Introduction
Fruit flies (Diptera: Tephritidae) represent one of the best models for the study of sexual behavior due to the wide ecological variation, evolutionary diversification, and complex mating systems they exhibit. Several tephritid species are among the most important pest species in the world, and the study of their biology, ecology, and behavior plays a key role in their regulation and control (Díaz-Fleischer and Aluja, Reference Díaz-Fleischer, Aluja, Aluja and Norrbom2000; Aluja and Mangan, Reference Aluja and Mangan2008; Ekesi et al., Reference Ekesi, De Meyer, Mohamed, Virgilo and Borgemeister2016). This knowledge is particularly relevant in the context of the sterile insect technique (SIT). The SIT is an environmentally friendly and effective pest management strategy, and its success depends on the mating of mass-reared sterile males with wild females (Knipling, Reference Knipling1955; Enkerlin, Reference Enkerlin, Dyck, Hendrichs and Robinson2005; Lance and McInnis, Reference Lance, McInnis, Dyck, Hendrichs and Robinson2005). Hence, the evaluation of wild female mate choice is crucial in determining the effectiveness of sterile males competing against wild males. Nonetheless, the processes required by the SIT (e.g. domestication, mass-rearing and irradiation) can lead to phenotypic changes, including male sexual behavior, that results in the loss of sexual competitiveness (Cayol, Reference Cayol, Aluja and Norrbom2000; Hasson and Rossler, Reference Hasson and Rossler2002; Weldon, Reference Weldon2005; Gómez-Cendra et al., Reference Gómez-Cendra, Segura, Alberti and Vilardi2014). Therefore, the effective implementation of the SIT requires considerable knowledge on the traits and factors relevant in sexual selection, particularly in mate choice, in addition to identifying possible differences between wild and mass reared populations that may lead to disparity in mating success (Robinson et al., Reference Robinson, Cayol and Hendrichs2002; de Souza et al., Reference De Souza, de Lima-Filho, Molina, de Almeida, de Gouveia, de Macêdo, Laumann and Paranhos2015; Shelly and McInnis, Reference Shelly and McInnis2016).
In many tephritids, including Anastrepha ludens (Loew) and A. obliqua (Macquart), males aggregate in leks, emitting pheromones and defending territories that do not contain resources critical to females. Then, females choose their mate from among males performing courtship displays (Shelly, Reference Shelly2018b). This choice has been associated with direct benefits for female (i.e., fecundity and longevity), and simultaneously indirect genetic benefits (‘good genes’ and the ‘sexy son’ hypotheses) (Kirkpatrick and Ryan, Reference Kirkpatrick and Ryan1991).
Despite a large number of studies on female choice in tephritid pest species, there is still no consensus regarding the traits and processes determining mate choice (Pérez-Staples et al., Reference Pérez-Staples, Shelly and Yuval2013; Shelly and Nishimoto, Reference Shelly and Nishimoto2017; Bachmann et al., Reference Bachmann, Devescovi, Nussenbaum, Milla, Todd, Cladera, Fernández, Vera and Segura2019). One of the traits widely studied in sexual selection is body size, due to its strong correlation with fitness components (Blanckenhorn, Reference Blanckenhorn2000). A larger body size is related to enhanced acoustic and chemical signals to attract a mate, in addition to being correlated with the ability to acquire and defend a territory (Webb et al., Reference Webb, Sivinski and Smittle1987; Benelli et al., Reference Benelli, Donati, Romano, Ragni, Bonsignori, Stefanini and Canale2015; Shelly, Reference Shelly2018a). Studies related to body size, such as morphometry and fluctuating asymmetry, have determined that shape, size and symmetry of some traits (e.g. thorax length, head features, wing width and setae) are key to tephritid copulatory success (Hunt et al., Reference Hunt, Crean, Wood and Gilburn1998; Rodriguero et al., Reference Rodriguero, Vera, Rial, Cayol and Vilardi2002a, Reference Rodriguero, Vilardi, Vera, Cayol and Rial2002b; Sciurano et al., Reference Sciurano, Segura, Rodriguero, Gómez Cendra, Allinghi, Cladera and Vilardi2007; Segura et al., Reference Segura, Petit-Marty, Sciurano, Vera, Calcagno, Allinghi, Gómez Cendra, Cladera and Vilardi2007).
There is evidence that size influences female choice, mainly for Ceratitis capitata (Wiedemann). Orozco and López (Reference Orozco, López, Aluja and Liedo1993) determined that, among sterile males, large individuals were more competitive than small individuals, although these differences were not found for wild populations. Churchill-Stanland et al. (Reference Churchill-Stanland, Stanland, Wong, Tanaka, McInnis and Dowell1986) found similar results in the same species, highlighting the importance of female size in mate choice, while Anjos-Duarte et al. (Reference Anjos-Duarte, Costa and Joachim-Bravo2011) indicated that large and small laboratory females preferred mating with large males. Preference for large males in C. capitata laboratory populations was also confirmed by de Aquino and Joachim-Bravo (Reference De Aquino and Joachim-Bravo2014), although this depended on the proportions of large: small males; however, wild females preferred wild males over laboratory males independent of their respective sizes. Likewise, in Bactrocera tryoni (Froggatt) younger and larger laboratory males had greater mating success than older and smaller ones (Ekanayake et al., Reference Ekanayake, Jayasundara, Peek, Clarke and Schutze2017). Benefits of a larger size has been found in A. suspensa (Loew), where the calling propensity was more frequent, and the calling song pulse train interval was significantly shorter in larger males (Burk and Webb, Reference Burk and Webb1983). In other species with a lek mating system, such as Drosophila melanogaster (Meigen), the mating success of the large male has also been reported. According to Jagadeeshan et al. (Reference Jagadeeshan, Shah, Chakrabarti and Singh2015) although the body size may not be a trait under selection by female choice, this trait likely amplifies male activity and courtship cues to influence female arousal threshold faster.
Notwithstanding evidence on the mating advantages of larger males, other studies have demonstrated the inconsistency of body size in mating success, indicating that although this feature may be related to intrasexual selection, this is not a determining factor in mate choice (Arita and Kaneshiro, Reference Arita and Kaneshiro1988; Norry et al., Reference Norry, Calcagno, Vera, Manso and Vilardi1999; Aluja et al., Reference Aluja, Pérez-Staples, Sivinski, Sánchez and Piñero2008; Niyazi et al., Reference Niyazi, Shuker and Wood2008). For example, Aluja et al. (Reference Aluja, Rull, Sivinski, Trujillo and Pérez-Staples2009) found that A. ludens and A. obliqua wild females did not discriminate effectively between males of different sizes. While recently, Tejeda et al. (Reference Tejeda, Arredondo, Díaz-Fleischer and Pérez-Staples2020) found that, while in A. obliqua large males achieved more matings than small ones, there was no difference in A. ludens. The relevance of body size in female choice remains controversial, especially given the possible variability in tephritid species. Thus, a more detailed studied on individual morphometric traits involved in mate choice is warranted.
Our objective here was to evaluate the effect of male body size on mating success in two economically important species: A. ludens and A. obliqua. In addition, we analyzed the relationship between morphometric traits and mating success for both species and differences in this relationship between mass-reared and wild populations. Initially, we planned to test wild flies from one representative host, sour orange (Citrus aurantium L.). However, at the time of the second experiment this host was not available, so we collected wild flies from matasano (Casimiroa edulis La Llave & Lex.). Because this host difference and changes in the experimental design we analyzed and report these as two independent experiments.
Materials and methods
Study insects
A. ludens and A. obliqua were derived from laboratory and wild populations. Laboratory flies of each species were obtained as pupae from mass-rearing colonies (bisexual strain) at the Moscafrut facility, located in Metapa de Dominguez, Chiapas, México (Orozco-Dávila et al., Reference Orozco-Dávila, Quintero, Hernández, Solís, Artiaga, Hernández, Ortega and Montoya2017). Wild flies were obtained as larvae from sour oranges (C. aurantium) and matasanos (Casimiroa edullis) for A. ludens, and from mangoes (Mangifera indica, cv. ‘coche’) for A. obliqua from different localities in the Soconusco region and the Comiteca-Tojolabal Plateau, in Chiapas, México.
Infested fruits were maintained in laboratory conditions (25 ± 2°C, 12:12 L:D, 65 ± 5% RH) in trays with vermiculite. When larvae reached third instar, they were extracted from the fruits and placed in containers with vermiculite at 60% humidity to promote pupation (Orozco-Dávila et al., Reference Orozco-Dávila, Quintero, Hernández, Solís, Artiaga, Hernández, Ortega and Montoya2017). Five days before adult emergence, pupae were sorted by size (see below) and placed in glass cages (30 × 30 × 30 cm) according to size. After adult emergence, flies were separated by sex and housed with 200–250 flies per cage. All flies were fed ad libitum with water, sugar and yeast hydrolysate (ICN Biomedicals, USA) in a 3:1 ratio.
