Introduction
Among the many challenges in sustaining crop productivity and nutritional security, direct and indirect damages by insect pests is of paramount importance. Pests such as aphids and thrips pose the dual problem of direct physical damage to crop plants as well as vectoring of many plant pathogenic viruses (Blackman & Eastop, Reference Blackman and Eastop2000; Mound, Reference Mound2005). Management of plant pathogens vectored by insect pests is all the more complex because of the factors influencing the epidemiology of these diseases. Among the many plant viruses transmitted by insects, aphid-transmitted viruses are the most numerous and predominant worldwide (Blackman & Eastop, Reference Blackman and Eastop2000). Aphids (Hemiptera: Aphididae), with a recorded diversity of about 5000 species, are small, soft-bodied insects with sucking mouth parts that feed mainly on phloem and are considered as economically-important, often invasive pests throughout the world (van Emden & Harrington, Reference van Emden and Harrington2007; Foottit et al., Reference Foottit, Maw, von Dohlen and Hebert2008). In light of this, a quick, accurate and timely identification of aphids is important for their management. However, the evolutionary tendency towards the loss of taxonomically useful characters and phenotypic plasticity due to host and environmental factors make their identification difficult (Foottit et al., Reference Foottit, Maw, von Dohlen and Hebert2008). In addition, the presence of unusual morphological forms of species on different host plants under various climatic conditions, complex life cycles, colour polymorphisms and a cyclically parthenogenetic mode of reproduction in the majority of species, often involving an alternation of hosts between a winter primary host and spring-summer secondary host/s (Dixon, Reference Dixon1998), add to the difficulty of precise identification. Furthermore, morphological examination of aphids to species is usually restricted to certain life stages or asexual forms, since there are generally no reliable keys for the identification of the immature stages (Henderson et al., Reference Henderson, Loxdale and Greenwood1976) or for that matter, the sexual morphs themselves, and which may prove difficult for a non-expert to use.
Considering all these factors, it is even so necessary to detect invasive quarantine pest species introduced into particular countries along with agricultural or horticultural products at the port of entry, where speed and accuracy of identification are paramount (Glover et al., Reference Glover, Collins, Walsh and Boonham2010). In this regard, Hebert et al. (Reference Hebert, Cywinska, Ball and Dewaard2003a, Reference Hebert, Ratnasignham and Dewaardb) proposed the concept of DNA barcoding as a rapid and precise way of species discrimination of a broad range of biological specimens using a selected 658 bp fragment of the 5′ end of the mitochondrial cytochrome oxidase-1 (CO-I) gene. DNA barcoding can be employed as a useful approach for molecular identification of species in their various life stages and forms (Foottit et al., Reference Foottit, Maw, Pike and Miller2010), host-associated genetic differences (Brunner et al., Reference Brunner, Chatzivassilious, Katis and Frey2004), discrimination of cryptic species (Smith et al., Reference Smith, Woodley, Janzen, Hallwachs and Hebert2006), as well as biotypes (Eastop, Reference Eastop and Lowe1973; Shufran et al., Reference Shufran, Burd, Anstead and Lushai2000). Potentially, DNA barcoding could be easily incorporated into pest management programmes involving pest complexes – such as in the case of the apple aphid, Aphis pomi (De Geer) and small raspberry aphid, Aphis spiraecola Patch, where both selection and timing of the management practices can be affected by the insect's polymorphism and overwintering host adaptation (Lowery et al., Reference Lowery, Smirle, Foottit and Beers2006; Footit et al., Reference Foottit, Maw, Pike and Miller2010).
The purpose of the present study was to discriminate 142 individual aphids representing 32 species collected on various host plants in South India using CO-I barcoding and to record the presence of cryptic species and host-associated genetic forms among these taxa, if any.
