Introduction
The preference-performance hypothesis (PPH) postulates that host range is mainly modulated by the ability of phytophagous insects, to recognize host quality (Jaenike, Reference Jaenike1978). As insect larvae have few opportunities to leave the host plant, adult females need to select an oviposition resource that secures high levels of offspring survival and suitable conditions for the development of reproductively competitive offspring (Thompson, Reference Thompson1988; Singer et al., Reference Singer, Rodrigues, Stireman and Carriere2004; Wetzel & Strong, Reference Wetzel and Strong2015; Wetzel et al., Reference Wetzel, Kharouba, Robinson, Holyoak and Karban2016). Both traits have been shown to evolve because of their joint evolution (Craig & Itami, Reference Craig, Itami and Tilmon2008) and are supposed to be modulated by an insect's ability to overcome plant defences (Dethier, Reference Dethier1954; Ehrlich & Raven, Reference Ehrlich and Raven1964), avoid natural enemies (Singer et al., Reference Singer, Rodrigues, Stireman and Carriere2004), avoid intra- or interspecific competition (Duyck et al., Reference Duyck, David, Junod, Brunel, Dupont and Quilici2006), and also as a result of an increased ability to locate hosts (Bernays, Reference Bernays2001).
The association between female preference and offspring performance seems to be highly correlated with diet specialization (Gripenberg et al., Reference Gripenberg, Mayhew, Parnell and Roslin2010; Hafsi et al., Reference Hafsi, Facon, Ravigné, Chiroleu, Quilici, Chermiti and Duyck2016). Specialists will mostly prefer hosts that maximize offspring fitness (Jaenike, Reference Jaenike1978, Reference Jaenike1990; Gripenberg et al., Reference Gripenberg, Mayhew, Parnell and Roslin2010; Clark et al., Reference Clark, Hartley and Johnson2011). In contrast, polyphagous species or generalists almost always show a hierarchical preference across the wide array of hosts used, which also differ in quality (i.e., variance in nutrients and plant defensive traits). Preference-performance correlations, in this case, will depend on the taxonomic diversity of host plants among the plant families used (Clark et al., Reference Clark, Hartley and Johnson2011; Clarke, Reference Clarke2016). Correlations between polyphagous female oviposition preference and larval performance appear to be less adjusted across and within plant families (Clark et al., Reference Clark, Hartley and Johnson2011; Clarke, Reference Clarke2016) than among genotypes in a single plant species (Balagawi et al., Reference Balagawi, Vijaysegaran, Drew and Raghu2005; Papachristos & Papadopoulos, Reference Papachristos and Papadopoulos2009; Rattanapun et al., Reference Rattanapun, Amornsak and Clarke2010; Muthuthantri & Clarke, Reference Muthuthantri and Clarke2012; Aluja et al., Reference Aluja, Birke, Ceymann, Guillén, Arrigoni, Baumgartner, Pascacio and Samietz2014a, Reference Aluja, Arredondo, Díaz-Fleischer, Birke, Rull, Niogret and Epskyb). Indeed, it seems that as closely related plant species have similar nutritional compounds and similar types of secondary metabolites and volatiles, polyphagous preference-performance outputs will be better correlated for a closely related group of plants than for taxonomically distant plants (Clark et al., Reference Clark, Hartley and Johnson2011; Loxdale et al., Reference Loxdale, Lushai and Harvey2011; Clarke, Reference Clarke2016; Cunningham et al., Reference Cunningham, Carlsson, Tommaso, Dekker and Clarke2016). Mismatches in oviposition preference and offspring performance of tephritid fruit flies suggest that other ecological, physiological, and behavioural factors may influence host-use patterns (Balagawi et al., Reference Balagawi, Drew and Clarke2013; Birke et al., Reference Birke, Acosta and Aluja2015). The latter, because acquiring a general-purpose-enzyme-system or having a diverse gut microbiota that can efficiently digest primary metabolites, or overcome all secondary metabolites or defence mechanisms, is highly unlikely (Behar et al., Reference Behar, Jurkevitch and Yuval2008; Loxdale et al., Reference Loxdale, Lushai and Harvey2011).
Host availability and host abundance, predation, and natural enemies (i.e., parasitoids) (Aluja & Mangan, Reference Aluja and Mangan2008), and female ability to select better oviposition resources among fruits and fruit parts (Tania et al., Reference Tania, Brandalha and Zucoloto2004; Rattanapun et al., Reference Rattanapun, Amornsak and Clarke2009) have also been shown to shape tephritid preference-performance associations. Additionally, sub-optimal ovipositional preferences in other insect taxa have been attributed to the limited ability of polyphagous insects to store environmental information compared with specialists (i.e., monophagous, oligophagous or stenophagous species), which are well adapted to a set of specific host cues (Bernays & Funk, Reference Bernays and Funk1999; Bernays, Reference Bernays2001; Bernays et al., Reference Bernays, Singer and Rodrigues2004; Egan & Funk, Reference Egan and Funk2006).
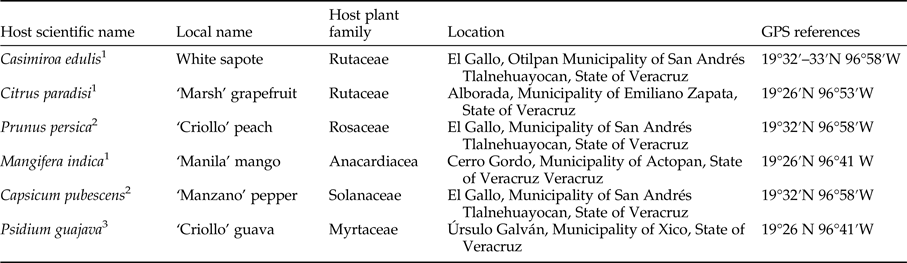
The Mexican Fruit Fly (Mexfly), Anastrepha ludens (Loew), is considered a polyphagous insect endemic to Mexico and a potential invader of temperate areas under climate change scenarios (Birke et al., Reference Birke, Guillén, Midgarden, Aluja and Peña2013; Aluja et al., Reference Aluja, Birke, Ceymann, Guillén, Arrigoni, Baumgartner, Pascacio and Samietz2014a). Mexfly preferentially uses mature-green or turning-yellow host fruit of 38 plant species belonging to 13 families (Thomas, Reference Thomas2004; Birke et al., Reference Birke, Guillén, Midgarden, Aluja and Peña2013). Mexfly larvae normally develop in the pulp of fruits, but due to their long ovipositor, flies can also lay their eggs in seeds of small wild fruits like yellow chapote (Casimiroa greggii [S. Watson] F. Chiang, Rutaceae), a native host endemic to the Sierra Madre of northeastern Mexico (Plummer et al., Reference Plummer, McPhail and Monk1941; Thomas, Reference Thomas2012). The natural habitat of this species includes subtropical to temperate transition areas, in which A. ludens encounter hosts within more than ten families that fruit simultaneously (Birke & Aluja, Reference Birke and Aluja2011). Under these circumstances, A. ludens females choose among a wide array of fruits with different degrees of suitability for offspring development. Infestation records show that fruit in the Rutaceae family are the most preferred, and among these, the native host Casimiroa edulis (La Llave & Lex) (white sapote) and exotic citrus fruit, mainly wild sweet oranges (Citrus sinensis Osbeck), sour oranges (Citrus aurantium L.), and grapefruit (Citrus paradisi Macfad.), are the most abundant (Aluja et al., Reference Aluja, Piñero, López, Ruíz, Zúñiga, Piedra, Díaz-Fleischer and Sivinski2000a). Based on infestation records, species preference in the Anacardiaceae, Rosaceae, and Solanaceae families are ranked as follows: mango (Mangifera indica L.), peach (Prunus persica (L.) Batsch), and ‘Manzano’ pepper (Capsicum pubescens Dunal) (Aluja et al., Reference Aluja, Piñero, López, Ruíz, Zúñiga, Piedra, Díaz-Fleischer and Sivinski2000a; Norrbom, Reference Norrbom2003; Thomas, Reference Thomas2004). A highly abundant non-host in Veracruz, Mexico or conditional host (sensu Aluja & Mangan, Reference Aluja and Mangan2008) is guava (Psidium guajava L.), a perennial tree distributed from the coast to the highlands (Padilla, Reference Padilla, González, Padilla, Reyes, Perales and Esquivel2003). Natural infestations of guava by A. ludens have never been recorded, and it is likely that both behavioural constraints and fruit suitability have modulated this non-association (Birke & Aluja, Reference Birke and Aluja2011; Birke et al., Reference Birke, Acosta and Aluja2015). Important to our aims here, although A. ludens larvae can develop in a wide array of fruit, guava represents a true limit to its host range (Aluja & Mangan, Reference Aluja and Mangan2008; Birke et al., Reference Birke, Acosta and Aluja2015).