Size treatments
Considering the high correlation between pupal size and adult size (McInnis, Reference McInnis1987, our own unpublished data), we used pupal diameter as the criterion to categorize the size of the males. The pupae were sorted out into ten size categories using a pupae sorting machine (FAO/IAEA/USDA, 2019). This machine consists of two horizontally positioned steel tubes that rotate in opposite directions, which are separated from each other by an adjustable distance, generating a gradually increasing gap through which the pupae pass and are separated into ten size categories according to their diameter. We established the optimal calibration (distance between tubes) of the pupae sorting machine by adjusting a mass-reared batch of ~5000 pupae to obtain a normal distribution for each species. The distance was measured with a 25 blade feeler gauge set (mm) (Powerbuilt model 648394). Most of the mass-reared pupae fell in the sixth size category, with the least number in the first and tenth categories. This distribution allowed us to establish our treatments based on size: small (lower end), control (average), and large (upper end). The defined intervals (gap between steel tubes) for the size classification of both laboratory and wild pupae of A. ludens were 1.84–2.98 and 1.72–2.76 mm for A. obliqua.
We assessed mating success of males of different size and origin: laboratory-large (LL), laboratory-small (LS), laboratory-control (LC) (average size) and wild (W). Although there were differences in the size distribution of wild and laboratory flies for both species, in all cases, the sixth category was the one with the greatest frequency (the mode) for males. In most case we used W males from this category, but when there were not enough, we used males from the fifth and seventh categories. Regarding the size categories of laboratory flies, LS were from the third and fourth, LC flies from the sixth, and LL from the seventh and eighth size categories, respectively. The size categories chosen depended on the number of males available. The extreme categories were discarded due to few pupae, low emergence (first and second) and few males (nineth and tenth) (table 1). For A. ludens, wild flies were from sour orange in the first experiment and from matasanos in the second experiment. In the second experiment, the LC treatment was removed to increase the differences between laboratory males, having only LS or LL males.
Table 1. Pupal diameter interval (mm) for each of the size treatments of each species

Mating competitiveness
Mating competitiveness tests were carried out in field cages (3 m diameter by 2 m high) with a citrus tree inside (Calkins and Webb, Reference Calkins and Webb1983). Laboratory flies were 10–14 days old, and wild flies from 17 to 20 days, all sexually mature. Two days before the test, the flies were marked with water-based paint (Vinci de Mexico S.A. de C.V., Mexico) on their thorax, using a different color for each treatment (Pérez-Staples and Aluja, Reference Pérez-Staples and Aluja2004). In each field cage, equal number of virgin males of each treatment were released and wild virgin females, in a sex ratio of two males per each female. In the first experiment with A. ludens we released 30 LL, 30 LS 30 LC and 30 W males with 60 wild females per cage. In the second experiment with A. ludens we released 20 LL, 20 LS and 20 W males with 30 wild females. In the case of A. obliqua, 30 LL, 30 LS, 30 LC and 30 W males with 60 wild females were released per cage. The variations in the number of insects per cage was determined by the number of wild insects available.
Males were released 30 min before the females to allow them to establish territories. Observations were performed during the period of sexual activity, for A. ludens: 16:00–19:00 h and for A. obliqua: 07:00–12:00 h (Aluja et al., Reference Aluja, Piñero, Jácome, Díaz-Fleischer, Sivinski, Aluja and Norrbom2000). Mating pairs were collected in vials. The treatment, mating latency (elapsed time between the release of the females and mating initiation) and copula duration were recorded. Once copulations ended, all unsuccessful males (not mated) and successful males (mated) were taken to the laboratory for morphometric measurements. For A. ludens, we evaluated seven field cages (replicates) in the first experiment and four in the second, while in A. obliqua we evaluated five field cages.
Morphometry
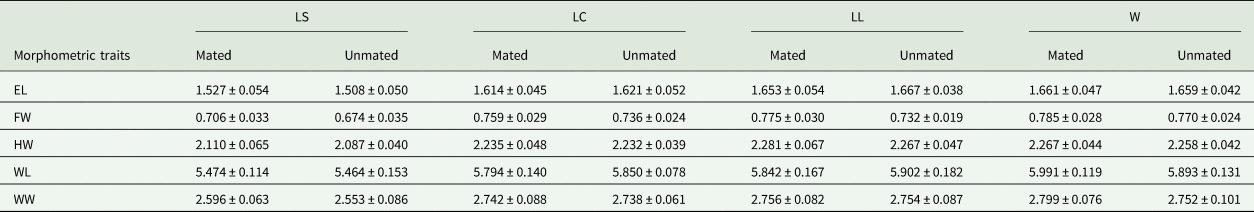
To ensure equal samples sizes for morphometric analysis, our target was to measure all mated males and a random sample of the same number of unsuccessful males. In few cases we were unable to measure all successful males because some of them were damaged. Both successful and unsuccessful males from each treatment were stored in microtubes with 70% ethanol. All flies were dissected in a Petri dish. Head, thorax, wings and legs were removed and photographed using a Zeiss Axiocam microscope (ERc 5s) (Carl Zeiss, Stemi 2000-C), following the landmarks described by Gómez-Cendra et al. (Reference Gómez-Cendra, Segura, Alberti and Vilardi2014, Reference Gómez-Cendra, Paulin, Oroño, Ovruski and Vilardi2016). In A. ludens, we measured a total of four traits in the 1st experiment: Face Width (FW), Head Width (HW), Wing Length (WL) and Wing Width (WW); while in the 2nd experiment we measured 12 traits, eight traits in addition to those considered in the first experiment, eye length (EL), thorax length (TL), fore femur (FF), fore tibia (FT), mid-femur (MF), mid-tibia (MT), hind femur (HF), hind tibia (HT). The reason for this was that we decided to increase efforts to analyze other presumably relevant traits in sexual selection. In A. obliqua, five traits were measured (EL, FW, HW, WW and WL) (fig. 1) since we found no need for the other morphometric traits. Measurements were made with AxioVision 3.6 software.
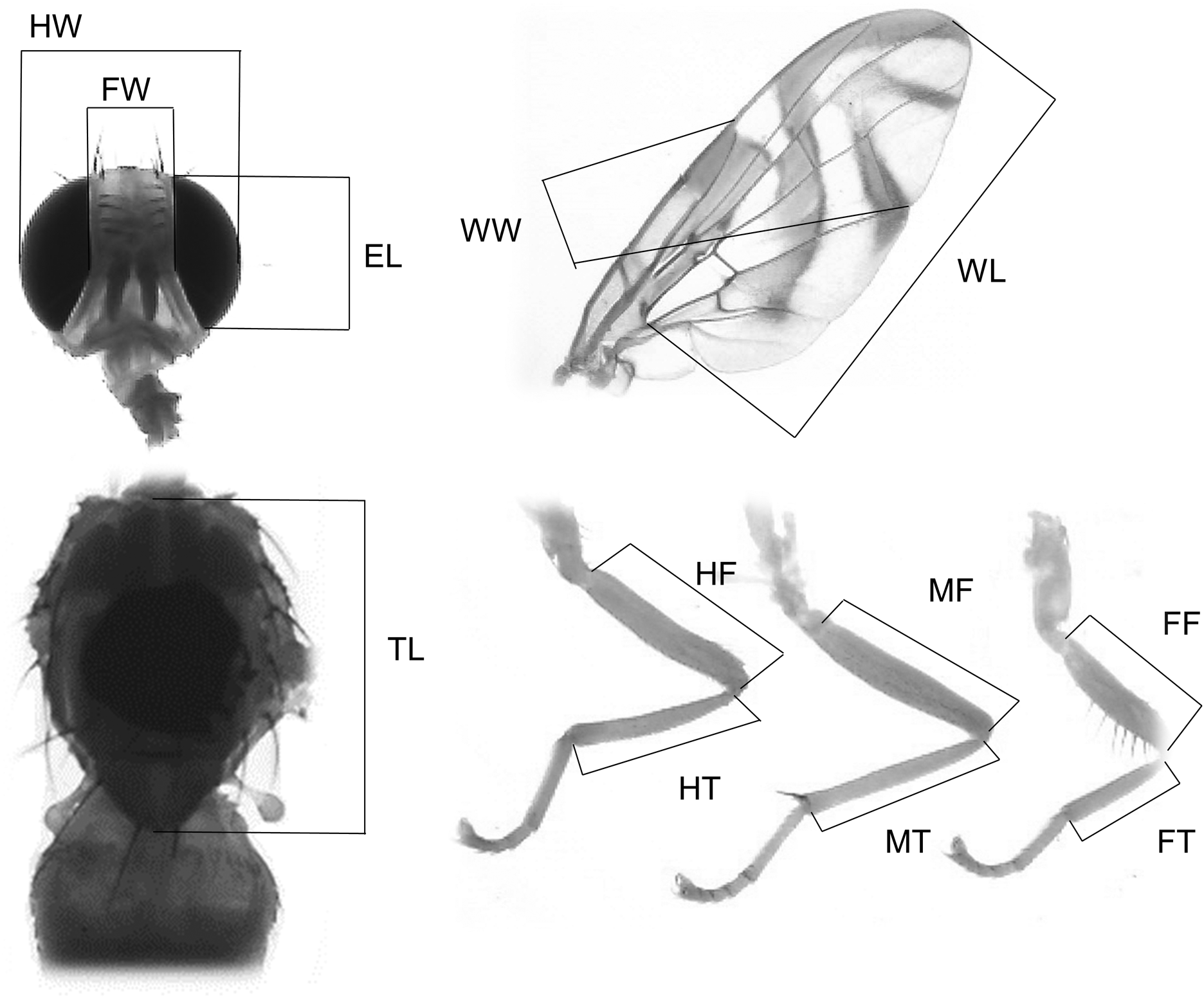
Figure 1. Representation of the morphometric traits measured in A. ludens and A. obliqua males, EL, eye length; FW, face width; HW, head width; TL, thorax length; WL, wing length; WW, wing width; FF, fore femur; FT, fore tibia; MF, mid femur; MT, mid tibia; HF, hind femur; HT: hind tibia.
