Introduction
The carnation tortrix moth, Cacoecimorpha pronubana (Lepidoptera: Tortricidae) (Roelofs and Brown, Reference Roelofs and Brown1982) is an economically important multivoltine polyphagous pest species affecting over 138 plant genera (Kacar and Ulusoy, Reference Kacar and Ulusoy2008; Penchevа and Yovkova, Reference Penchevа and Yovkova2016; Reddy, Reference Reddy2016). Damage is caused by the larva, which feeds mainly on the foliage. It spins a shelter of silk on the upper or lower surface of leaves, or ties the leaves together, and feeds between the leaf surfaces (Fisher, Reference Fisher1924; Bestagno, Reference Bestagno1955). In ornamental plant production, the moth affects mainly plants in the families Aceraceae, Apiaceae, Asteraceae, Brassicaceae, Caryophyllaceae, Ericaceae, Fabaceae, Geraniaceae, Oleaceae, Rutaceae, Rosaceae, Salicaceae, Solanaceae (EPPO, 2014; Beitia et al., Reference Beitia, Asis, De Pedro, Goula and Tormos2016). It naturally occurs in the Mediterranean area, but has been present in Britain since at least 1905 (Fisher, Reference Fisher1924; EPPO, 2014).
While different methods have been used to control C. pronubana, the use of synthetic pesticides remains the most frequently used approach (Solomon et al., Reference Solomon, Jay, Innocenzi, Fitzgerald, Crook, Crook, Easterbrook and Cross2001; Rehman et al., Reference Rehman, Aziz, Saggu, Abbas, Mohan and Ansari2014; Wang et al., Reference Wang, Wu, Cang, Yang, Yu, Zhao, Wang and Cai2014). This is despite fact that insecticides, such as neonicotinoids, pyrethroids, organophosphates and carbamates, are known, to be harmful to natural enemies of this pest, such as Trichogramma evanescens, a parasitic wasp of lepidopteran eggs (Wang et al., Reference Wang, Wu, Cang, Yang, Yu, Zhao, Wang and Cai2014). In addition, pyrethroid and neonicotinoid insecticides are harmful to pollinators such as the European honey bee, Apis mellifera, and the alfalfa leafcutting bee, Megachile rotundata (Whitehorn et al., Reference Whitehorn, Wallace and Vallejo-Marin2017; Piccolomini et al., Reference Piccolomini, Whiten, Flenniken, O'Neill and Peterson2018).
Although most agricultural production relies on the use of synthetic chemical pesticides to maintain high crop yields (Margni et al., Reference Margni, Rossier, Crettaz and Jolliet2002), integrated pest management (IPM) systems have become one of the most extensively researched methods of sustainable crop production (Stenberg, Reference Stenberg2017). IPM systems are typically based on crop monitoring, use of economic thresholds, manipulation of biotic and abiotic factors to prevent pest problems wherever possible, and where required, the application of bio-pesticides or natural enemies (Gross and Gündermann, Reference Gross, Gündermann, Horowitz and Ishaaya2016; Lamichhane et al., Reference Lamichhane, Dachbrodt-Saaydeh, Kudsk and Messéan2016; Lemes et al., Reference Lemes, Zanuncio, Serrão and Lawson2017; Ramsden et al., Reference Ramsden, Menendez, Leather and Wäckers2017; Rodrigues et al., Reference Rodrigues, Batista, Nave, Matos and da Costa2017). Host plant suitability plays an important role in shaping the feeding behaviour of a larva, and determining the larval and adult performance in terms of its longevity, body size, fecundity and mating and ovipositional behaviour (Brewer et al., Reference Brewer, Capinera, Deshon and Walmsley1985; Lance, Reference Lance and Ahmad2012; Moreau et al., Reference Moreau, Desouhant, Louâpre, Goubault, Rajon, Jarrige, Menu and Thiéry2017). Even where an insect herbivore is polyphagous, individuals may still display improved performance on one specific host plant, family or order, in terms of reproductive success and population growth (Lance, Reference Lance and Ahmad2012).
Little is known about the influence of the host plant species on the performance of C. pronubana, and how the components of its life stage correlate with each other. Knowledge of whether host plant influences performance of this pest would inform whether or not crop protection approaches should be tailored to specific crops (Bell et al., Reference Bell, Marris, Prickett and Edwards2005). Improved understanding of the life history of C. pronubana may also allow for the development of models of fitness for this species that can be used to predict the reproductive success of this moth within a specific crop environment, for example, and further determine the economic impact that this species may have and could be used in the development of improved pest management of this insect. Similar relationships have previously been studied, for example, for the diamondback moth, Plutella xylostella (Verkerk and Wright, Reference Verkerk and Wright1996) and the maize weevil, Sitophilus zeamais (Ojo and Omoloye, Reference Ojo and Omoloye2016), and used to explain observed plasticity in life-history traits (Nylin and Gotthard, Reference Nylin and Gotthard1998).
The aim of this study was to investigate the effect of the host plant on the larval development of C. pronubana, and the relationships between the larval stage duration and the weight of pupa of the insect. Improved understanding of larval development on different ornamental host plant species and how this affects further the moth development may be used to determine crop-specific population dynamics of the moth, and management of crop infestations more effectively within an IPM system.
Materials and methods
Host plants
Six, economically important plant species that are commonly attacked by C. pronubana were obtained for the experiment from an ornamental plant nursery in Shropshire, UK (52°37′20″ N, 2°15′20″ W; 119 m a.s.l.). These were: Mexican orange, Choisya ternata Kunth ‘Lich’ (Rutaceae), Japanese spindle tree, Euonymus japonicus Thunb. var. Ovatus Aureus (Celastraceae), New Zealand broadleaf, Griselinia littoralis Raoul (Griseliniaceae), Christmas berry, Photinia Lindl. ‘Red Robin’ (Rosaceae), cherry laurel, Prunus laurocerasus var. schipkaensis Späth ex H.L.Späth (Rosaceae), and firethorn, Pyracantha angustifolia (Franch.) C.K.Schneid. (Rosaceae). Plants were propagated from softwood cuttings two years before the experiment. Cuttings were collected, propagated and grown in 9-cm diameter black plastic pots filled with Sinclair® All Purpose Growing Medium Compost (Sinclair Pro, UK) under protected glasshouse conditions at the same ornamental plants nursery. At the time of the experiment, plant's height ranged from 20 to 25 cm, and their mid-width measured approximately 12 cm.