We designed two experiments to ascertain whether host preference of A. ludens in nature is related to offspring performance following the PPH in terms of female use of high-quality hosts that enhance the survival of offspring, or follows the information-processing hypothesis (IPH) in terms of the polyphagous female's inability to process a variable array of host cues. First, PPH was tested under natural conditions by exposing fruit across five families to ovipositing A. ludens females and analyzing (i) the effect of host plant suitability on host preference, and fruit physical and chemical features on (ii) offspring performance (fitness correlates) extended to F1 fecundity, fertility, and longevity to determine if plant suitability hinders offspring reproduction. Then, IPH was tested by contrasting accuracy and speed in foraging for a host between the polyphagous Anastrepha ludens and the oligophagous Anastrepha striata. If the PPH was true for our study system, we would predict that females’ preference would correlate positively with offspring performance, particularly for closely related hosts. Should the IPH apply, we predicted that a lack of accuracy or mismatches were a result of neuronal constraints in A. ludens that would hinder females from ‘correctly’ or ‘efficiently’ choosing the preferred host (Bernays & Funk, Reference Bernays and Funk1999; Egan & Funk, Reference Egan and Funk2006; Clarke, Reference Clarke2016).
Materials and methods
Insects
A. ludens pupae stemmed from a semi-wild laboratory colony originally collected from field-infested Citrus aurantium (sour orange) in Miradores and Alborada, Veracruz (F4, F12–14), following methods outlined in Birke et al. (Reference Birke, Acosta and Aluja2015). A. striata pupae stemmed from the first generation of laboratory-reared flies collected from field-infested guava located in Jamapa, Veracruz, Mexico. Adult flies were kept in Plexiglas cages (30 × 30 × 30 cm), and environmental conditions were maintained at 26 ± 1°C, 60 ± 5% relative humidity (RH), and 12:12 h (L:D) photoperiod with light provided by 36 Watt Philips® daylight bulbs. Food (a mixture of 3:1 sugar: enzymatic protein hydrolysate) and water were offered ad libitum. Once females had mated (ca. 15 days), they were offered natural hosts as an oviposition substrate for larval development (i.e., grapefruit [A. ludens] or guava [A. striata]).
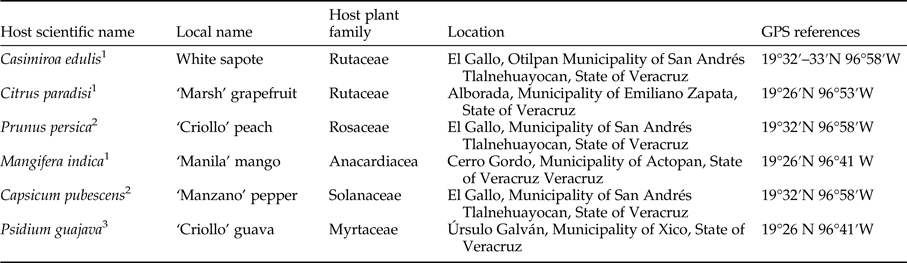
Forced infestations were performed in natural environments as this approach is the most precise way to determine host status and host resistance to tephritid attack (Aluja & Mangan, Reference Aluja and Mangan2008). Trials were performed from May to November at sites close to Xalapa where A. ludens and host plants naturally occur (table 1).
Table 1. Host scientific name, local name, host plant family, location, and global positioning system (GPS) references of host fruit species evaluated to the forced exposure of Anastrepha ludens females under semi-natural conditions.
1 natural preferred host.
2 marginal host.
3 conditional (artificial) host (Aluja & Mangan, Reference Aluja and Mangan2008).
Fruit characteristics
Ten fruit per host species were selected to measure weight, size, firmness, and sugar content. Fruit firmness measured as rind resistance to puncture (Newtons), was determined using a Penetrometer with a 1 mm flat-tip metal probe (four equatorial punctures per fruit) connected to a force gauge (Accuforce gauge III, model AF3010CE, Ametek, Mansfield & Green Division, Largo, FL) on a motorized test stand (model 4665, Ametek, Mansfield & Green Division, Largo, FL).
The fruit was weighed using a standard electronic digital precision balance (Sartorius, CP64). Fruit size was determined by measuring fruit length and height using a Vernier caliper (dialMax®, Mexico City). Sugar content was measured from a drop of fruit juice using a hand-held refractometer (ATAGO Mod. 34Z US).
Host nutritional content
Samples of 400 g of each host species at the most preferred maturity stage (green-yellowing mature fruits) for A. ludens females were subjected to bromatological analyses at a certified analytical laboratory (‘Laboratorios de Alta Tecnología de Xalapa, S.C. – LATEX’), in Xalapa, Veracruz, Mexico. Protein content was determined by the Kjeldahl method, lipids by Soxhlet extractor and total carbohydrates were calculated as nitrogen-free extract following Mexican government approved guidelines NOM-051-SCFI-1994 1994 (AOAC, 1975).
Experiment 1. PPH models: assessment of preference-offspring performance of A. ludens
Host plant species
Hosts used were catalogued as preferred hosts (white sapote, ‘Marsh’ grapefruit and ‘Manila’ mango), marginal hosts (‘Criollo’ peach and ‘Manzano’ pepper), and conditional hosts (guava) (sensu Aluja & Mangan, Reference Aluja and Mangan2008). These categories were based on our annual local sampling records across different taxonomic families that varied in fruit physical and chemical characteristics (Birke & Aluja, Reference Birke and Aluja2011). We also selected host species that were fruiting almost simultaneously to render our experimental approach more robust (Birke & Aluja, Reference Birke and Aluja2011).
Experimental design
Fruit from each of five trees (replicates) for each of six host plant species (treatments) was randomly selected in localities near of Xalapa, Veracruz (see table 1).
Forced infestations followed the protocol of Aluja & Mangan (Reference Aluja and Mangan2008). We selected one–two branches harbouring five or ten fruits for each of our five trees. In these trials fly density was adjusted according to host weight and host size to avoid a density effect on offspring performance. We used a 1:1 fruit: female ratio for small fruit (white sapote, peach, pepper, and guava ranging in weight from 25 to 50 g) (six fruits-six females), a 1:2 ratio for mid-sized fruit (mango 260 ± 30 g) (three fruits-six females), and a 1:3 ratio for large fruit (grapefruit 430 ± 30 g) (two fruits-six females). Fruit was exposed to gravid females (15–20 d old-females) for a 48-h period in field cages. Flies were then recaptured and placed in a 70% alcohol solution.