Data analysis
The proportion of matings for each treatment and mating latency were analyzed by analysis of variance (ANOVA). Means were compared by Tukey' multiple mean comparison test (α = 0.05). Due to the significant interaction between size treatment × field cage, copula duration was analyzed by linear mixed-effects models (REML), where the treatments were considered fixed effects, and field cages were considered random effects.
The morphometric data (mean ± SE) of each treatment (mated and unmated) was analyzed by a factorial multivariate analysis of variance (MANOVA), with Pillai tests. Subsequently, a canonical biplot was conducted to analyze the interactions between the size treatments and the contributions of the morphometric traits on mating success of each treatment. The biplot was performed in two representative canonical dimensions (can 1 and can 2). The vector length indicates the variability of the data, while the orientation of each size treatment (confidence circle) toward a certain trait (vector) indicates the influence of the trait. The differences between the treatments are indicated by the non-intersection of the confidence circles. The missing data of the morphometric traits (due to wing damage) were estimated using the imputation method by random forests. To analyze the differences among morphometric traits and mating success (regardless of the size treatment) a canonical discriminant analysis was performed. In this analysis, the structure on the first canonical axis (can1) shows the relative importance (vector length) for each morphometric trait; where the length and direction of the vector is proportional to their contribution to explain the variability between the groups (mated and unmated). The relationship among morphometric traits measured in mated and unmated males was analyzed with a Spearman coefficient (r). The packages used were lme4 (Bates et al., Reference Bates, Maechler, Bolker and Walker2020), random forests (Liaw and Wiener, Reference Liaw and Wiener2018), car (Fox and Weisberg, Reference Fox and Weisberg2020) and candisc (Friendly and Fox, Reference Friendly and Fox2020). Analysis were carried out with Rcmdr (Fox and Bouchet-Valat, Reference Fox and Bouchet-Valat2020) using R software v.3.6.1 (R Core Team, 2020).
Results
Mating competitiveness
A. ludens
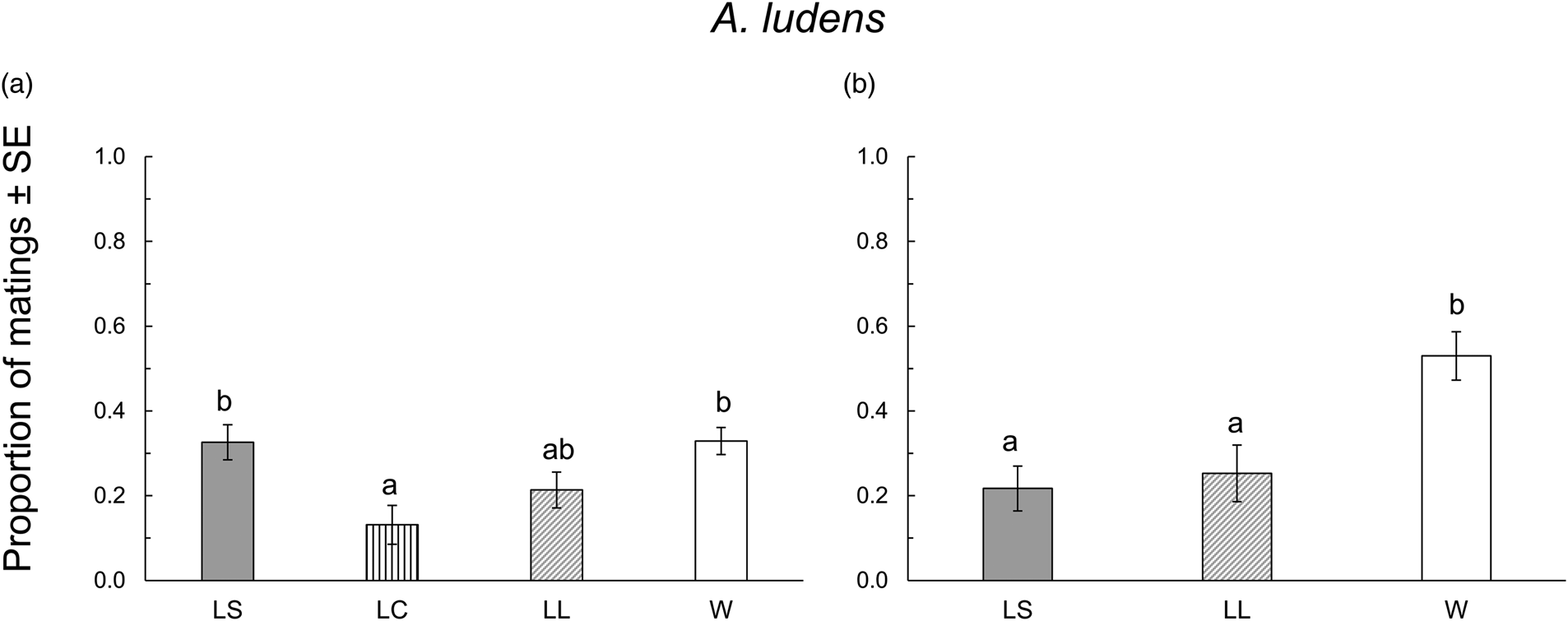
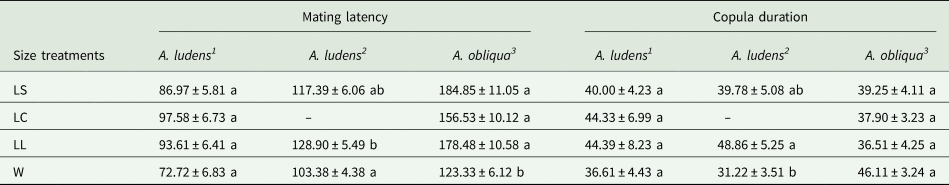
When A. ludens collected from sour oranges, we obtained a total of 89 mated males from the seven replicates. LC males showed the lowest proportion of matings, while LS and W males were the most successful, and LL males were intermediate (F 3,24 = 5.52, P = 0.004) (fig. 2a). We found no significant difference in mating latency and copula duration (F 3,85 = 2.55, P = 0.061 and F 3,78 = 0.44, P = 0.720, respectively; table 2). When A. ludens collected from matasano, we obtained 84 matings. In this test, LC treatment was not evaluated, and W males were more successful than either LS or LL males (F 2,9 = 8.36, P = 0.008) (fig. 2b). We found significant differences in both mating latency and copula duration (F 2,81 = 6.43, P = 0.002 and F 2,78 = 4.58, P = 0.013, respectively). W males showed shorter mating latency and copula duration, followed by LS and LL males. However, mean comparison tests revealed only differences between W and LL (table 2).
Figure 2. Proportion of matings of A. ludens in the 1st (a) evaluation and the 2nd (b) evaluation in field cage tests for each size treatment: LS, laboratory-small; LC, laboratory-control; LL, laboratory-large; W, wild from sour oranges (a) or from matasanos (b). Different letters indicate statistical differences. P < 0.05 (Tukey test, HSD).
Table 2. Mean (±) SE mating latency and copula duration (min) of wild female A. ludens and A. obliqua mated with different size treatments in field cage tests.
Size treatments: LS, laboratory-small; LC, laboratory-control; LL, laboratory-large, W, wild males from 1sour oranges
2 matasano
3 mangoes.
Different letters indicate statistical differences. P < 0.05 (Tukey test, HSD).
A. obliqua
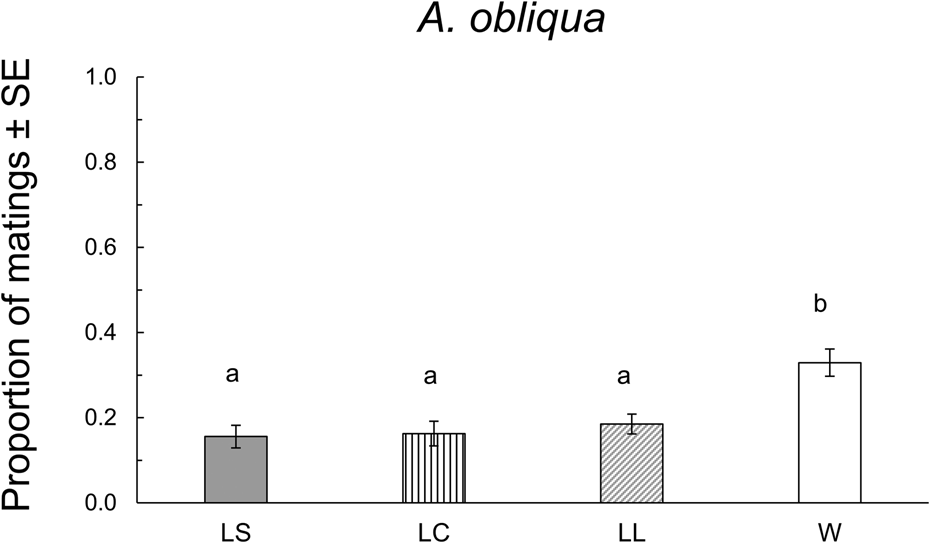
In this species, 178 matings were obtained. W males were significantly more successful than L males, regardless of their size (F 3,16 = 33.07, P < 0.0001 (fig. 3). We found significant differences in mating latency (F 3,174 = 12.35, P < 0.0001). The W males mated significantly earlier than L males. There were no significant differences in copula duration between W or L males irrespective of their size (F 3,170 = 1.69, P = 0.169) (table 2).