Insects
Cultures of C. pronubana were established using adult moths caught between April and May 2018 at the same ornamental plants nursery in Shropshire, UK, from which the plants were obtained. The moth culture was established in a BugDorm-4D® Insect Rearing Cage (47.5 × 47.5 × 47.5 cm) (MegaView, Taiwan) placed in a Fitotron® SGR plant growth room (Weiss Technik, UK) maintained at a constant temperature 20°C, 60% RH and 16L:8D photoperiod. Moths were reared on a mix of ornamental plants belonging to Caprifoliaceae, Hypericaceae, Pittosporaceae, Plantaginaceae and Rhamnaceae. Plant species and plant families that are commonly affected by the pest were carefully selected for rearing the moth culture to mimic diverse range of plants grown within an ornamental nursery and to ensure fitness of the moths used in the experiment (Merz, Reference Merz1959; Freeland, Reference Freeland1975; Hägele and Rowell-Rahier, Reference Hägele and Rowell-Rahier1999). Plants were grown and sourced from the same ornamental plant nursery, from which the host plants were obtained. The moth colony was maintained from May to November 2018. No additional individuals were introduced after the initial establishment and during the maintenance of the culture. Second-generation neonates (0–34 h old) were collected for the experiment.
Experiments
The experiment was conducted in a Fitotron® SGR ‘Walk-in’ plant growth room under the same controlled environmental conditions used to maintain the culture of C. pronubana. The treatments consisted of the six host plants – Choisya, Euonymus, Griselinia, Photinia, Prunus and Pyracantha. Each replicate consisted of a single plant and six replicates were laid out using a Latin Square design (Fisher, Reference Fisher1925). Plants were stood on a black PVC tray and were watered as required from beneath. Five neonates collected from the culture were placed at the same time on each plant using a fine paintbrush, after which each plant was covered with a nylon mesh sleeve (43 cm long by 13 cm diameter; mesh aperture area = 1.6 × 10−5 metre, with the open area of a mesh (A°) = 50%) closed at the top and the bottom. Time of application was recorded for each host plant. Pupae were transferred separately to Petri dishes (90 mm diameter × 15 mm height), and placed in the same room under the same constant environmental conditions. Each Petri dish had a circular piece of dry plain white paper towel placed on the bottom of the dish. The following data were recorded for each individual moth: the cumulative duration of larval stage from neonate to pupa; the weight of pupa after the integument hardened and turned brown; as well as the date of emergence, sex and weight of adult. The adult measurements were taken between 6 and 12 h after eclosion. Sex was determined using a Microtec HM-3® stereo microscope (TEC Microscopes, UK).
After eclosion and measurements one male and up to three female moths were placed into one clear Petri dish (all from the same host plant species) and were allowed to mate. Females were allowed to lay eggs on the inside walls of the dish until their death. Only the total number of eggs laid per host plant species, and the total number of egg clutches per host plant species was recorded. The females were allowed to lay eggs up to their death; during this period eggs were counted every 48 h using a Microtec HM-3® stereo microscope.
Statistical analysis
Statistical analyses were performed with R version 3.6.1 (R Core Team, 2019), at α = 0.05. Larval survivorship (numbers survived to pupal stage/number introduced) was compared between six host plant species, and pupal survivorship between five host plant species (analyses did not include Euonymus) using Kruskal–Wallis test, and post hoc pairwise comparisons [with P values adjusted by Benjamini and Hochberg (BH) method] were performed with Wilcoxon test. One-way analysis of variance (ANOVA) was used to detect differences between the duration of the larval or pupal stage across the host plant treatments. Wilcoxon signed-rank test with continuity correction was used to test the difference between the total number of larvae applied initially and the larvae survived, as well as between the pupae that was collected and the pupae that survived. Pupal weight was compared between six host plant species using Kruskal–Wallis test, and post hoc pairwise comparisons was tested using Wilcoxon test with P value adjusted by BH method.
Correlation between duration of larval stage and pupal weight between sexes on different host plant species, its strength and the direction was tested with Pearson's product−moment correlation coefficient (PPMCC). When the data were not normally distributed the Kendall rank correlation coefficient (tau) test was used to estimate a rank-based measure of association. When the correlation was significant (P < 0.05), the R-squared was used to indicate the strength of predictive accuracy. When the host plant species did not affect the sexes, the data were pooled from all host plants in order to analyse the correlation between the overall duration of larval stage and the overall pupal weight between sexes. The duration of larval stage and the weight of pupa between males and females was compared using non-parametric unpaired two-samples Wilcoxon test.
Fecundity on different host plants was determined by the mean number of eggs laid inside Petri dishes as well as the number of eggs per clutch laid. In order to determine the differences between the mean fecundity of moths that had developed on the five different host plants we compared the observed numbers of eggs laid with the expected values based on an equal distribution of eggs laid by adults from the five different host plants. The null hypothesis was that there is no significant difference between the observed and the expected values, and it was tested with Pearson's chi-square goodness of fit test. Kruskal–Wallis rank-sum test was used to test the differences between the number of eggs laid per clutch between five different host plants, and the differences between the groups of host plants with pairwise comparisons using Wilcoxon test with P value adjusted by BH method.