Experiment 1.1 measurement of female host preference
Female oviposition preference was assessed regarding oviposition response (clutch size and proportion of exposed infested fruit) following Aluja et al. (Reference Aluja, Arredondo, Díaz-Fleischer, Birke, Rull, Niogret and Epsky2014b). One fruit of each branch, replicated five times for each treatment, was harvested to determine the number of clutches and number of eggs per clutch. The fruit was taken to the laboratory, then peeled and sliced. Oxidized points in fruits were dissected under a Zeiss stereomicroscope (SMDSZ) to locate eggs and record the number of egg clutches per fruit.
Experiment 1.2 effect of maternal host choice on offspring performance
Measurement of host effect on infestation level
Fruit was collected from the tree over a 10–15-d period from the initial moment of female release. We note that the A. ludens egg-pupae development time varies depending on the host fruit and can range from 10 to over 40 days under laboratory conditions (Leyva et al., Reference Leyva, Browning and Gilstrap1991; Birke et al., Reference Birke, Guillén, Midgarden, Aluja and Peña2013). All remaining fruit was removed after a 15-d period (mostly all fruits were collected and maintained in separate individual plastic containers with moist vermiculite [SUNGRO®, USA]). The fruit was maintained until it completely decomposed, and larvae and pupae were recovered daily to assess egg to pupae developmental time. Finally, fruit was dissected to ascertain if any larvae had pupated within the fruit or died. Infestation level was assessed as pupae/fruit or pupae/g fruit. Pupal weight was determined individually 4 days after pupation by using an analytical balance (Sartorius CP64), hollow pupae were discarded, and adult emergence was recorded daily to determine pupae to adult development time.
Measurement of host effect on F1 fitness correlates
Fitness reproductive correlates were determined by selecting randomly 15 pairs (female and male) of adults stemming from each host species. Individual pairs were placed in a ½ liter plastic container with water and food provided ad libitum as described above. After a 15-d period, once mating had occurred and A. ludens females began to lay eggs (following the protocol of Jácome et al., Reference Jácome, Aluja and Liedo1999), fecundity (number of eggs laid every day) and fertility (hatched eggs) was determined by placing daily an artificial oviposition device in each plastic container. The egg collection devices (3.5 cm-diameter agar spheres) were prepared with Bacteriological Agar (BD Bioxon™, Becton Dickinson de México, Cuautitlán Izcalli, Edo. de Mex., Mexico) and dyed with green food color (McCormick-Hérdez, Mexico) following Jácome et al. (Reference Jácome, Aluja and Liedo1999). Spheres were wrapped in Parafilm™ (American National Can/Tm, Greenwich, Connecticut, USA) and offered to the flies for periods of 24 h, then removed for dissection (egg harvest and count), and replaced with new ones. Egg hatch was measured each day by placing a sample of 30 eggs recovered during sphere dissection in an incubation chamber (a Petri dish with the bottom filled with cotton moistened with a 1% borax solution and covered with a round piece of black cloth to facilitate observation of the eggs). The number of hatched eggs was recorded after 4 days using a stereomicroscope (Nikon SZM). Fecundity (number of eggs laid per day per female) and egg hatch were recorded for a 15-d period, after which flies remained in cages until death to assess longevity.
Experiment 2. IPH: accuracy of host-selection under field cage conditions
The IPH was tested at a cohort-level with individuals of the highly polyphagous A. ludens and oligophagous A. striata regarding host use specificity, efficiency, and accuracy following Bernays & Funk (Reference Bernays and Funk1999).
Cohorts of 25 A. ludens and A. striata females were released in a very large field cage that covered one small-sized grapefruit and one guava mid-sized tree (host and non-host, respectively). Variables measured were total visits (which included repeat visits to a fruit that were recorded separately) and numbers of the visited fruit of each type, the number of oviposition in a 6-h period in its host, and the percentage of errors in ovipositing a non-host. The trees were 2 m apart from each other and were enclosed by a 13 m × 7 m high × 7 m width field-cage. A total of 125, fully developed and physiologically mature fruit, were chosen on each tree; remaining fruit was removed (Birke et al., Reference Birke, Acosta and Aluja2015). Selected fruit was covered with white paper bags (Kraft de México, S.A., Mexico City) (Aluja & Mangan, Reference Aluja and Mangan2008). On each observation day (5-d observation period), 25 bags per tree (50 total) were removed and 50 gravid females (25 A. striata and 25 A. ludens females [1:1 female per fruit per fly species]) were released in the field-cage. To identify females and capture them at the end of the observation day, flies were marked on the pronotum using different colours of acrylic paint for each observation day (Vinci®, Grupo Dixo, S.A. de C.V., Mexico City) (Aluja et al., Reference Aluja, Jácome and Macías-Ordoñez2001).
Observations took place from 09:00 to 16:00 h, which covered the peak of oviposition activity of both species (Aluja et al., Reference Aluja, Piñero, Jácome, Díaz-Fleischer, Sivinski, Aluja and Norrbom2000b). To test the IPH, two observers at each tree recorded the following parameters using a scan sampling observation method (Opp & Prokopy, Reference Opp, Prokopy, Miller and Miller1986): (a) female choosiness by recording fly visits (landing on host or non-host/conditional hosts), oviposition attempts (host or non-host/conditional hosts), oviposition attempt duration, successful ovipositions followed by ovipositor dragging on host and non-host/conditional host, and oviposition time, (b) female accuracy, by counting wrong ‘decisions’ (landings on a non-host/conditional host), (c) female efficiency, by counting number of landings and ovipositions in a 6-h period. After the 6-h observation period, the fruit was covered again with white paper bags, and all flies were captured and removed. Tests were replicated five times using a new cohort of flies and fruit on each occasion. All fruit was harvested after a 15-d period and transported to the laboratory.
Fruit infestation by A. ludens and A. striata
Harvested fruit recovered from trials was placed individually in plastic containers with moist vermiculite and covered with a mesh. The fruit was maintained until completely decomposed, and larvae and pupae were recovered. Pupae were placed in small plastic containers (1/4 liter) with moist vermiculite and were weighed individually with an analytical balance (Sartorius CP64) after 4 days (Aluja et al., Reference Aluja, Piñero, Jácome, Díaz-Fleischer, Sivinski, Aluja and Norrbom2000b).
Data analyses
Statistical analyses were performed using STATISTICA Version 7 (Statsoft, 1998). All data were checked for normality and homogeneity of variance after fitting the model (normal plot of residuals and pattern in the residuals vs. predicted values) (Fox et al., Reference Fox, Negrete-Yankelevich and Sosa2015). Data on skin firmness, fruit weight, sugar content, infestation levels (larvae per fruit), host preference (number of eggs per clutch, number of clutches), pupal weight (mg), and egg to pupae developmental time (days), were subjected to analysis of variance (ANOVA) with a hierarchical structure, nesting fruit into tree and using a general linear model. Fertility (hatching percentage), was normalized by arcsine transformation (Montgomery, Reference Montgomery2006) prior to a one-way ANOVA test. Fertility and adult survival were subjected to rank transformation (Conover & Iman, Reference Conover and Iman1981) prior to one-way and two-way ANOVAs, respectively. Post-hoc Tukey tests were performed when appropriate. Contingency tables and Mann–Whitney U-test analyses were used to compare numbers of fruit selection errors, fruit visits, and oviposition attempts. Oviposition-bout duration and attempted oviposition duration were compared by t-tests.