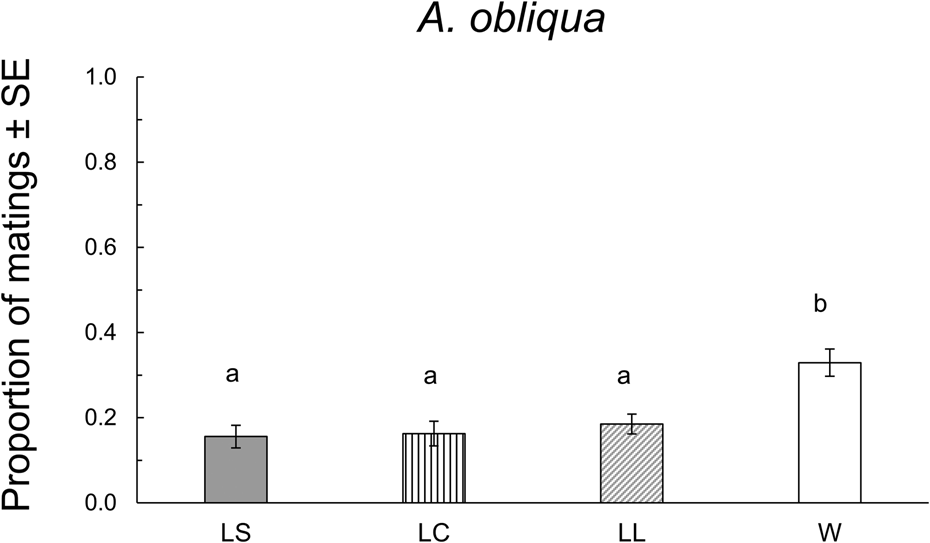
Figure 3. Proportion of matings of A. obliqua in field cage tests for each size treatment: LS: laboratory-small; LC, laboratory-control; LL, laboratory-large, W, wild. Different letters indicate statistical differences. P < 0.05 (Tukey test, HSD).
Morphometry
A. ludens
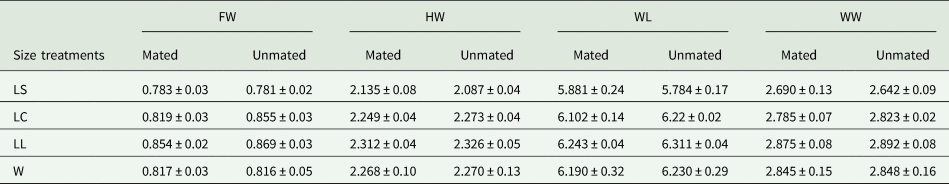
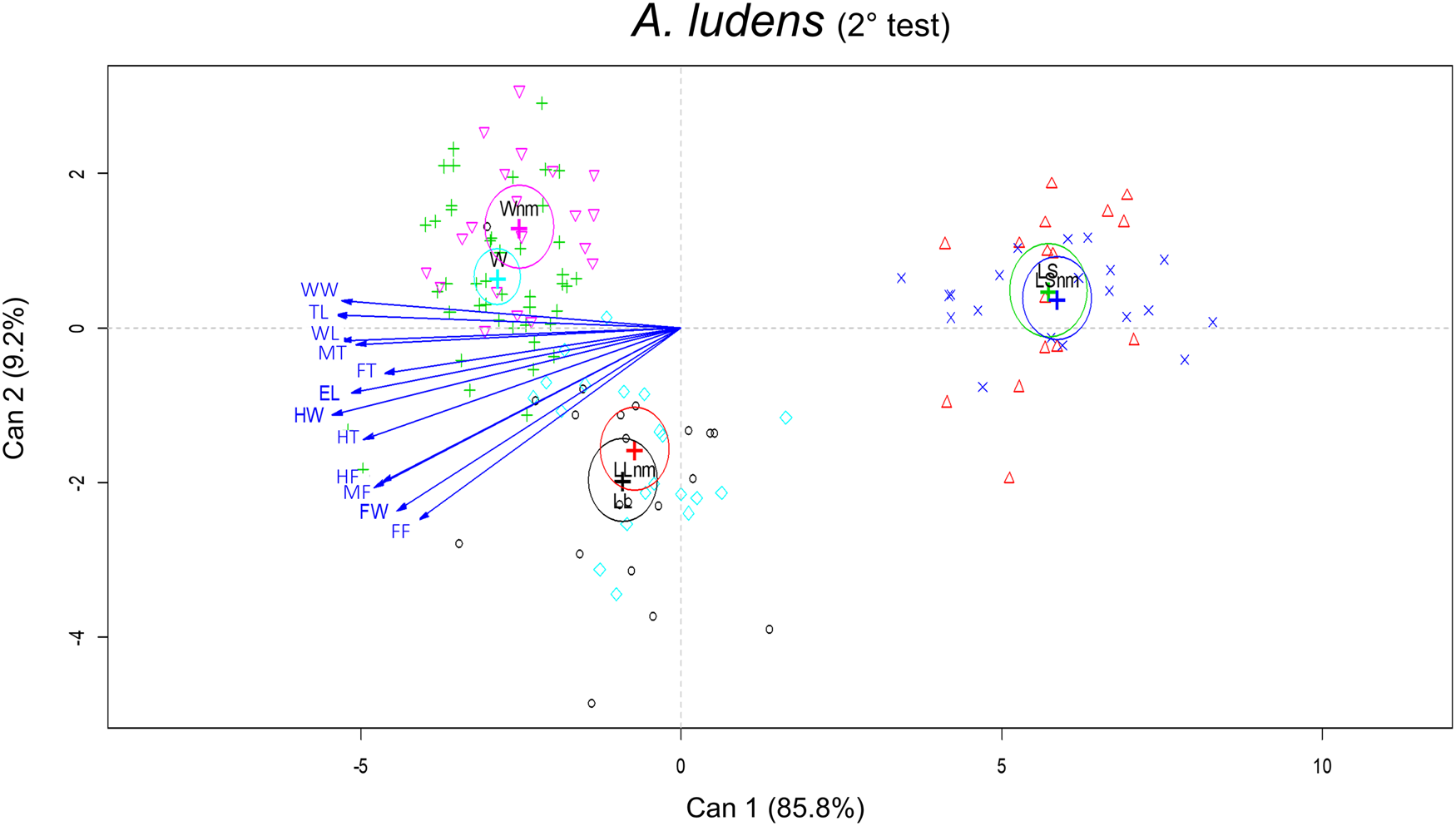
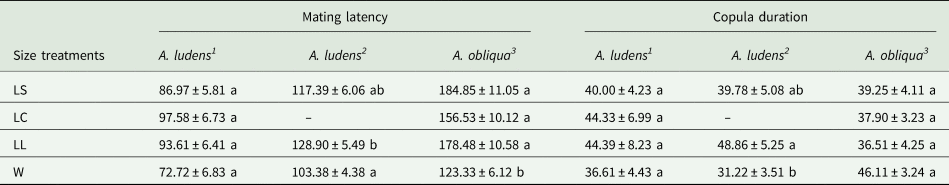
When wild A. ludens were from sour oranges, the morphometric study was carried out with a subset of 99% of mated males and 16% of non-mated males. There were significant differences in the morphometric traits between size treatments (F 12,474 = 12.81, P < 0.0001) and male mating success (mated /not mated) (F 4,156 = 4.09, P = 0.003). The interaction between male size and success was not significant (F 12,474 = 1.21, P = 0.272). According to the canonical biplot, the only significant difference observed between mated and unmated males of each treatment was in LC. LS mated and unmated males were different from all other males. We found also that LL mated and unmated and LC unmated males were different from W mated males. FW was longer in LL males compared to W males (fig. 4 and table 3). Overall, the morphometric features of the mated and unmated males indicated significant differences (F 4,162 = 4.91; P < 0.0001); according to the canonical analysis the relevant trait was FW. Mated males had wider faces than unmated ones (fig. 5 and table 3). All morphometric traits were positively and significantly correlated with each other (P < 0.0001). The most correlated variables were WL with WW and HW with WL (table 1 supplementary material).

Figure 4. Canonical biplot representing the interactions between size treatments and morphometric traits in relation to male mating success of A. ludens (1st field test). The vectors are representing morphometric the traits (mm): FW, face width; HW, head width; WL, wing length; WW, wing width. Mated and unmated (nm) males of each size treatment: LS, laboratory-small; LC, laboratory-control; LL, laboratory-large; W, wild (from sour oranges).