Results
Larval and pupal survivorship
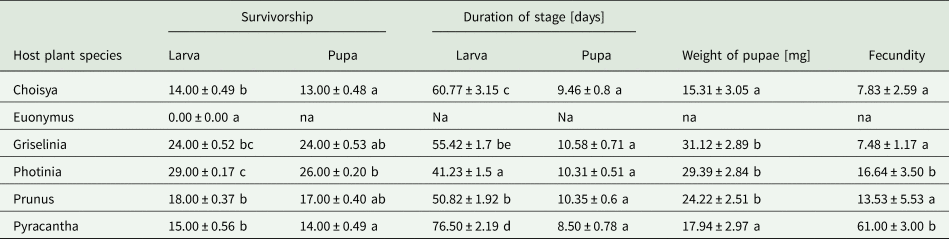
Larval survivorship was determined by pupae collected from five different host plant species − Choisya, Euonymus, Griselinia, Photinia, Prunus and Pyracantha. Survival rates differed significantly between host plant species for larvae (Kruskal–Wallis (KW): χ2 = 26.06, df = 5, P < 0.0001), and for pupae (KW: χ2 = 13.28, df = 4, P = 0.009). Multiple pairwise comparisons indicated significant differences in larval survivorship between eight pairs of host plants (P < 0.05) (Table 1).
Table 1. Four life history traits of Cacoecimorpha pronubana reared on six different host plants in terms of mean numbers (±SE) of larval and pupal survivorship, duration of larval and pupal stage determined in days, weight of pupa in milligrams, and fecundity defined by the number of eggs in each clutch; na – not applicable (larvae did not survive on Euonymus); values in each column with the same letter are not significantly different (P > 0.05)
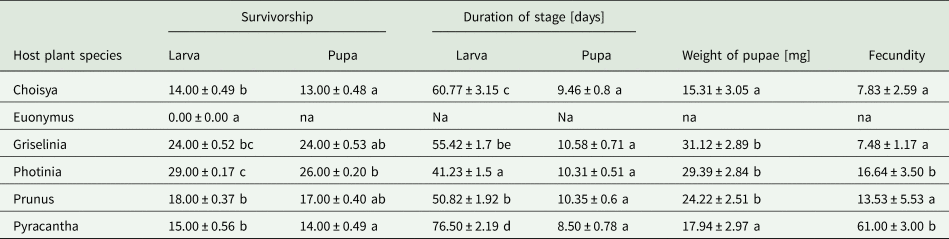
The difference between the total number of neonate larvae placed on each plant at the start of the experiment, and the numbers of larvae that survived was statistically significant (Wilcoxon signed-rank test with continuity correction (WRS): V = 406, P < 0.0001), and there was no difference between the numbers of pupae collected, and the numbers of pupae that survived (WRS: V = 10, P = 0.072). It was concluded that the median number of the larvae placed on each plant at the start of the experiment was significantly different from the median number of larvae that survived, however, the median number of pupae collected was not significantly different from the median number of pupae that survived.
Duration of larval and pupal stage
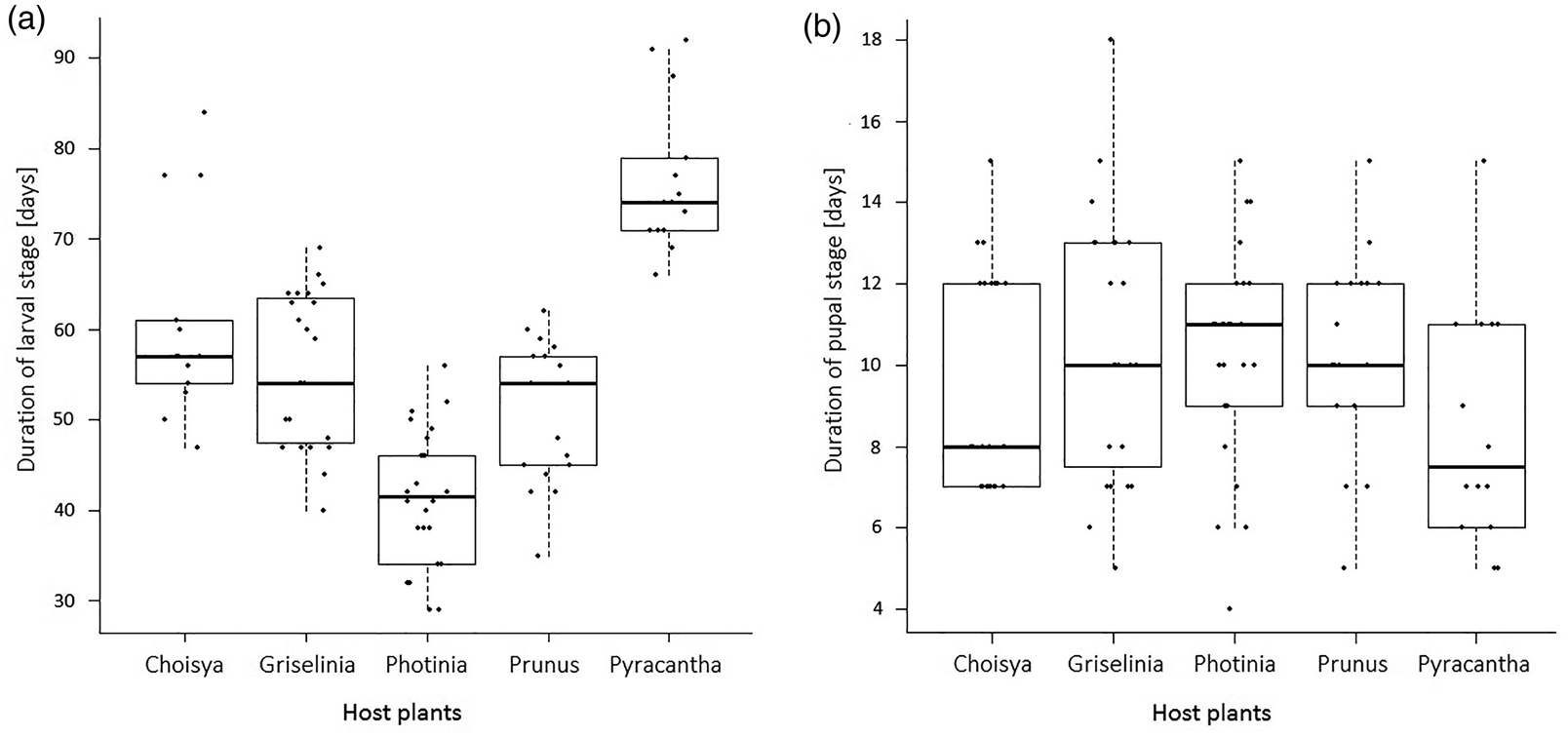
The mean duration of the larval stage (± 0.82 days; deviation of ± 1.50% from the mean) was (mean ± SEM) 54.54 ± 1.50, and pupal stage was 10.00 ± 0.30 days. Host plant species had a significant effect on larval duration (one-way ANOVA: df = 4, F = 41.6, P < 0.0001), but not on the duration of pupal stage (KW: χ2 = 5.83, df = 4, P = 0.212; fig. 1b). Tukey pairwise comparison indicated that the duration of larval stage between host plants differed significantly except between Griselinia and Prunus, and Griselinia and Choisya (fig. 1a).