Results
Experiment 1. effect of maternal host preference and offspring performance
Fruit physical and chemical characteristics
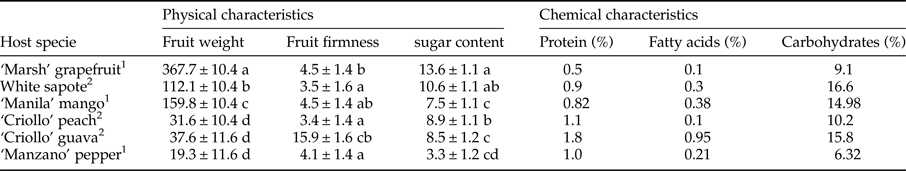
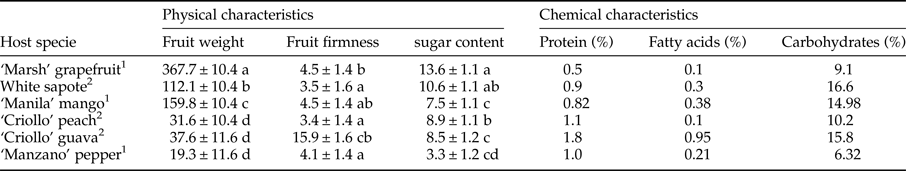
Significant differences in fruit weight (nested ANOVA, F 5,223 = 662.2, P < 0.001), firmness (nested ANOVA, F 5,220 = 5.33, P < 0.001), and sugar content (nested ANOVA, F 5,210 = 104.72, P < 0.001) were observed among host species (table 2). Guava fruit firmness was found to be ca. twofold greater than grapefruit firmness and threefold greater than the other host species (table 2). Sugar content was highest for grapefruit and significantly lower for pepper. Mango, white sapote, guava, and peach had similar sugar contents (table 2). Guava, peach, and pepper had the highest protein content, whereas guava and mango had the highest lipid and carbohydrate contents (table 2).
Table 2. Measurement of fruit physical characteristics (mean ± SE) (fruit weight [g], rind firmness [Newtons]) and chemical characteristics (sugar content [brix]) and bromatological references (protein contain, fatty acids, carbohydrates in %) of six host plant species [Citrus paradisi (‘Marsh’ grapefruit), Casimiroa edulis, (white sapote), Mangifera indica (‘Manila’ mango), Prunus persica, (‘Criollo’ peach), Capsicum pubescens (‘Manzano’ pepper), Psidium guajava (‘Criollo’ guava)].
1 USDA National Nutrient Database for Standard Reference http://www.nal.usda.gov/fnic/foodcomp/search/.
2 Bromatological References, Latex, Xalapa, Veracruz.
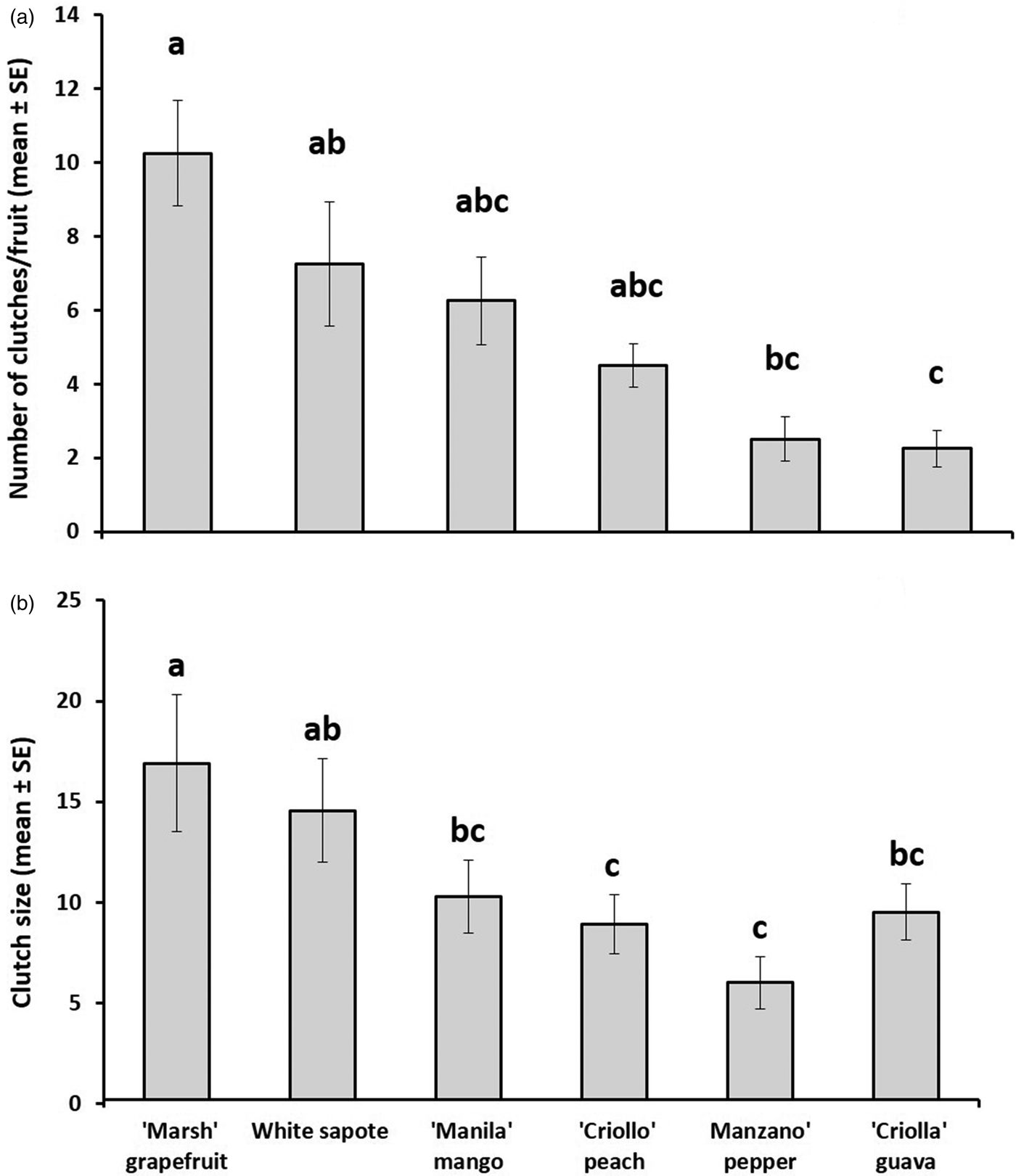
Total number of eggs laid per fruit differed markedly among host species (fig. 1a, b). Pepper and guava received the lowest number of clutches per fruit (2.5 ± 0.6, n = 10; 2.25 ± 0.6, n = 12, respectively) (ANOVA, F 5,18 = 6.72, P < 0.001) and pepper the smallest clutch size (6.8 ± 1.6, n = 10) followed by peach and guava (9.2 ± 1.1, n = 22; 9.5 ± 1.9, n = 12, respectively) (ANOVA, F 5,115 = 11.3, P < 0.001), whereas grapefruit, mango, and white sapote received the largest number of clutches (10.3 ± 1.03, n = 24; 7.3 ± 1.4, n = 23; 6.3 ± 1.6; n = 17, respectively), and grapefruit and white sapote the largest clutch size (16.9 ± 0.8, n = 23; 14.6 ± 1.24, n = 17, respectively).