Figure 5. Canonical discriminant analysis for morphometric traits in mated and unmated males in A. ludens (1st field test). The vectors are representing morphometric the traits (mm): FW, face width; HW, head width; WL, wing length; WW, wing width.
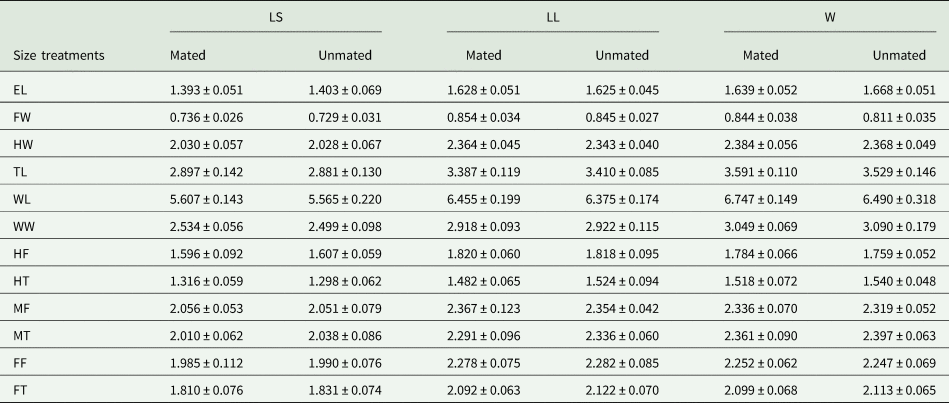
Table 3. Mean (±) SD (mm) morphometric traits of mated and unmated A. ludens males in field cages (1st test).
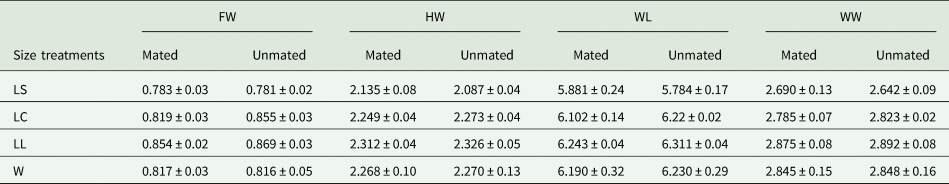
Morphometric traits: FW, face width; HW, head width; WL, wing length; WW, wing width.
Size treatments: LS, laboratory-small; LC, laboratory-control; LL, laboratory-large, W, wild males from sour oranges.
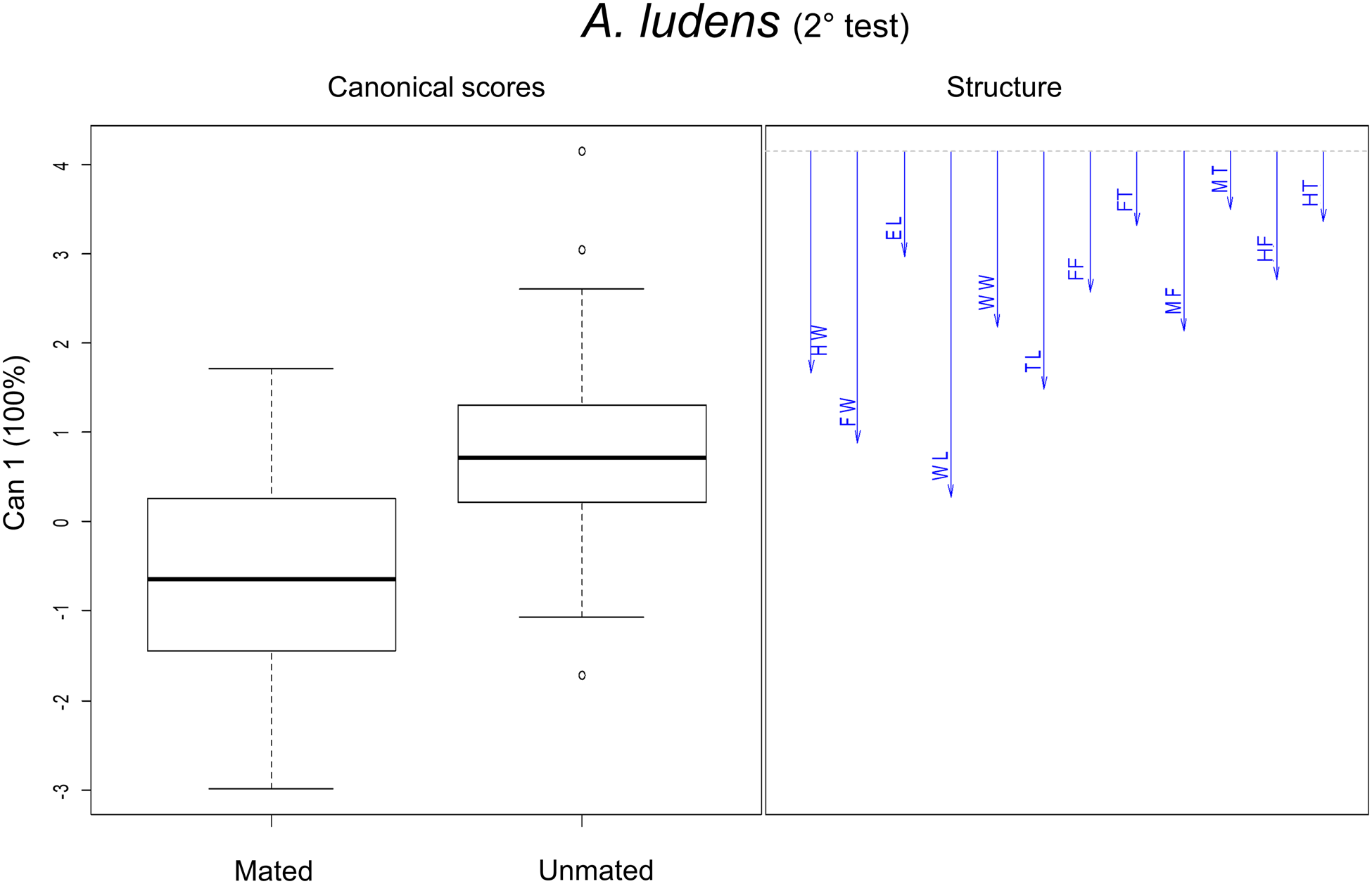
When wild A. ludens were from matasano, we analyzed 95% of mated and 30% of unmated males. There were significant differences in the morphometric traits between sizes treatments (F 24,248 = 31.19; P < 0.0001) and male mating success (F 12,123 = 4.95, P < 0.0001). The interaction between size and mating success was not significant (F 24,248 = 1.52, P = 0.058). According to the canonical biplot, the main differences between treatments were due to W mated and unmated males which were associated with TL, WL and WW traits; while LL (mated and unmated) were associated with FW and femur traits (FF, MF and HF). Differences between mated and unmated males were only found between W mated and unmated males. The W mated males had the larger measurements for FW, HW, TL and WL traits than unmated ones (fig. 6 and table 4). In the canonical analysis between mated and unmated males, regardless of the treatments, we found that mated males were larger in FW, HW, TL and WL than unmated ones (F 12,127 = 4.86, P < 0.0001, fig. 7). Spearman' correlation coefficients indicated that all traits were positively and significantly correlated with each other, with r ranging from 0.50 to 0.83 (P < 0.0001) (Table 2 supplementary material).
Figure 6. Canonical biplot representing the interactions between size treatments and morphometric traits in relation to male mating success of A. ludens (2nd field test). The vectors are representing the morphometric traits (mm): EL, eye length; FW, face width; HW, head width; TL, thorax length; WL, wing length; WW, wing width; FF, fore femur; FT, fore tibia; MF, mid femur; MT, mid tibia; HF, hind femur; HT, hind tibia. Mated and unmated (nm) of each size treatment: LS, laboratory-small; LL, laboratory-large, W, wild (from matasano).

Figure 7. Canonical discriminant analysis for morphometric traits in mated and unmated males in A. ludens (2nd field test). The vectors are representing the morphometric traits (mm): EL, eye length; FW, face width; HW, head width; TL, thorax length; WL, wing length; WW, wing width; FF, fore femur; FT, fore tibia; MF, mid femur; MT, mid tibia; HF, hind femur; HT, hind tibia.
Table 4. Mean (±) SD (mm) for morphometric traits measured for mated and unmated A. ludens males in field cages (2nd test).