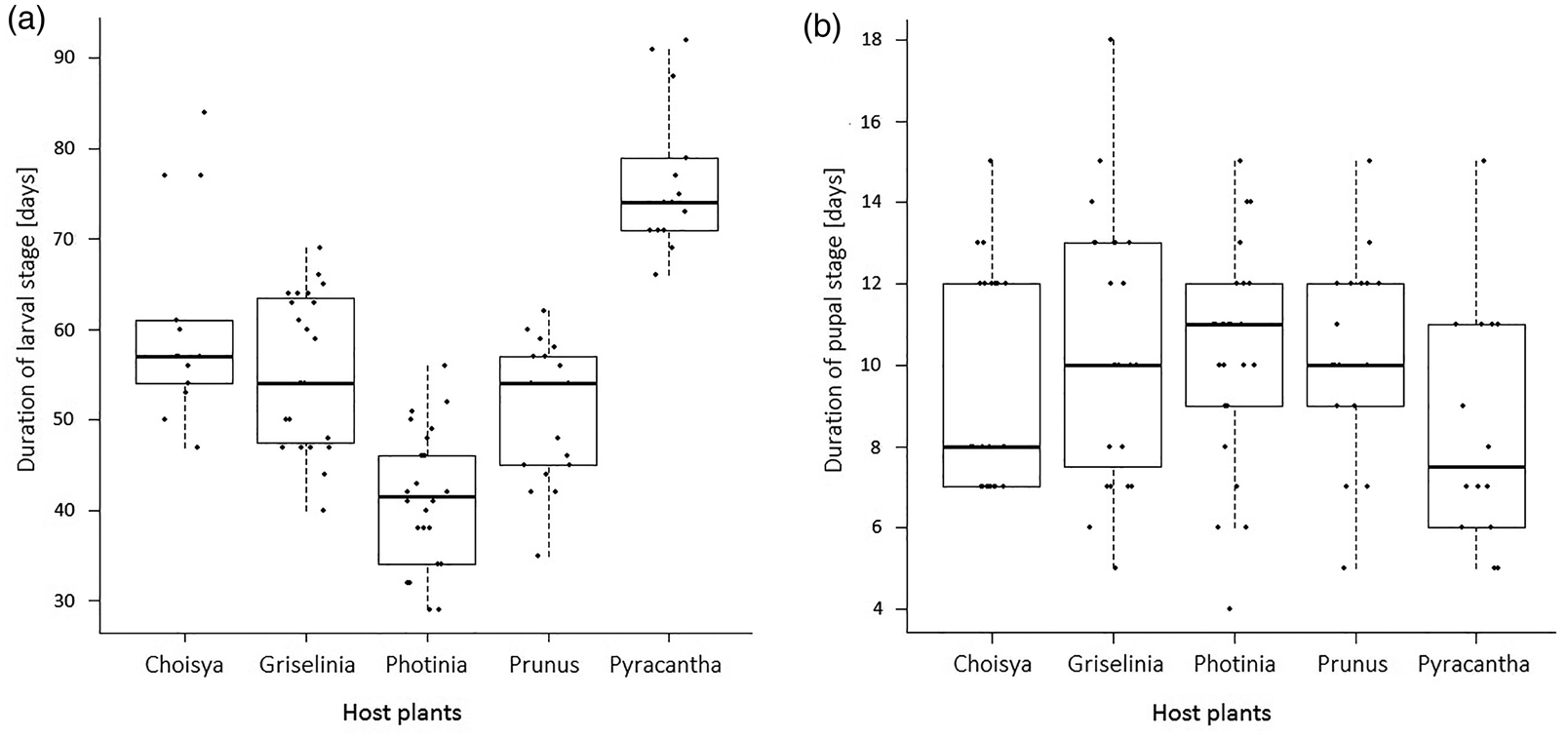
Figure 1. Duration of larval (A) and pupal stage (B) for Cacoecimorpha pronubana from egg hatch to adult emergence with distinction between five different host plant species; for larvae to grow from hatching to fully formed pupae, and for pupae to undergo pupation up to adult eclosion. Boxes represent the interquartile range (IQR) with a thick horizontal line at the median and whiskers extending to the largest or smallest observation falling within 1.5 IQRs of the upper or lower quantiles. The scattered black dots plotted on each box & whisker represent data distribution along the timeline of the duration of the larval stage on each host plant; the data points were intentionally horizontally spread in order to avoid the points being obscured by each other.