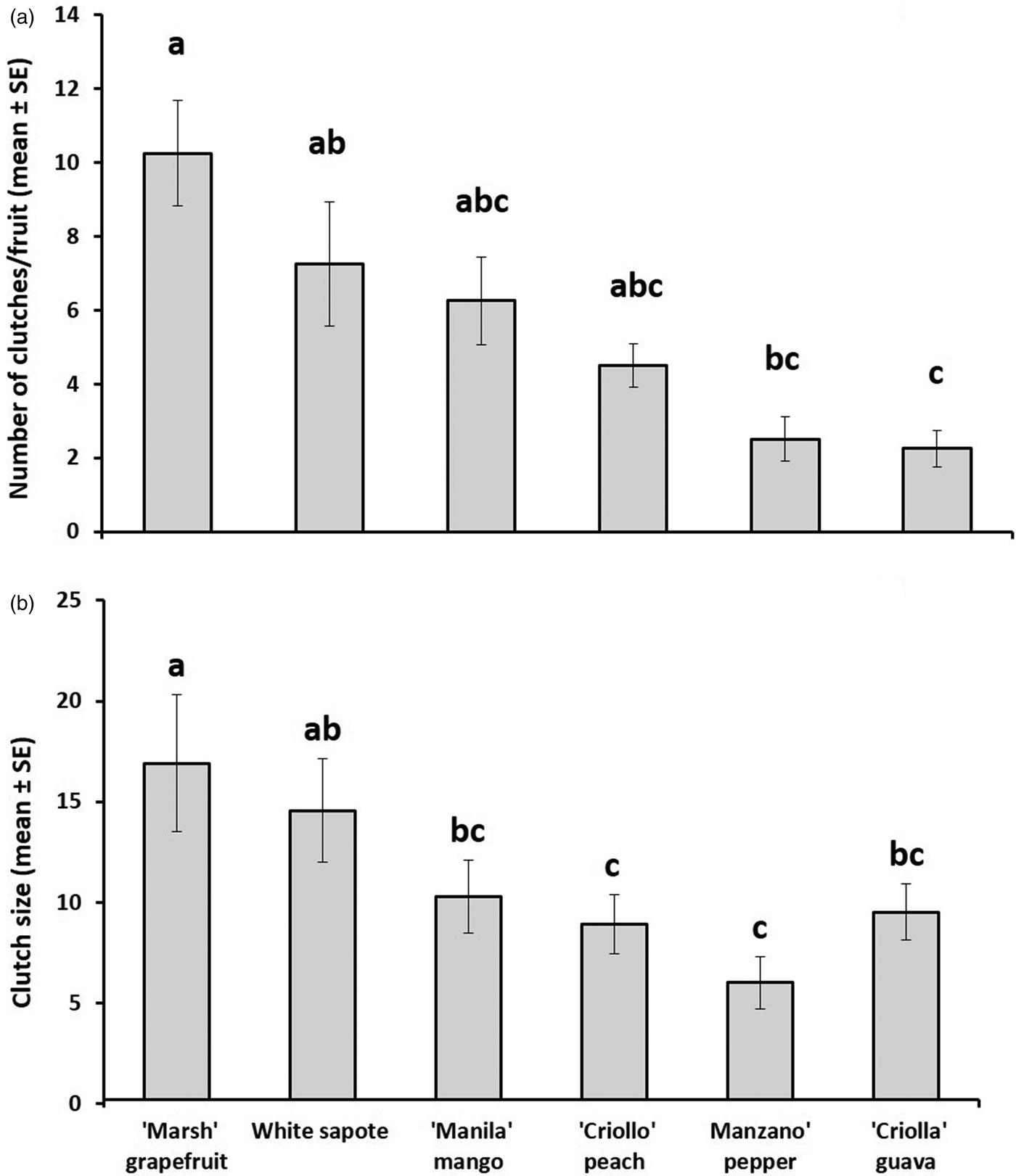
Fig. 1. (a) Number of clutches (mean ± SE) laid per fruit and (b) clutch size (mean (±SE) determined for six host plant species [Casimiroa edulis, (white sapote), Mangifera indica (‘Manila’ mango), Prunus persica, (‘Criollo’ peach), Capsicum pubescens (‘Manzano’ pepper), Psidium guajava (‘Criollo’ guava) and Citrus paradisi (‘Marsh’ grapefruit)] artificially exposed to wild-reared flies in enclosed fruit-bearing branches under field conditions.
Effect of maternal host choice on offspring performance
Assessment of fruit infestation
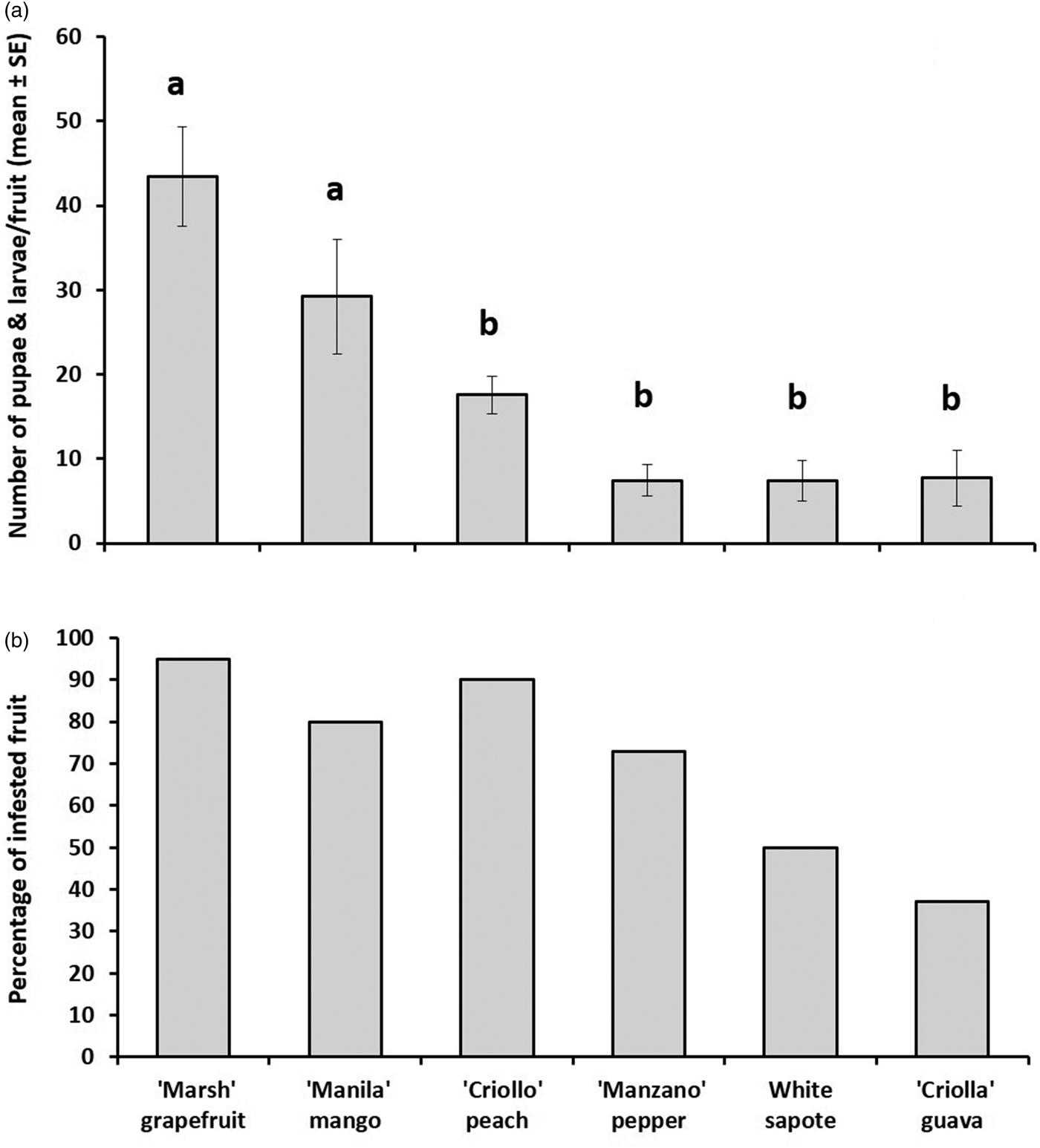
Guava, pepper, peach, and white sapote harbored significantly fewer larvae and yielded significantly fewer pupae than mango and grapefruit (nested ANOVA, F 5,130 = 12.75, P < 0.0001) (fig. 2a, b). However, guavas were the most resistant hosts to infestation, with only 36% of all the exposed fruit being infested. In sharp contrast, almost all mangoes and grapefruits were infested (fig. 2b). The prevalence of infestation in peach (n = 42), pepper (n = 34), and white sapote (n = 39) were 90, 73, and 50%, respectively. Pepper, white sapote, and guava yielded the lowest numbers of larvae and pupae (fig. 2a).