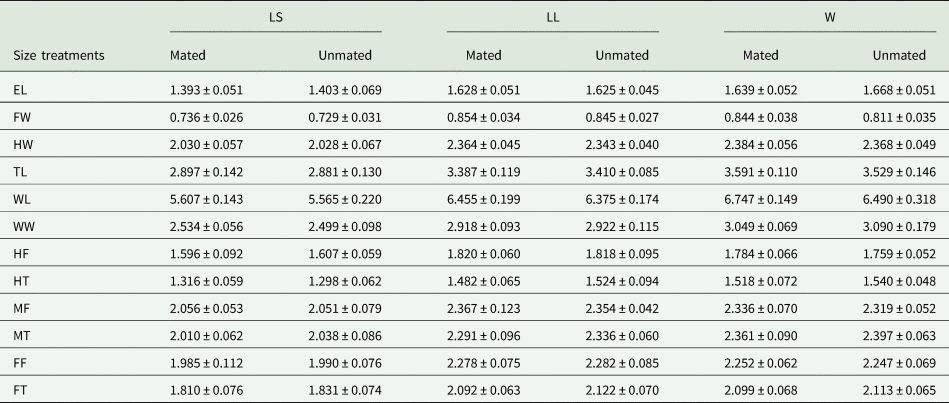
Morphometric traits: EL, eye length; FW, face width; HW, head width; TL, thorax length; WL, wing length; WW, wing width; FF, fore femur; FT, fore tibia; MF, mid femur; MT, mid tibia; HF, hind femur; HT, hind tibia.
Size treatments: LS, laboratory-small; LC, laboratory-control; LL, laboratory-large, W, wild males from matasano.
A. obliqua
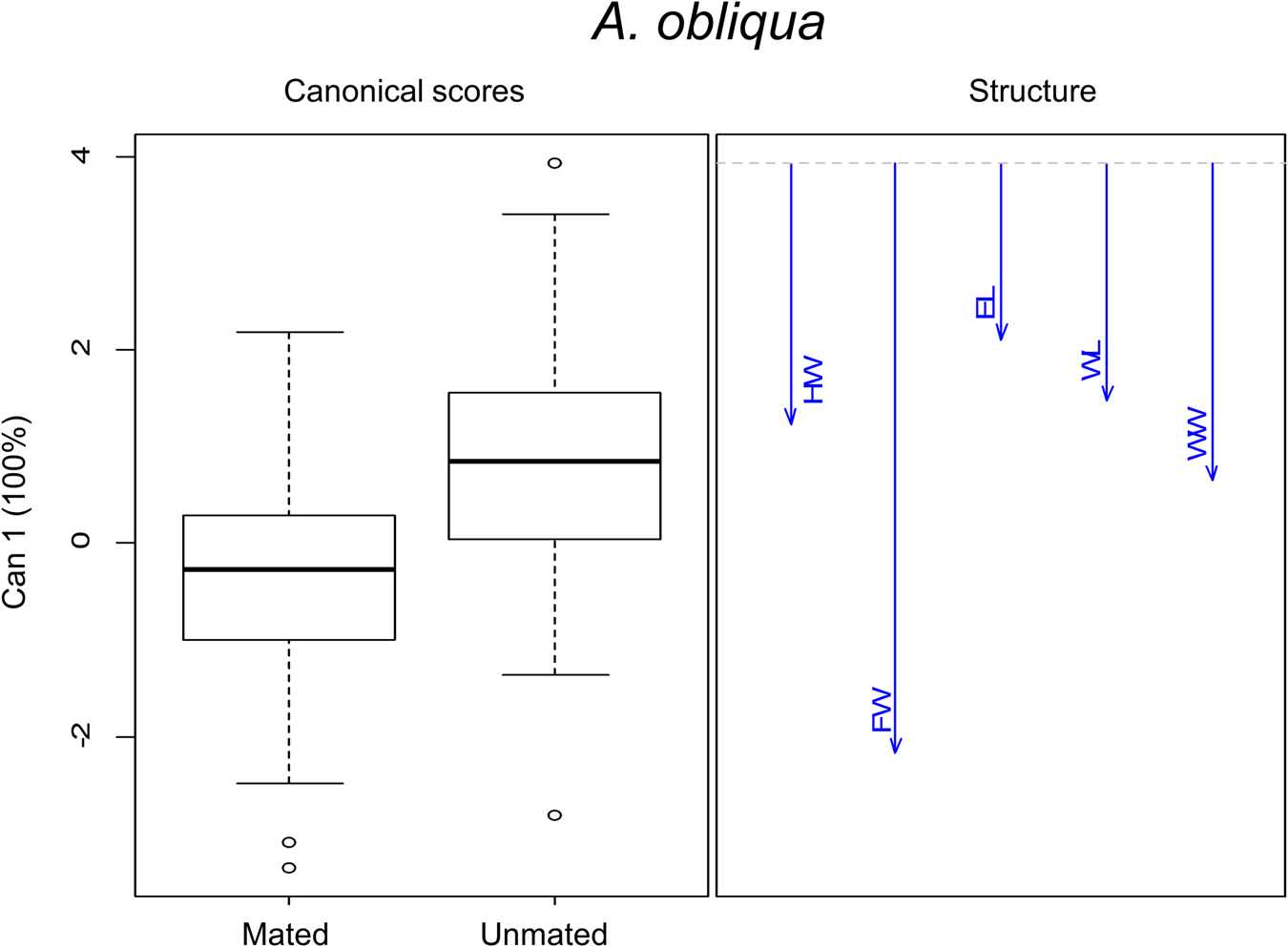
Morphometric analysis in this species was performed with 96% of mated males and 13% unmated males. There were significant differences in morphometric traits between size treatments (F 15,726 = 22.96, P < 0.0001) and male mating success (F 5,240 = 12.43, P < 0.0001). The interaction of both factors was significant (F 15,726 = 2.28, P < 0.0001). These differences are shown in the canonical biplot, where the LL and W mated males were different from each other. LL and LC mated males were different from their unmated ones. Overall, we found differences in the association to morphometric features between wild mated males and L treatments (LL and LC) mated and unmated ones. W mated males were larger in WL, WW and TL than L males (mated and unmated) (LS, LC and LL) (fig. 8 and table 5). Regardless of treatment, we found morphometric differences between mated and unmated males. According to the canonical analyses, the relevant feature was FW. Mated males had longer FW than unmated ones (fig. 9). Spearman' correlation coefficients indicated that all traits were positively and significantly correlated with each other, with r ranging from 0.54 to 0.86, (P < 0.0001) (Table 3 supplementary material).
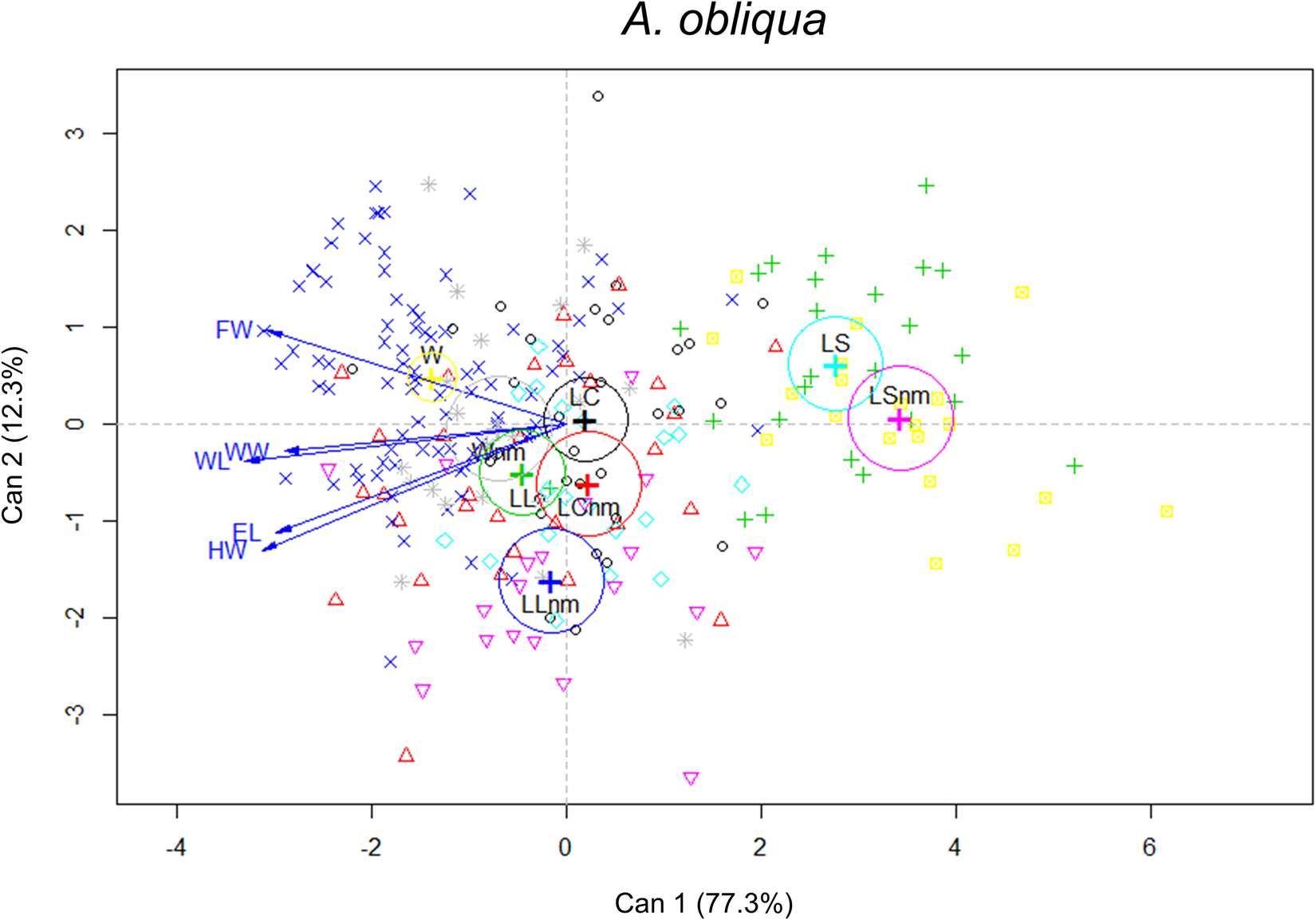
Figure 8. Canonical biplot representing the interactions between size treatments and morphometric traits in relation to male mating success of A. obliqua. The vectors are representing the morphometric traits (mm): EL, eye length; FW, face width; HW, head width; WL, wing length; WW, wing width. Mated and unmated (nm) males of each treatment: LS, laboratory-small; LC, laboratory-control; LL, laboratory-large, W, wild (from mangoes).