Correlation between duration of larval stage and pupal weight between sexes
Overall, the weights of pupae collected from five different host plant species significantly differed between the plant species (KW: χ2 = 32.44, df = 4, P < 0.0001, table 1). The weights of male pupae was also significantly different between plant species (One-way ANOVA: df = 4, F = 28.65, P < 0.0001), but female pupal weights did not differ between the host plants (KW: χ2 = 9.38, df = 4, P = 0.052).
There was no significant correlation observed between the duration of the larval stage and the weight of pupae for female larvae on any of the five studied host plant species. This was also true for males on four different plant species, however, there was a significant (negative) relationship between the duration of the larval stage and the weight of pupae for male larvae only on Photinia (Pearson's correlation: t = −3.60, d.f. = 14, P = 0.003; PPMCC: r = −0.69). The mean duration of the larval stage and weight of the pupae for male moths (± SEM) developing on Photinia were 38.8 ± 1.92 days and 19.00 ± 0.59 mg, respectively.
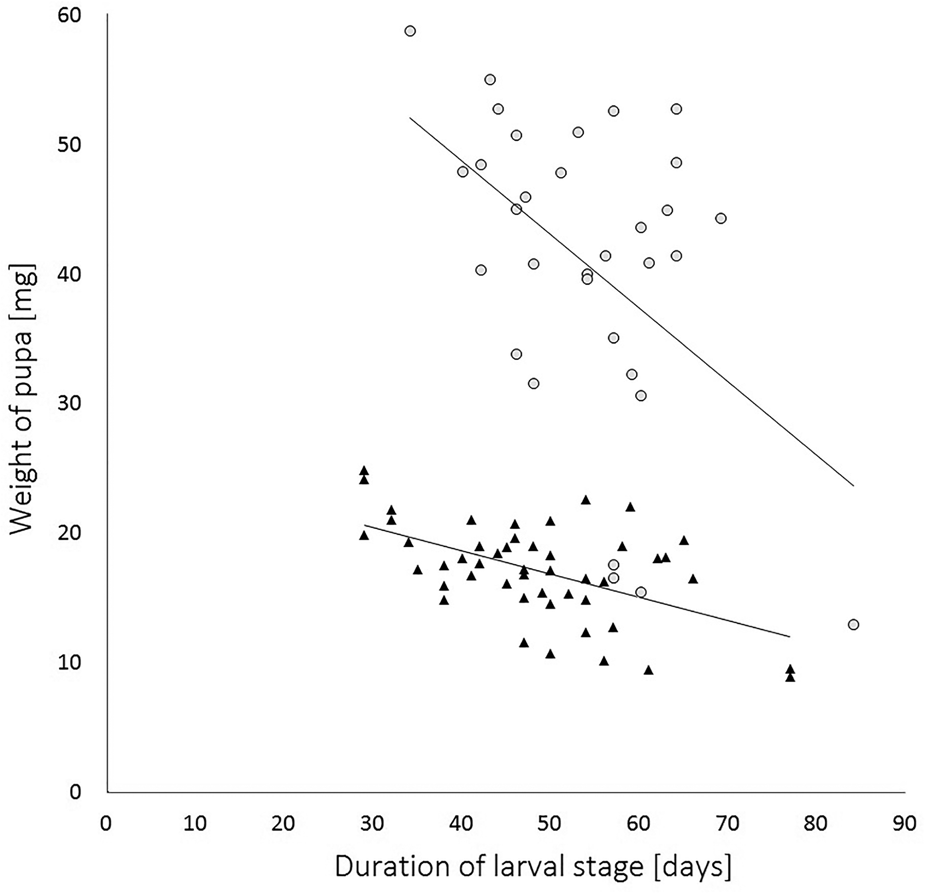
The data, both for duration of the larval stage and the weight of the pupae, were then analysed across the whole experiment against sex of the moth. The mean duration of the larval stage and the mean weight of the pupae for male moths (± SEM) was 53.73 ± 2.02 days and 16.40 ± 0.49 mg, and for female moths was 55.91 ± 1.94 days and 40.20 ± 1.99 mg, respectively. The duration of the larval stage was significantly longer for males than for females (Wilcoxon rank-sum test with continuity correction: W = 1405.5, P < 0.0001). The weight of female pupae was significantly heavier than those of males (Wilcoxon rank-sum test with continuity correction: W = 1405.5, P < 0.0001). The duration of the larval stage and the weight of pupae for males were highly statistically correlated with each other (Pearson's correlation: t = −5.83, d.f. = 57, P < 0.0001) displaying a moderate negative relationship (Pearson's product−moment correlation coefficient (PPMCC: r = −0.61; fig. 2). For females the correlation between the duration of the larval stage and the weight of pupae was also statistically significant (Kendall rank correlation: z = −2.21, P = 0.04, τ = −0.26) with a moderate negative relationship (PPMCC: r = −0.48; fig. 2).
