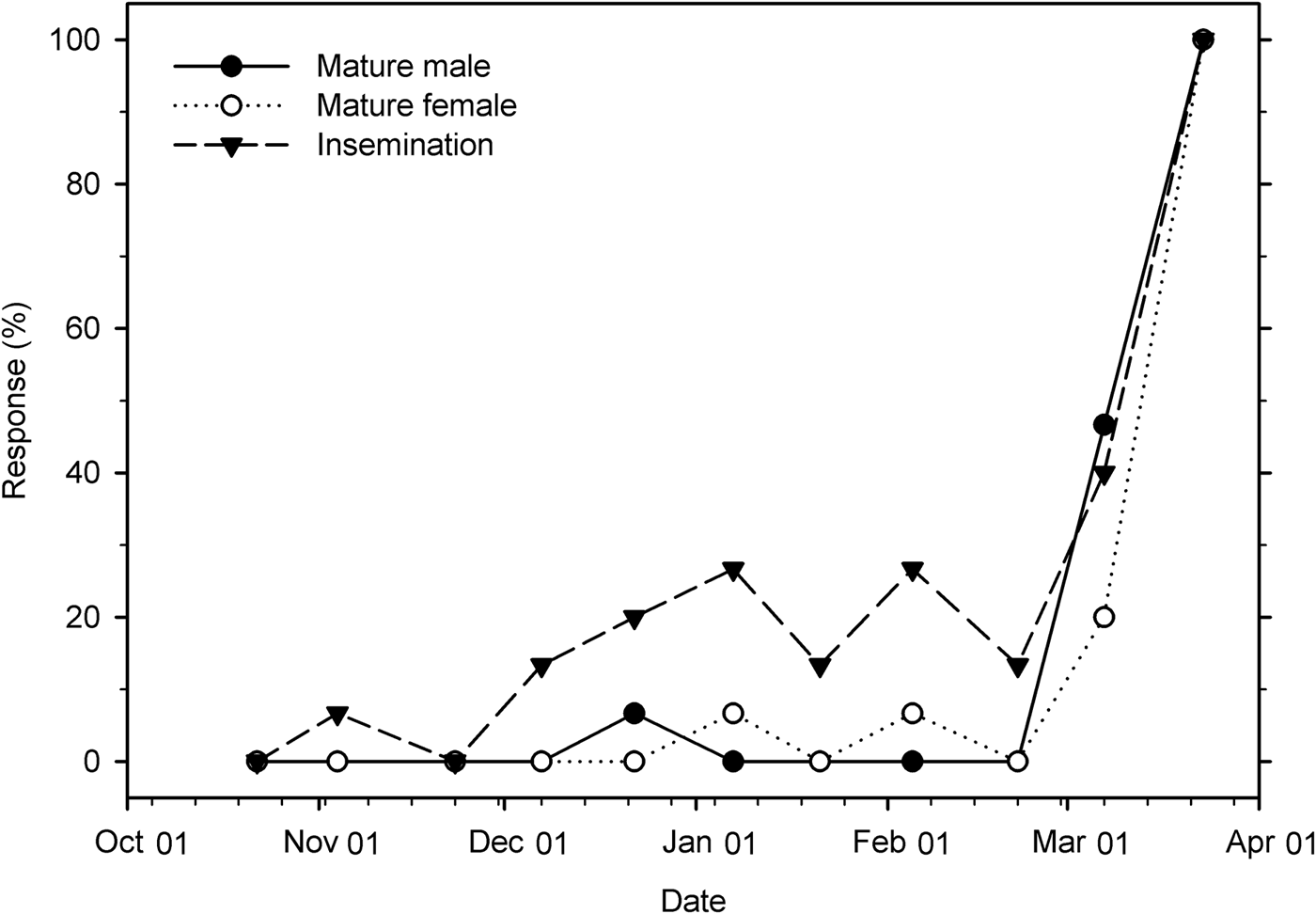
Fig. 9. Percentage of reproductively mature male and female Listronotus maculicollis and insemination rate in the field from 22 October 2015 to 23 March 2016 (n = 15 for each point).
Discussion
In our previous investigations, a prolonged period of chilling during winter synchronized diapause termination and advanced reproductive development in ABW (Wu et al., Reference Wu, Kostromytska, Xue and Koppenhöfer2018). The current study showed that temperature regulated the post-diapause reproductive growth in both male and female ABW, and higher temperatures facilitated the developmental process in early spring. Our findings are consistent with the studies by Rothwell (Reference Rothwell2003), who observed greater female ovarian development and larger sizes of male organs under a regime of LD 8:16 and 22°C than at LD 16:8 and 7°C in spring-emerging ABW. The use of several temperatures combined with the same photoperiod (L:D 12:12) in a series of experiments in our study more clearly demonstrated the role of temperature in post-diapause development in this weevil.