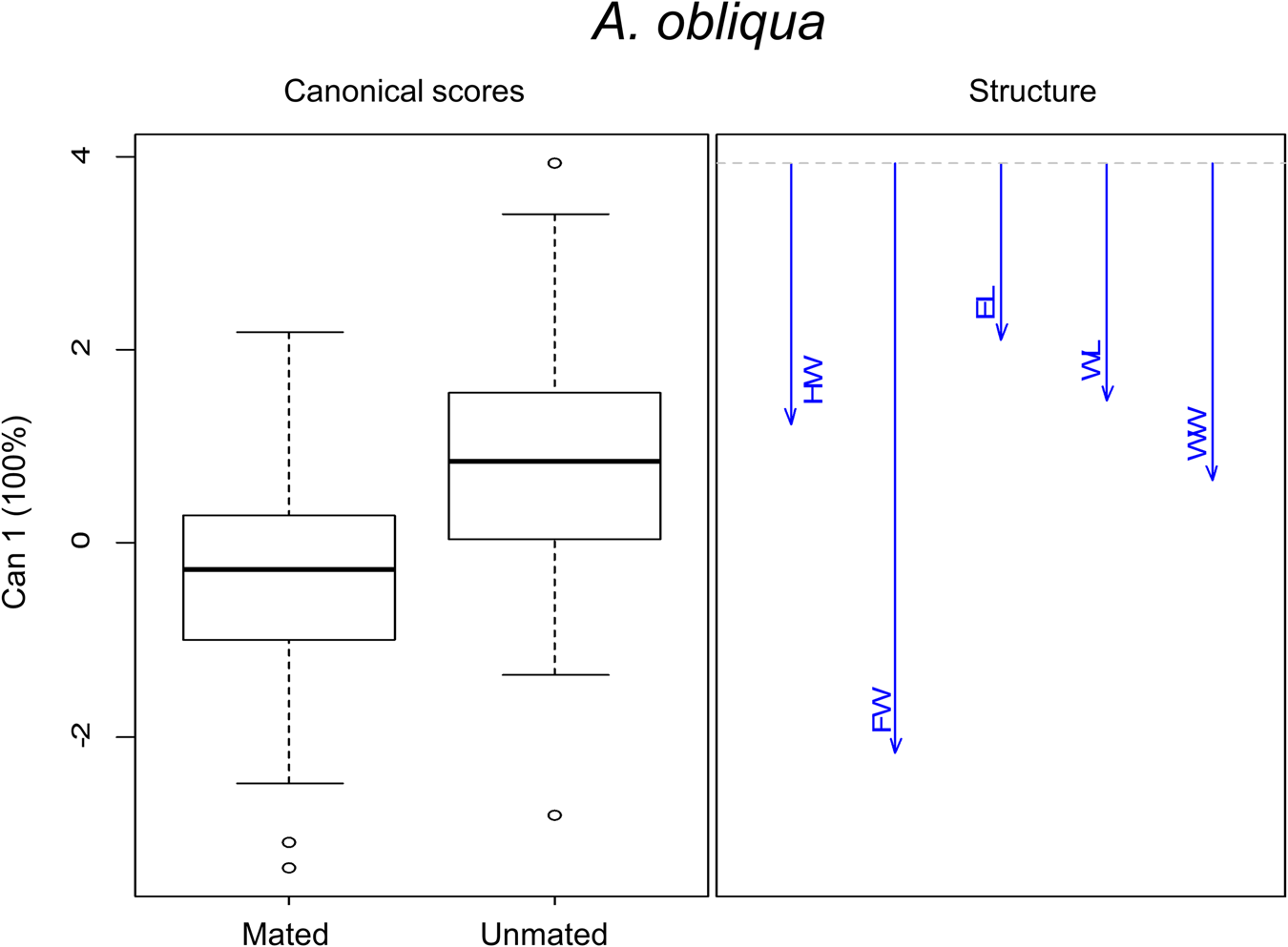
Figure 9. Canonical discriminant analysis for morphometric traits in mated and unmated males in A. obliqua. The vectors are representing the morphometric traits: EL, eye length; FW, face width; HW, head width; WL, wing length; WW, wing width.
Table 5. Mean (±) SD (mm) for morphometric traits measured on mated and unmated males of A. obliqua evaluated in field cage tests
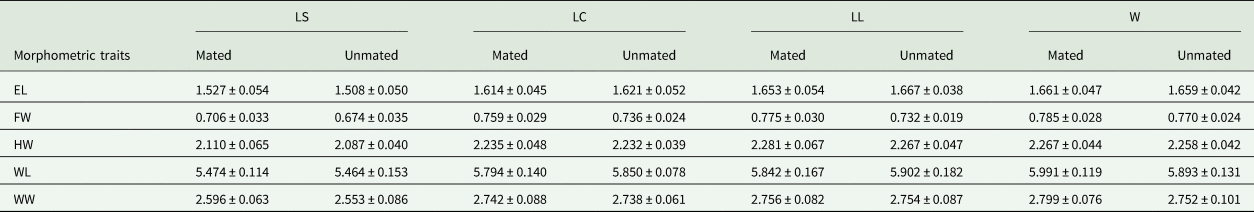
Morphometric traits: EL, eye length; FW, face width; HW, head width; WL, wing length; WW, wing width.
Size treatments: LS, laboratory-small; LC, laboratory-control; LL, laboratory-large, W, wild males from matasano.
Discussion
Here, we determined the effect of male size on mating success in two lekking species, A. ludens and A. obliqua, from mass-reared and wild in semi-natural conditions. Additionally, we evaluated several morphometric traits related with mating success, such as EL (eye length), FW (face width), HW (head width), TL (thorax length), WL (wing length), WW (wing width), FF (fore femur), FT (fore tibia), MF (mid-femur), MT (mid-tibia), HF (hind femur) and HT (hind tibia). All L males were obtained at controlled larval densities (FAO/IAEA/USDA, 2019), while in the W population, we used flies from two different hosts because limitations in their availability. For this reason and because the experimental design was slightly modified, we analyzed these two tests as independent experiments. However, the use of two different hosts for the wild flies allowed us to observe different competition scenarios that mass-reared males may face with wild males of different sizes.
Regarding mating competitiveness, we found that male overall body size did not have a determining effect on the mating success of A. ludens or A. obliqua. When A. ludens were from sour orange, LS and LL males were as competitive as W males, a result not seen with the control average sized L males. However, when males were from matasano, W females preferred to mate with W males, and no preference for LL males was found over LS ones, under the ‘bigger is better’ assumption. This confirms that for A. ludens the wild condition for the male determined mating success, while body size did not. Our results are consistent with other studies in this species and related Anastrepha species, that have shown that male size, either measured as adult thorax length or pupal size (diameter), does not determine mating success in either wild or mass-reared laboratory males (Robacker et al., Reference Robacker, Mangan, Moreno and Tarshis Moreno1991; Segura et al., Reference Segura, Petit-Marty, Sciurano, Vera, Calcagno, Allinghi, Gómez Cendra, Cladera and Vilardi2007; Aluja et al., Reference Aluja, Pérez-Staples, Sivinski, Sánchez and Piñero2008, Reference Aluja, Rull, Sivinski, Trujillo and Pérez-Staples2009; Tejeda et al., Reference Tejeda, Arredondo, Díaz-Fleischer and Pérez-Staples2020). The consistency in results on the effect of body size on male sexual performance of A. ludens, indicates that body size is not relevant to the mating success in this species.
For A. obliqua, we found similar results to those reported in A. ludens. W males were more competitive than L males regardless of body size, and no differences were found between the L males of different sizes. Sexual competitiveness of W males from mango with respect to mass-reared males was very similar to those reported by Hernández et al. (Reference Hernández, Ruíz-Montoya, Toledo, Montoya, Liedo, Aceituno-Medina and Perales2019). These results also showed that male size is not an important factor in the mating success of A. obliqua, which contrasts with what was reported by Tejeda et al. (Reference Tejeda, Arredondo, Díaz-Fleischer and Pérez-Staples2020). In their study, an effect of size on mating success was demonstrated, even among wild flies. On the other hand, Aluja et al. (Reference Aluja, Pérez-Staples, Sivinski, Sánchez and Piñero2008, Reference Aluja, Rull, Sivinski, Trujillo and Pérez-Staples2009) indicated that size was not a determining factor in the mating success of this species without ruling out the possibility that male size may be important in intrasexual selection (Burk and Webb, Reference Burk and Webb1983; Liedo et al., Reference Liedo, Carey, Celedonio and Guillen1992). Therefore, the evidence seems to indicate that size is not a consistent aspect that can determine intersexual selection per se.