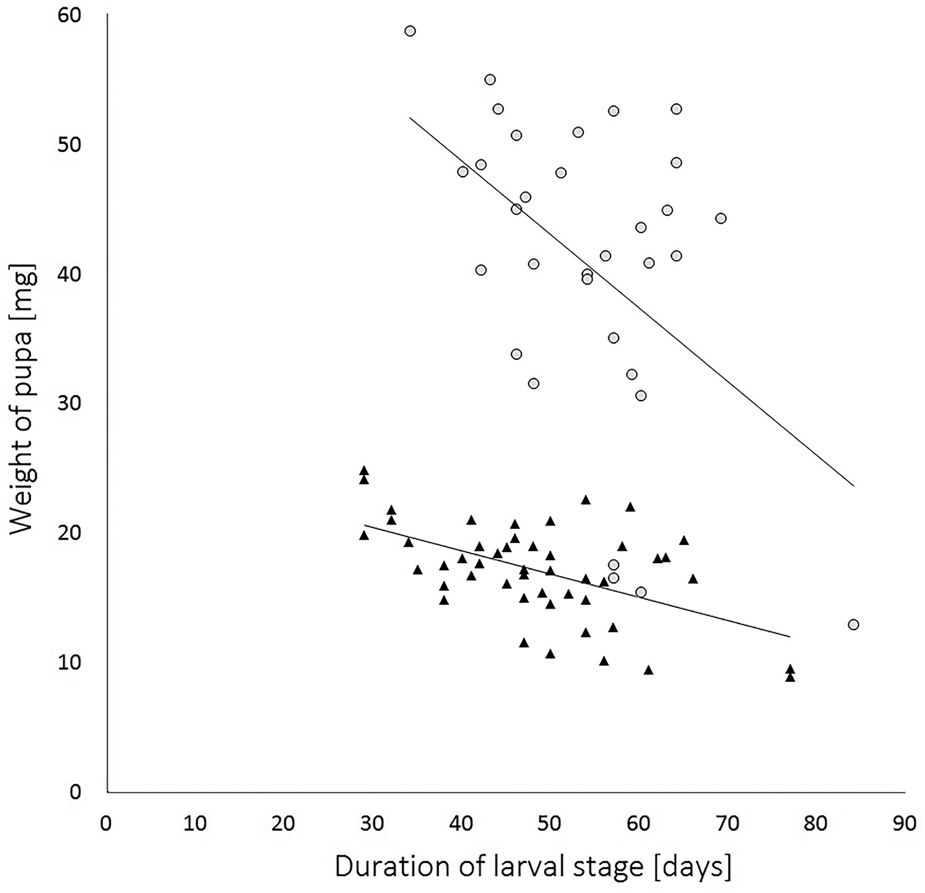
Figure 2. Correlation of the duration of larval stage and pupal weights of Cacoecimorpha pronubana. There is a moderate negative r = −0.61, y = −0.149x + 24.4, and weak negative correlation r = −0.46, y = −0.475x + 66.7, for male (triangle-shaped data points) and for female (circle-shaped data points), respectively. R-squared indicates that in both instances, either for male (R 2 = 0.37; P < 0.0001) or female (R 2 = 0.21; P < 0.05) the duration of larval stage has a weak predictive accuracy on the weight of pupa.
Fecundity on different host plants
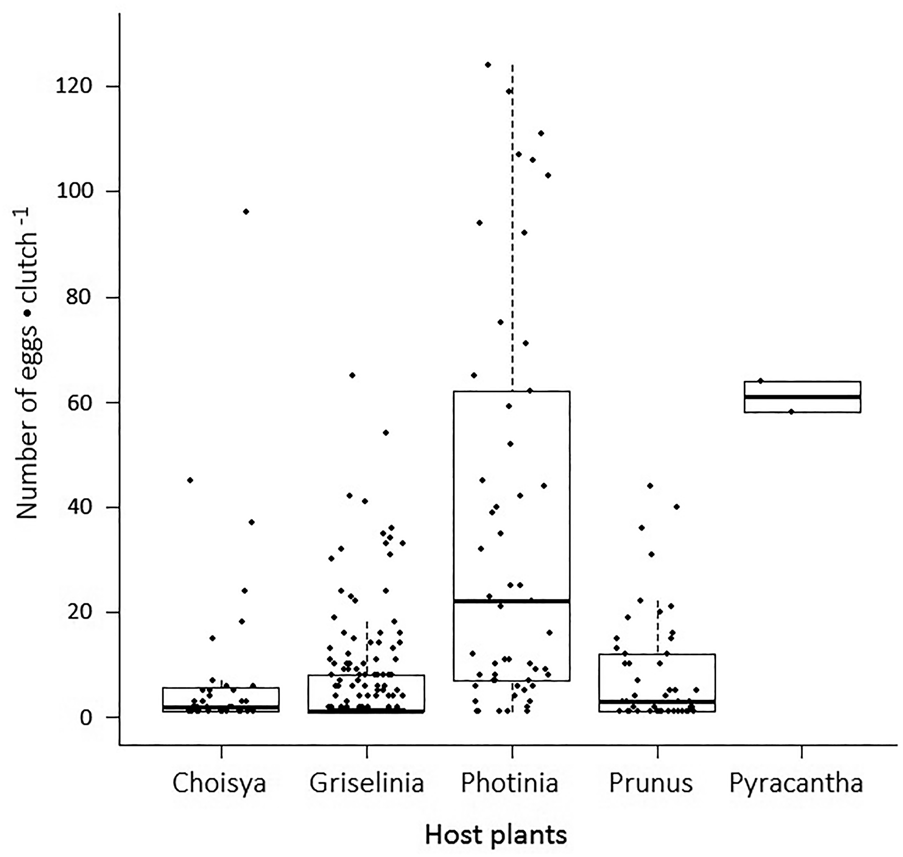
The total numbers of eggs laid by females that had developed on Choisya, Griselinia, Photinia, Prunus and Pyracantha were 329, 1212, 2016, 717 and 122, respectively. The mean number of eggs laid per host plant species were 66, 101, 202, 143 and 41, respectively, and they differed significantly among host plants (Pearson's chi-squared test goodness of fit: χ2 = 147.64, d.f. = 4, P < 0.001). There was a significant difference between the number of eggs laid per clutch for moths reared on the five different host plants (KW: χ2 = 64.77, df = 4, P < 0.001). Clutch sizes were significantly different among the six pairs of host plants (P < 0.05) (Pairwise comparisons using Wilcoxon rank-sum test with P value adjusted by BH method; fig. 3).