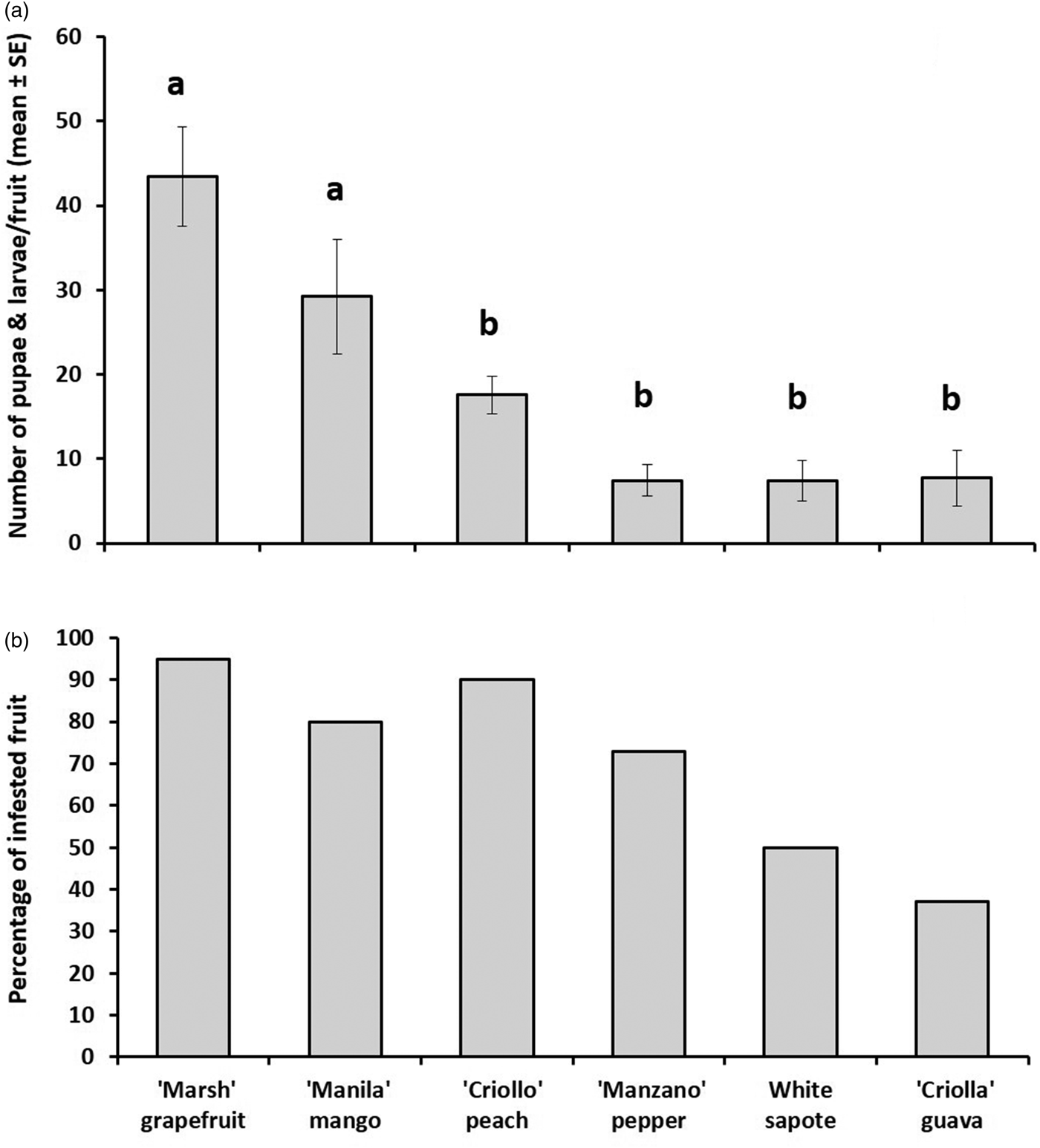
Fig. 2. (a). Number of Anastrepha ludens larvae/fruit (mean ± SE) and (b) percentage of infested fruit obtained from six host plant species [Casimiroa edulis, (white sapote), Mangifera indica (‘Manila’ mango), Prunus persica (‘Criollo’ peach), Capsicum pubescens (‘Manzano’ pepper), Psidium guajava (‘Criollo’ guava) and Citrus paradisi (‘Marsh’ grapefruit)] artificially exposed to wild-reared flies in enclosed fruit-bearing branches under field conditions.
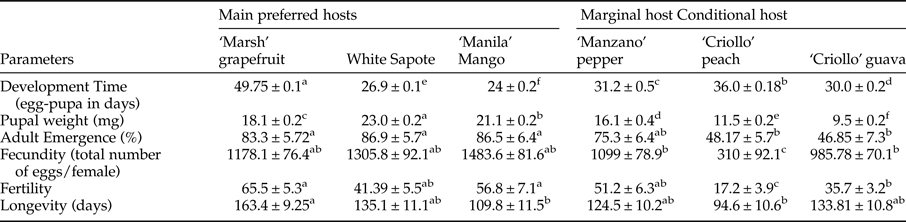
Effect on pupal weight and larval developmental time
Mean pupal weight differed significantly among treatments (nested ANOVA, F 5,2397 = 463.06, P < 0.0001). Highest pupal weights were measured in pupae from white sapote (23.02 ± 0.19 mg, n = 256) and mango (21.25 ± 0.16 mg, n = 399) followed by pupae obtained from grapefruit (18.23 ± 0.15 mg, n = 713), pepper (16.12 ± 0.35 mg, n = 128), peach (11.55 ± 0.25 mg, n = 699), and guava (9.45 ± 0.15 mg, n = 252) (table 3).
Table 3. Developmental and fitness traits (mean ± SE) recorded for Anastrepha ludens progeny obtained from six host plant species [Citrus paradisi (‘Marsh’ grapefruit), Casimiroa edulis, (white sapote), Mangifera indica (‘Manila’ mango), Prunus persica, (‘Criollo’ peach), Capsicum pubescens (‘Manzano’ pepper) and Psidium guajava (‘Criollo’ guava) exposed to wild Anastrepha ludens flies in enclosed branches.
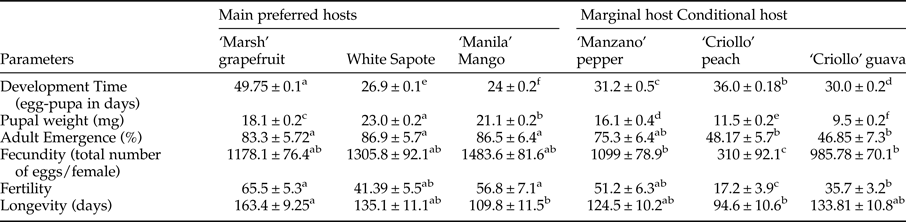
Different letters within one row indicate significant differences among hosts (Tukey HSD test significance P < 0.05).