At higher temperatures (14 and 21°C in the first and second experiments carried out in winter 2014–2015; 13 and 15°C in the third experiment in winter 2015–2016), both male and female ABWs had significantly larger reproductive organs in general. However, in the experiment in which diapausing weevils were exposed to 7, 14 and 21°C starting on 10 December 2014, reproductive development in both male and female adults was not evident until 28 DAT; compared with the low temperature (7°C), higher temperatures (14 and 21°C) did not facilitate the organ development before 28 DAT (figs 1 and 2). In contrast, in the experiments started on 26 January in 2015 and 2016, 14 and 21°C and 13 and 15°C, respectively, induced significantly larger reproductive systems than 7°C within 7–14 DAT in both sexes (figs 3–6). This difference reflects how the time of transfer from cold to warm conditions, or length of chilling exposure, affected the response to temperature treatments. In a previous study, a period of chilling was a necessity for diapause termination in this weevil, and the termination process was not initiated until January by exposure to warm conditions (Wu et al., Reference Wu, Kostromytska, Xue and Koppenhöfer2018). Hence, in the experiment started on 10 December 2014, reproductive development was delayed because the required length of chilling time for diapause termination had not been reached, yet. In this experiment, a significant but transient decrease in the size of male reproductive organs was observed at 49 DAT (fig. 1), which was probably due to caging effects as discussed by Wu et al. (Reference Wu, Kostromytska, Xue and Koppenhöfer2018).
In the two experiments conducted at 7, 14 and 21°C, the female reproductive system did not significantly develop at 7°C, whereas male organs did grow in the later phase of the experiments, albeit less than at the higher temperatures (figs 1–4). This indicates that male weevils might have a lower threshold than females in response to temperature, which was verified in the more detailed follow-up experiments. The five temperatures from 7 to 15°C had a dramatically different impact on the development of male and female reproductive systems. Reproductive organ sizes and developmental rate did not increase significantly over time at 7°C in male weevils and at 7 and 9°C in females (figs 5–7). These findings suggest that male ABW may develop and emerge earlier than the females in the spring. Under field conditions, a higher percentage of males (80%) than females (53%) terminated diapause and resumed reproductive growth in January (Wu et al., Reference Wu, Kostromytska, Xue and Koppenhöfer2018), and also a higher proportion of males than of females reached maturity in early March (47% vs. 20%) (fig. 9). However, in the study by Diaz et al. (Reference Diaz, Seto and Peck2008), earlier appearance of male vs. female ABW was not evident in the field from mid-April onwards, although a significantly male-skewed sex ratio was observed on the rough vs. the fairway. However, there remains a possibility of earlier emergence of males prior to the sampling dates.
A base temperature of 10°C has been used in the GDD model for monitoring population dynamics of ABW (Vittum, Reference Vittum1980) and L. bonariensis (Goldson et al., Reference Goldson, Penman and Pottinger1982), whereas in L. oregonensis 7°C is commonly used (Simonet & Davenport, Reference Simonet and Davenport1981; Boivin, Reference Boivin1988). Rothwell (Reference Rothwell2003) determined that the optimal temperature for adult activity is 20°C, and the threshold temperature for larval growth is 13.3°C which was used as the base temperature in the GDD model to predict initial larval activity. However, the exact threshold temperature for predicting overwintered adult emergence and oviposition remains unclear. Jarošík et al. (Reference Jarošík, Honěk, Magarey and Skuhrovec2011) found that related species require similar thermal threshold and degree days for development from the summary of 1054 insect and mite species. In species related to L. maculicollis, the lower threshold temperature for development from egg to adult was 7°C (623 degree days) in L. oregonensis (Simonet & Davenport, Reference Simonet and Davenport1981) and was 13.3°C (435 degree days) in L. texanus (Woodson & Edelson, Reference Woodson and Edelson1988); the latter was close to the observation of Rothwell (Reference Rothwell2003) in L. maculicollis. In L. oregonensis, Simonet & Davenport (Reference Simonet and Davenport1981) determined a threshold temperature of 14.8°C for reproductive development and 131 cumulative degree days for oviposition based on laboratory studies, but they observed 40 degree days for oviposition in overwintered adults in the field. In the current study, we found that the threshold temperature for post-diapause development in ABW was 7.8°C in males, based on which the accumulated degree-days required for 50% of males developing were 61.7 GDD7.8 (from 1 January 2016) (fig. 8). Under field conditions, this coincided with 18 March in spring 2016 for males, although females might have delayed development. This was 1 week before reaching fully reproductive maturity on 23 March 2016 when GDD7.8 was 70 and when GDD10 reached 34.2 (from 1 January 2016), which was 1 week before reaching 56–67 GDD10 when control agents against the overwintered adults are normally applied in the field (Koppenhöfer AM, unpublished). Due to the short residual time of commonly used adulticides in the field (Koppenhöfer et al., Reference Koppenhöfer, Alm, Cowles, McGraw, Swier and Vittum2012), the optimal time to apply chemical insecticides should be around the peak of adult weevil densities on the turfgrass surface but before significant oviposition has started. This optimal timing reduced the need for repeated applications. Weather conditions allowing, the control agents should ideally be applied when 50% weevils reach full maturity, but the required value of GDD accumulated at that time needs to be further verified. The predictive accuracy of the two GDD models needs to be further examined in the field with more frequent samplings.
To conclude, the temperature may serve as the major environmental cue in regulating diapause termination and post-diapause development in ABW, although other factors may also play a role. In late January after the required chilling period was met and diapause was terminated, higher temperatures facilitated reproductive development. Male weevils might have a lower threshold temperature than the females for triggering the development of the reproductive system. The accuracy of using the threshold temperature for monitoring male adult emergence needs to be verified under field conditions in the spring, and an effective model for monitoring overwintered female activities needs to be developed.
Acknowledgments
This research was funded by the Rutgers Center for Turfgrass Scientific Research (New Brunswick, NJ) and by the USDA National Institute of Food and Agriculture Hatch Multistate projects 0206130 through the New Jersey Agricultural Experiment Station, Hatch Multistate projects NJ08295.