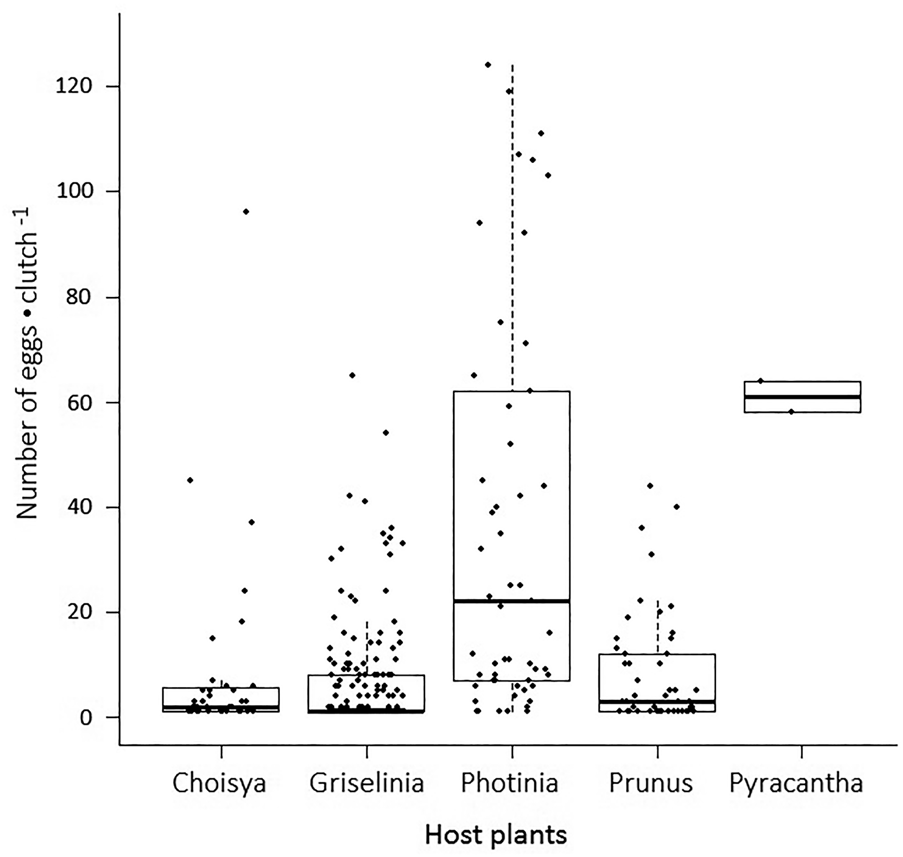
Figure 3. Fecundity of the female Cacoecimorpha pronubana reared on five different host plants. Fecundity is defined by the eggs•clutch −1. Each data point represents a clutch of eggs, where eggs•clutch −1 is determined on the y-axis.
Discussion
Survival, duration of larval stage, pupal weight and fecundity of C. pronubana is significantly affected by larval host plant as has been shown for other species of Lepidoptera (Barbosa et al., Reference Barbosa, Waldvogel, Martinat and Douglass1983; Thiery and Moreau, Reference Thiery and Moreau2005; Saeed et al., Reference Saeed, Sayyed, Shad and Zaka2010). The faster developing larvae reared on Photinia (Rosaceae), Prunus (Roseceae), and Griselinia (Griseliniaceae) resulted in heavier pupae, while the slower developing larvae on Pyracantha (Rosaceae) and Choisya (Rutaceae) resulted in lighter pupae. A similar phenomenon was observed for P. xylostella by Kahuthia-Gathu et al. (Reference Kahuthia-Gathu, Loehr and Poehling2008). Kahuthia-Gathu et al. (Reference Kahuthia-Gathu, Loehr and Poehling2008) showed that the duration of larval development and pupal weight of the diamondback moth was significantly affected by different Brassicaceae species. As larval performance is dependent on the plant on which it develops, it would appear to be a suitable criteria on which to evaluate host preference (Mayhew, Reference Mayhew1998; Gripenberg et al., Reference Gripenberg, Mayhew, Parnell and Roslin2010). It is known that the development and survival of a moth is affected by ovipositional preferences (Leather, Reference Leather1985; Sarfraz et al., Reference Sarfraz, Dosdall, Keddie and Myers2011), it is yet to be demonstrated, however, whether survival, development, and fecundity of a moth can predict larval host preference (Bentancourt et al., Reference Bentancourt, Scatoni, Gonzalez and Franco2003; Lance, Reference Lance and Ahmad2012; Kong et al., Reference Kong, Wang, Guo, Chai, Li and Ma2020).
No significant differences in the duration of pupal stages are reported here for C. pronubana between host plants. This phenomenon can be also observed among other moth species (Xue et al., Reference Xue, Pang, Wang, Li and Liu2010; Kirichenko et al., Reference Kirichenko, Flament, Baranchikov and Grégoire2011; Razmjou et al., Reference Razmjou, Naseri and Hemati2014; Reigada et al., Reference Reigada, Guimarães and Parra2016).
This study found that the neonatal larvae of C. pronubana were unable to survive on Euonymus japonicus Thunb. var. Ovatus Aureus. It is possible that the first instar larvae were unable to chew through the thick leaf epidermis, and that in the natural environment late-instar larvae migrating from other hosts might infest the host plant (Shelomi et al., Reference Shelomi, Perkins, Cribb and Zalucki2010; Lance, Reference Lance and Ahmad2012). Such larvae may perhaps use the more mature foliage as a shelter against predators, where they could develop and emerge as an adult (Bittencourt-Rodrigues and Zucoloto, Reference Bittencourt-Rodrigues and Zucoloto2009). Nevertheless, it is not entirely clear, how the above-mentioned host affects the larvae differently in natural environments.