Developmental time from egg to pupae varied significantly among host species (nested ANOVA, F 5,2408 = 2761.27, P < 0.0001). Egg to pupae development took much longer in ‘Marsh’ grapefruit (49.75 ± 0.13 days, n = 725) when compared with the other fruit tested (table 3). Development was twofold faster when eggs and larvae developed in mango (24 ± 0.15 days, n = 398).
Effect of maternal host on F1 offspring performance
Proportion of adult emergence
Proportion of adult emergence was significantly lower in guava (48 ± 5%, n = 130 adults, 62 ♀ and 68 ♂) and peach (47 ± 6%, n = 269 adults, 145 ♀ and 124 ♂), followed by grapefruit (70 ± 5%, n = 705, 358 ♀ and 347 ♂), pepper (75 ± 6%, n = 188, 94 ♀ and 94 ♂), mango (86 ± 6%, n = 467 adults, 231 ♀ and 236 ♂), and white sapote (87 ± 7%, n = 229 adults, 111 ♀ and 118 ♂) (ANOVA, F 5,20 = 6.31, P = 0.001) (table 3).
Effect of maternal host fruit on fertility, fecundity, and longevity of adult offspring
Significant differences in fecundity and fertility of adults obtained from six different host fruit were recorded. Gravid females obtained from ‘Criollo’ guavas and ‘Criollo’ peaches laid significantly fewer eggs than females recovered from ‘Marsh’ grapefruit, white sapote, ‘Manila’ mango, and ‘Manzano’ pepper (ANOVA, F 5,70 = 34.42, P < 0.001). The same pattern was detected for egg hatch (fertility). Females obtained from ‘Criollo’ guavas and ‘Criollo’ peaches exhibited lower fertility than females stemming from other hosts (ANOVA, F 5,70 = 56.42, P < 0.001) (table 3). In relation to longevity, females were in general less long-lived than males (ANOVA, F 5,204 = 38.83, P < 0.0001) and only offspring from grapefruit (163.4 ± 14.3 days, n = 36) lived significantly longer than offspring stemming from other hosts (ANOVA, F 5,204 = 4.78, P < 0.0004) (table 3).
Experiment 2. effect of information processing hypothesis. accuracy in host-selection comparing A. ludens and A. striata
When host selection trials were performed in the large field enclosure using free-standing guava and grapefruit trees, we never observed A. striata females on the non-host (grapefruit). In the case of A. ludens females, we registered few visits (six) on its conditional host (guava) compared with the higher total number of visits recorded for grapefruit (34). Frequency, in this case, included repeated visits to the same fruit. When total visited fruit per day (recorded only once) were registered, only 4% of guavas were visited during the complete observation period, compared with 22% for grapefruit. Mean visits per day in guavas was 0.8 (±0.6 SE) compared with 4.4 (±0.1 SE) for grapefruits (χ2 = 57.85; P < 0.001) (table 4).
Table 4. Mean number of visited fruits, mean number of oviposition attempts, mean number of ovipositions and mean oviposition duration (±SE) by Anastrepha striata and Anastrepha ludens on natural hosts (H) and non-hosts (NH) in presence of two trees per day.

1 Non-host in Veracruz, or conditional (artificial host) sensu Aluja & Mangan (Reference Aluja and Mangan2008).
The duration of oviposition attempts by A. ludens on its non-host (guava) was significantly longer than on its natural host (grapefruit). More preferential hosts were used for oviposition by the stenophagous species than by the polyphagous species (χ2 = 83; P < 0.001) (table 4). Measuring female efficiency by counting the number of oviposited fruit per day indicated that the stenophagous species (11.6 ± 1.6 fruits) was significantly more accurate and efficient than the polyphagous species (4.2 ± 1.6 fruits) (Mann–Whitney U-test, P < 0.01) (table 4).
Costs of using a non-host were noticed for A. ludens, as only two adults were obtained from guavas compared with 42 from grapefruit. In contrast, a total of 205 A. striata adults were recorded from guavas. A. ludens pupae stemming from grapefruits weighted significantly more (20.8 mg ± 0.5) than those obtained from guavas (15.7 mg ± 3.6) (Student t-test P < 0.001).
Discussion
Our study is one of the very few to have experimentally assessed how the combined effects of host quality, plant defences, and insect behavioural and neurological aspects modulate polyphagous frugivorous insect interactions in a wide range of hosts of different families. The fruit used ranged from species that were never infested in the field but accepted by females under artificial conditions, to highly preferred hosts. We tested two hypotheses (PPH and IPH) showing how both contribute to the knowledge of insect–plant interaction, and deepens our understanding of the role of plant resistance/defence in the context of the latter two hypotheses. In sum, when offspring fitness traits (pupal weight, adult emergence, fecundity, and fertility) were compared among host plants, A. ludens offspring performed better on ‘Marsh’ grapefruit, white sapote, and ‘Manila’ mango, followed by ‘Manzano’ pepper and lastly ‘Criollo’ guava and ‘Criollo’ peach. This study also contributes to the recent debate related to understanding ‘generalism in insects’ (Loxdale et al., Reference Loxdale, Lushai and Harvey2011; Clarke, Reference Clarke2016). In contrast with Bactrocera generalists (Clarke, Reference Clarke2016), A. ludens might be a case of a specialist that used only two fruit species within one genera (Casimiroa edulis and C. greggii) as its main hosts in the past, but has recently expanded its host range to other species within the Rutaceae, Anacardiaceae, Rosaceae, and Solanaceae families eventually becoming a generalist. We also considered a complete suite of fitness correlates measuring F1 fertility and fecundity with the aim of ascertaining if the resulting population would be viable or would decline. This was the case of guavas in our study: highly reduced number of offspring and extremely low fertility and fecundity.