The effect of male body size on mating success remains uncertain since several studies have indicated female preference for large males over small males in different species of tephritids: C. capitata (Churchill-Stanland et al., Reference Churchill-Stanland, Stanland, Wong, Tanaka, McInnis and Dowell1986; Orozco and López, Reference Orozco, López, Aluja and Liedo1993; Anjos-Duarte et al., Reference Anjos-Duarte, Costa and Joachim-Bravo2011; de Aquino and Joachim-Bravo, Reference De Aquino and Joachim-Bravo2014), B. tryoni (Ekanayake et al., Reference Ekanayake, Jayasundara, Peek, Clarke and Schutze2017), B. oleae (Rossi) (Benelli et al., Reference Benelli, Donati, Romano, Ragni, Bonsignori, Stefanini and Canale2015), A. suspensa (Burk and Webb, Reference Burk and Webb1983), and A. obliqua (Tejeda et al., Reference Tejeda, Arredondo, Díaz-Fleischer and Pérez-Staples2020). Other findings, such as those reported by Arita and Kaneshiro (Reference Arita and Kaneshiro1988), Aluja et al. (Reference Aluja, Pérez-Staples, Sivinski, Sánchez and Piñero2008, Reference Aluja, Rull, Sivinski, Trujillo and Pérez-Staples2009) and our results, point out that body size is not a factor that determines mating success, suggesting that female choice may be due to a complex interaction between morphological and behavioral traits as well as male−male agonistic encounters.
As for mating latency of A. ludens, wild males mated earlier than large laboratory males. Although this trend was observed with wild males from the two hosts, significant differences were only reported for flies from matasano. W males showed shorter latencies than L males, and there were no differences attributed to size. Variations in mating latency between wild and mass-reared populations are indicative of behavioral changes related to the environmental conditions of each population (Meza-Hernández and Díaz-Fleischer, Reference Meza-Hernández and Díaz-Fleischer2006). According to Schutze et al. (Reference Schutze, Dammalage, Jessup, Vreysen, Wornoayporn and Clarke2015), differences in mating latency between mass-reared and wild flies are associated with the behavioral response to sunset light. A. ludens is a dusk-mating species and therefore mass-rearing could certainly affect their behavior. For A. obliqua, W males had shorter mating latencies than laboratory males regardless of male size. Given that W males of A. obliqua mated 30 min earlier than L males, differences in male calling and courtship probably had a significant effect on the mating success of W males, which represents a great disadvantage for sterile males of this species.
As to copula duration, we only found differences related to male size in A. ludens from matasanos. W males had shorter copula duration than LL males. Although it has been argued that this variable is controlled by the female (Field and Yuval, Reference Field and Yuval1999; Joachim-Bravo et al., Reference Joachim-Bravo, Anjos and Costa2009; Pérez-Staples et al., Reference Pérez-Staples, Weldon, Radhakrishnan, Prenter and Taylor2010), it is also known that the interaction between the diet quality (host fruit) of the males and female size can affect copula duration (Aluja et al., Reference Aluja, Pérez-Staples, Sivinski, Sánchez and Piñero2008).
Female preference goes beyond overall body size; however, some morphometric studies in several tephritid species have allowed us to elucidate specific morphometric traits correlated with mate choice (Norry et al., Reference Norry, Calcagno, Vera, Manso and Vilardi1999; Rodriguero et al., Reference Rodriguero, Vera, Rial, Cayol and Vilardi2002a, Reference Rodriguero, Vilardi, Vera, Cayol and Rial2002b; Sciurano et al., Reference Sciurano, Segura, Rodriguero, Gómez Cendra, Allinghi, Cladera and Vilardi2007; Segura et al., Reference Segura, Petit-Marty, Sciurano, Vera, Calcagno, Allinghi, Gómez Cendra, Cladera and Vilardi2007). Here, we established male size treatments a priori and analyzed some morphometric traits in mated and unmated males. The morphometric study performed in successful and unsuccessful males of A. ludens and A. obliqua confirmed the following: (1) Size was not a factor in determining mating success; (2) The were significant differences in the morphometric traits between male size; (3) The size of the large and small laboratory males was significant different, this allowed us to be sure of having evaluated males of noticeably different sizes; and (4) Males of different origin were associated with certain morphometric traits. Because male size treatment was determined a priori, differences between the morphometric traits of different treatments were expected. However, the morphometric differences between males of different origin (L and W) were nonlinear and probably attributed to other factors. In A. ludens, W males were more closely associated with traits such as WW, WL and TL; while in LL males they were associated with FW and FF. Regarding morphometric features between the mated and unmated males, we found that the traits that contributed to these differences were FW, WL, TL and HW, these larger traits were correlated with mating success. In A. obliqua, we observed a similar pattern. LS males (mated and unmated) were different with the rest of the treatments. We found differences between mating success (mated and unmated) of W and LL males. Both L and W mated males were larger in FW and WL than unmated males, respectively; therefore, morphometric variations between treatments also appear to be non-linear. Morphometric variations between wild and laboratory males are not related to size. In A. obliqua, we found that face width (FW), wing length and width (WL, WW) and head width (HW) were related with the mating success regardless the overall size of males.
In general, our results indicate morphometric variation among males of different origin. Similar to our results, A. fraterculus (Wiedemann) laboratory males had a significantly larger HW and EL and smaller WW than wild males (Gómez-Cendra et al., Reference Gómez-Cendra, Segura, Alberti and Vilardi2014). The arguments for these morphological differences maintain that adaptation to laboratory conditions, where short-distance interactions are common, can favor through sexual selection, the increase in head and face features contrary to the features of the wings due to the short flight distances. Therefore, a scenario under natural conditions, where flight is important, would explain the associated traits in a wild population. Since wings movement is important in fruit flies courtship behavior (Roriz et al., Reference Roriz, Japyassú and Joachim-Bravo2018, Reference Roriz, Japyassú, Cáceres, Teresa and Joachim-Bravo2019) the differences in the measurements of the wings can explain the lower competitiveness of laboratory males compared to wild ones.
Morphological changes under a context of sexual selection can occur when a trait confers a direct advantage on sexual competence. Positive allometry can evolve resulting in stronger directional selection in trait size than body size when there is a combined effect of sexual and viability selection on trait size (Bonduriansky & Day, Reference Bonduriansky and Day2003). Here we determined for both species, morphometric traits that are possibly targeted by sexual selection. Even though male size was not relevant in terms of the number of matings obtained, we found an association between specific traits such as FW, HW, TL, WW, and WL with mating success. In A. ludens, we found contrasting results in the field tests. FW was significant in both evaluations. When they emerged from sour oranges it was negatively correlated with mating success, while when they were from matasanos, FW, HW, TL and WL were positively related to male mating success. In A. obliqua, we also found a relationship between FW, WL, WW and HW, and mating success. Likewise in A. fraterculus, WW, TL, and FL were target features of sexual selection (Sciurano et al., Reference Sciurano, Segura, Rodriguero, Gómez Cendra, Allinghi, Cladera and Vilardi2007), while in C. capitata FW, TL, and EL were found to be important in mating success (Rodriguero et al., Reference Rodriguero, Vera, Rial, Cayol and Vilardi2002a). Overall these results suggest that although certain morphometric traits, such as EL, were not significant across species, other traits such as FW and TL are more consistent predictors of mating success. In summary, the effect of body size or morphometric traits associated with intrasexual and/or intersexual selection, constitutes a relevant factor in the sexual performance of fruit flies. We found that overall size was not a good predictor of male mating success, although specific traits, such as FW, TL and WL, seemed to be associated factors in male mating success across species and populations. However, in a lek-polygamous mating system, where the interactions are complex, female criteria for mate choice can depend on male courtship skills (acoustic or chemical), so female choice goes beyond the specific phenotypic trait of size. For example, Segura et al. (Reference Segura, Petit-Marty, Sciurano, Vera, Calcagno, Allinghi, Gómez Cendra, Cladera and Vilardi2007) found that the mating success of A. fraterculus is related to the calling activity, but body size was not related to this key behavior. A deep understanding of the morphometric traits involved in intersexual selection can contribute to optimize mass-rearing processes for SIT, particularly strategies to mitigate the adverse effects of selective pressures that impact not only on male behavior but possibly on phenotypic traits that are relevant in mate choice. In addition, our results enrich current knowledge on the effect of male body size and sexual selection in complex lek-polygamy mating systems.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485321000833.
Acknowledgements
We specially thank to Oscar Carmona for his technical support. Special thanks to Dina Orozco, Yeudiel Gómez, Emilio Hernández, José Arredondo, Marco Tejeda, Patricia López and the technical staff of the technological validation area of MOSCAFRUT (SAGARPA-SENASICA) mass-rearing facilities. This study was funded by project SEP-CONACYT 26004 and IAEA Research Contract No: 22676 and was carried out as a partial requirement for MSR' doctoral thesis. MSR also thanks CONACyT for scholarship support.