Significant correlation between the duration of larval stage and the pupal weight between sexes among the host plant species was only observed on Photinia for males. The results from this example showed that the longer duration of larval stage is, or the less suitable food resources are, the greater the negative effect on the development of the adult moth may be. It is possible that C. pronubana adult body weight and its reserves are determined by larval food supply (Briegel, Reference Briegel1990). We further observed that correlations between duration of larval stage and pupal weight in this study differed between sexes. The correlation between the duration of larval stage and the pupal weight for males was moderate, and it was weak among the females. Similar correlations were observed for the fall armyworm moth Spodoptera frugiperda (Pencoe and Martin, Reference Pencoe and Martin1982). Female pupae were also significantly heavier than those of males as is common in species of Lepidoptera (Lederhouse et al., Reference Lederhouse, Finke and Scriber1982; Haukioja and Neuvonen, Reference Haukioja and Neuvonen1985; Leather et al., Reference Leather, Beare, Cooke and Fellowes1998). It is possible that the correlation between duration of the larval stage and pupal weight occurs because of stress caused by unsuitable food resources. A similar phenomenon was observed for the greater wax moth Galleria mellonella (Krams et al., Reference Krams, Kecko, Kangassalo, Moore, Jankevics, Inashkina, Krama, Lietuvietis, Meija and Rantala2015). It has also been shown that nutritional value of a host plant increases the size and the reproductive performance of adult European grapevine moth Lobesia botrana (Thiery and Moreau, Reference Thiery and Moreau2005). Such a phenomenon may be a consequence of a higher host plant nitrogen content that accelerates larval development and subsequently weight of adults (Lindroth et al., Reference Lindroth, Klein, Hemming and Feuker1997). It may be possible, that as a result of improving the long-term application of nitrogen fertilizers (or generally synthetic fertilizers), the herbivore feeding preferences, food consumption, and survival, as well as growth, reproduction, and population density of C. pronubana may be reduced (Lu et al., Reference Lu, Yu, Heong and Cui2007). Before applying a control measure to reduce the moth population, it may, however, be economically more viable to estimate, for example, the duration of the larval period for a specific crop prior to selecting the most appropriate control strategy. This may be important where the natural enemy, for example, is effective against only certain larval instars as in the case of the ectoparasitic wasp, Eulophus pennicornis which may be used against the tomato moth, Lacanobia oleracea (Bell et al., Reference Bell, Marris, Prickett and Edwards2005).
Although fecundity, in terms of the total number of eggs laid per host plant, was highest on Photinia, and the lowest on Pyracantha, the highest number of egg clutches was found on Griselinia and Photinia and the lowest on Pyracantha. Results presented here showed that more fecund adults were observed from the same larval host plants on which the shorter duration of larval development produced heavier pupae. Pupal weight in Lepidoptera is recognized as a good indicator of the fitness of adults and their offspring, and it is known that heavier female adults are more fecund and that they are produced from heavier pupae (Danthanarayana, Reference Danthanarayana1975; Sarfraz et al., Reference Sarfraz, Dosdall and Keddie2007). Similar relationships were shown for the diamondback moth, P. xylostella females reared on the wild Brassicaceae species R. nudiuscula Thell. (highest mean fecundity), and on cultivated Brassica oleracea L. cultivars (lowest mean fecundity) (Kahuthia-Gathu et al., Reference Kahuthia-Gathu, Loehr and Poehling2008). Following the Hopkins Host Selection Principle (Hopkins, Reference Hopkins1917) it is possible that the oviposition shown in this study are influenced by the larval host plant (Verschut et al., Reference Verschut, Blažytė-Čereškienė, Apšegaitė, Mozūraitis and Hambäck2017; Gámez and León, Reference Gámez and León2018).
It is possible that the differences in oviposition behaviour seen in this study, in terms of the size of egg clutches, may also be as a consequence of the foliage density or/and the size of leaves of host plants on which larvae would develop. This phenomenon may be a form of adaptation in terms of a host plant exploitation by the larvae (Davies and Gilbert, Reference Davies and Gilbert1985). It is possible that the female retains information about the host plant and this affects her oviposition behaviour in developing an oviposition strategy for increased egg survival. Brown and Cameron (Reference Brown and Cameron1979), for example, showed that there is a strong correlation between decreased size of egg clutches and increased parasitism on the Gypsy moth Lymantria dispar dispar (Linnaeus, 1758) eggs. The density factors may play a significant role in the insect's ovipositional behaviour (Miller and McDougall, Reference Miller and McDougall1973; Bigger and Fox, Reference Bigger and Fox1997; Myers et al., Reference Myers, Boettner and Elkinton1998), but further study on this aspect of the biology of C. pronubana is needed.
In natural environments there are many different cues that influence the reproductive choices of an insect (Kagata and Ohgushi, Reference Kagata and Ohgushi2002; Mescher and Pearse, Reference Mescher and Pearse2016; Helms et al., Reference Helms, De Moraes, Tröger, Alborn, Francke, Tooker and Mescher2017), and subsequently the survival, development and reproductive potential of her offspring (Reavey and Gaston, Reference Reavey and Gaston1991). The performance of the moth, however, reflects the suitability of a host plant for the larva to feed on, the nutritional value of the available food, and the shelter for the larva to survive (Stockhoff, Reference Stockhoff1991; Roth et al., Reference Roth, Knorr and Lindroth1997; Chen, Reference Chen2008; Krams et al., Reference Krams, Kecko, Kangassalo, Moore, Jankevics, Inashkina, Krama, Lietuvietis, Meija and Rantala2015). Moth performance is equally likely to be linked to factors such as secondary metabolites, micronutrients, mechanical properties of a host plant or abiotic factors (Du et al., Reference Du, Poppy, Powell, Pickett, Wadhams and Woodcock1998; Dudareva et al., Reference Dudareva, Pichersky and Gershenzon2004; Colasurdo et al., Reference Colasurdo, Gélinas and Despland2009; War et al., Reference War, Paulraj, Ahmad, Buhroo, Hussain, Ignacimuthu and Sharma2012). Assessing and/or determining these factors may be an important aspect in managing IPM systems better. Results from this study support the hypothesis that different host plants have different effects on the performance of individual C. pronubana.