In contrast with Wetzel et al. (Reference Wetzel, Kharouba, Robinson, Holyoak and Karban2016), who suggested that nutritional content of hosts is more important than resistance/defence mechanisms, here we show that this probably differs according to the defence/resistance mechanism harbored by each host plant species and may not apply to all frugivorous tephritids, as some polyphagous tephritids use ripe and fully ripe fruit as oviposition substrates (Rattanapun et al., Reference Rattanapun, Amornsak and Clarke2009) that exhibit a high pulp, water, and nutritional content and decreased secondary metabolite composition (Clarke, Reference Clarke2016). As mentioned above, A. ludens, in contrast to other polyphagous species only ovipositing into pulp (e.g., Bactrocera spp.) (Rattanapun et al., Reference Rattanapun, Amornsak and Clarke2009; Clarke, Reference Clarke2016), also lays eggs into seeds of small, mature-green ‘yellow chapote’ fruit (Plummer et al., Reference Plummer, McPhail and Monk1941). Further studies are needed to compare the seed vs. pulp chemistry to fully understand how A. ludens larvae deal with different types of chemical compounds. Although it was not the main objective of our study, we noticed that defensive mechanisms of hosts like white sapote (i.e., egg encapsulation) and guavas, significantly reduced the number of offspring per fruit. Notably, approximately 50% of egg clutches (195 eggs) laid in white sapote were encapsulated and these eggs did not hatch. Preferred hosts like white sapote that are taxonomically related to Citrus (e.g., grapefruit) did not result in higher fitness correlates, but mango of the Anacardiaceae family did. The ‘Manzano’ pepper (Solanaceae) has a low nutritional but was a better host than peach (Rosaceae), probably due to the high phenolic content of this fruit, whereas guavas (Myrtaceae) have a high nutritional content, but flies developing in this fruit exhibited the worst fitness outcomes (table 2). In other words, fruit fly–host interactions in these types of insects are highly complex, and respond to a complex array of factors, many so far underestimated or never addressed but that merit further study as this system may shed light into the evolution and metabolical pathways involved in the process. For example, in both Rhagoletis pomonella, a specialist on crab apples and other Rosaceae, and A. ludens a generalist rarely infesting apples, larval development was nil or minimal as a consequence of the high total phenolic content of this fruit (Pree, Reference Pree1977; Aluja et al., Reference Aluja, Birke, Ceymann, Guillén, Arrigoni, Baumgartner, Pascacio and Samietz2014a). What is lacking that hinders these unrelated flies the detoxification of these secondary metabolites? Similarly, A. suspensa larvae in citrus were killed by toxic essential oils, particularly coumarins (Greany et al., Reference Greany, Styer, Davis, Shaw and Chambers1983; Salvatore et al., Reference Salvatore, Borkosky, Willink and Bardon2004), whereas resin ducts, sap content, and high tannin levels were negatively correlated with A. ludens, Anastrepha obliqua, and Bactrocera dorsalis Hendel infestation of mango (Guillen et al., Reference Guillen, Adaime, Birke, Velázquez, Angeles, Ortega and Aluja2017; Rashmi et al., Reference Rashmi, Verghese, Shivashankar, Chakravarthy, Sumathi and Kandakoor2017). Studies on Ceratitis fasciventris, a polyphagous tephritid, documented an adverse effect of plant alkaloids that reduced pupal weight and adult size but did not affect adult emergence (Erbout et al., Reference Erbout, De Meyer, Vangestel and Lens2009). All these examples highlight the complexity of the phenomenon and the need to further study it. In our study here, we suggest that high phenolic content in the fruit probably decreased A. ludens larval development in peach and guava and will, therefore, follow this in future studies. As suggested previously, phenolic compounds exert an antinutritive effect, the magnitude of which likely depends on the nutritional content of host fruit (Aluja et al., Reference Aluja, Birke, Ceymann, Guillén, Arrigoni, Baumgartner, Pascacio and Samietz2014a; Pascacio-Villafán et al., Reference Pascacio-Villafán, Lapointe, Williams, Sivinski, Niedz and Aluja2014, Reference Pascacio-Villafán, Williams, Birke and Aluja2016). Indeed, this might be a reason why our results only partially explained host preference-offspring performance correlations for A. ludens in white sapote, peach, and guava.
As predicted, good quality hosts, such as ‘Marsh’ grapefruit and ‘Manila’ mangoes, were preferentially selected and exhibited the highest infestation rates (measured as pupae/fruit and proportion of infested fruit) and offspring fitness indicators (fecundity and fertility). These cultivars have a low content of secondary metabolites and weak defence mechanisms when compared with other genotypes of the same species (Guillen et al., Reference Guillen, Adaime, Birke, Velázquez, Angeles, Ortega and Aluja2017, Birke et al., personal observation). Differences among genotypes of the same species may help to explain how fruit resistance or defence modulates offspring production in tephritids and therefore merits further study. Previous studies assessing preference-performance correlations in phytophagous insects have also tended to report a weak relationship between female oviposition preference or host quality and offspring performance for other polyphagous species that might also be related to plant resistance or defence traits (Gripenberg et al., Reference Gripenberg, Mayhew, Parnell and Roslin2010; Balagawi et al., Reference Balagawi, Drew and Clarke2013).
Testing the neuronal constraint hypothesis between two fruit flies with different diet breadth showed that, as predicted, polyphagous A. ludens females were significantly less accurate when selecting a host and spent more time searching than the specialist A. striata. Natural hosts were preferentially used for oviposition by A. striata (n = 205) compared with the polyphagous species (n = 42). Total female foraging efficiency and accuracy (100%) was only observed in the specialist species (A. striata). Our study presents additional evidence of the inability of generalists to process a wide range of sensory cues offered by different hosts as it was clearly noticed that host specificity was weak, accuracy low, and A. ludens females searched for hosts much longer when compared with A. striata, as had been previously described for other phytophagous insects (Bernays & Funk, Reference Bernays and Funk1999; Egan & Funk, Reference Egan and Funk2006).
Other studies performed in controlled laboratory settings have shown that preference for low ranking hosts can increase with female age, egg-load, and accumulated search time (Fitt, Reference Fitt1986a, Reference Fittb; Courtney, Reference Courtney1988; Leyva et al., Reference Leyva, Browning and Gilstrap1991), or if antennal receptors age or change (Tallamy et al., Reference Tallamy, Mullin and Frazier1999). Trade-offs of this nature influence female behaviour and can also explain the lack of correlation between host preference and offspring performance.
Two contrasting patterns emerge from our studies. On the one hand, and in contrast to recent studies, variability of host nutrients on which larvae feed did not influence adult size or adult emergence (Clarke, Reference Clarke2016; Wetzel et al., Reference Wetzel, Kharouba, Robinson, Holyoak and Karban2016), and offspring performance was likely influenced by secondary metabolites or other fruit traits (Greany et al., Reference Greany, Styer, Davis, Shaw and Chambers1983; Erbout et al., Reference Erbout, De Meyer, Vangestel and Lens2009; Aluja et al., Reference Aluja, Birke, Ceymann, Guillén, Arrigoni, Baumgartner, Pascacio and Samietz2014a; Guillen et al., Reference Guillen, Adaime, Birke, Velázquez, Angeles, Ortega and Aluja2017). Alkaloids such as capsaicin in ‘Manzano’ pepper did not seem to limit A. ludens larval development. However, we suggest that the phenolic content of peach and guava may have affected larval development, as suggested in previous studies (Aluja et al., Reference Aluja, Birke, Ceymann, Guillén, Arrigoni, Baumgartner, Pascacio and Samietz2014a; Pascacio-Villafán et al., Reference Pascacio-Villafán, Lapointe, Williams, Sivinski, Niedz and Aluja2014, Reference Pascacio-Villafán, Williams, Birke and Aluja2016). We believe that secondary metabolites and physiological plant defensive mechanisms (encapsulation) may have intervened in regulating offspring performance. Our results support the IPH as A. ludens females were less accurate and less efficient in selecting its preferential hosts.
In conclusion, our findings indicate that both hypotheses, PPH and IPH, are complementary and can partially explain host use by A. ludens. However, we also consider that the complexity of polyphagous insect–plant interactions needs to be approached by focusing on other factors such as plant resistance/defence mechanisms, including secondary plant chemicals, which at present limit both hypotheses for those tephritids that do not use ripe hosts as oviposition substrates.
Acknowledgements
Funding was provided by the Campaña Nacional Contra Moscas de la Fruta SAGARPA-IICA-INECOL, the Asociación de Productores y Empacadores de Aguacate de Michoacán (APEAM) and by the Instituto de Ecología, A.C. (INECOL). The authors thank Emilio Acosta, Nicolás Jimarez and Rafael Ortega (INECOL) for technical support and Trevor Williams, Juan Rull, Diana Pérez-Staples, Nicoletta Righini and Carlos Pascacio-Villafán for critical comments on earlier versions of the manuscript. This study is part of the doctoral dissertation of AB (Universidad Veracruzana, Instituto de Neuroetología) directed by MA. The authors also sincerely appreciate the insightful and critical comments on an earlier draft of this manuscript by two anonymous referees and the editor.
Author contributions
AB and MA conceived and designed the experiments. AB conducted fieldwork and performed the laboratory experiments. AB analyzed the data. AB, MA wrote the manuscript.