Materials and methods
Taxon sampling
Taxon assignments were performed according to the Current World Catalogue of Aphids (Remaudiere & Remaudiere, Reference Remaudiere and Remaudiere1997). Specimens were collected in 95% ethanol during 2008–2012 and kept at −80 °C until DNA testing. Prior to molecular work, aphid species were identified morphologically by Dr. Sunil Joshi of the National Bureau of Agriculturally Important Insects (NBAII), Bangalore, India. The complete data set, including 142 individual specimens representing 32 species of aphids, is listed in table 1. In order to understand and document intraspecific variations in the barcoding region of each species (Meyer & Paulay, Reference Meyer and Paulay2005), we analysed all the sequences for aphids available from NCBI-GenBank. Specimen details and sequences are available in BOLD (www.barcodingoflife.org, ‘Barcoding of aphids in Karnataka’, project) and also in NCBI-GenBank.
Table 1. Analysed samples of Aphid species with description of the sampling locations, GenBank accession numbers, name, date and voucher specimen details.

DNA extraction and Polymerase Chain Reaction (PCR)
Total genomic DNA was extracted from individual aphids using a non-destructive method (Rowley et al., Reference Rowley, Coddington, Gates, Norrbom, Ochoa, Vandenberg and Greenstone2007), while at the same time voucher specimens were mounted on glass slides and deposited with the National Pusa Collection (NPC), Indian Agricultural Research Institute (IARI) Delhi. Depending on the concentration, the DNA samples were diluted with sterile distilled water in order to obtain a working solution of 20–25 ng μl−1 purified DNA. A portion of the total DNA was preserved in glycerol (10%) at −80 °C for future reference. Standard protocols were followed for PCR, cloning, sequencing of the CO-I region, and sequence alignment (Toda & Komazaki, Reference Toda and Komazaki2002; Hajibabaei et al., Reference Hajibabaei, Janzen, Burns, Hallwachs and Hebert2006).
PCR was performed in a thermal cycler (ABI-Applied Biosystems, Veriti, USA) using the following cycling parameters; an initial denaturation step at 94 °C for 4 min followed by 35 cycles at 94 °C for 30 s, an annealing step at 47 °C for 45 s, an extension step at 72 °C for 45 s and a final extension step at 72 °C for 20 min using the universal CO-I primers: LCO-1490; 5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′ and HCO-2198; 5′- TAA ACT TCA GGG TGA CCA AAA AAT CA-3′ (Hebert et al., Reference Hebert, Cywinska, Ball and Dewaard2003a, Reference Hebert, Ratnasignham and Dewaardb). The total reaction volume of 25 μl contained ∼20 picomoles of each primer, 10 mM Tris/HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 0.25 mM of each dNTP and 0.5 units of Taq DNA polymerase (Fermentas Life Sciences, UK). The amplified products were resolved on 1.0% agarose gel, stained with ethidium bromide (10 μg ml−1) and visualized in a gel documentation system (UVP).
Sequencing and sequence analysis
The amplified products were eluted using a gel extraction kit (Nucleospin® Extract II, Macherey Nagel, Germany) according to the manufacturer's protocol, whilst the eluted products were ligated into general purpose cloning vector, InsT/A clone (Fermentas Life Sciences, UK), again according to the manufacturer's protocol. Blue–white selection was carried out and plasmids were isolated using GenJET™ plasmid MiniPrep kit (Fermentas Life Sciences, UK), according to the manufacturer's protocol from the overnight culture of positive clones cultured in LB broth. Sequencing was performed in triplicates of the above clones in an automated sequencer (ABI prism® 3730 XL DNA Analyzer; Applied Biosystems, USA) using M13 universal primers, both in the forward and reverse directions. A homology search was done using NCBI-BLAST (http://blast.ncbi.nlm.nih.gov/) and sequence alignment was performed using BioEdit version 7.0.9.0 (Hall, Reference Hall1999). All the sequences generated were deposited in NCBI-GenBank (Supplementary material 1) and also accessible in BOLD.
CO-I sequences were aligned using the Clustal W program in BioEdit.7.0. The sequences were further analysed using MEGA.5.0 (Kumar et al., Reference Kumar, Tamura and Nei1993) to obtain conspecific and congeneric distances, while Neighbour-Joining (NJ) trees were constructed using the Kimura-2-parameter (K2P) distance model (Kimura, Reference Kimura1980; Saitou & Nei, Reference Saitou and Nei1987).
Results
Data analysis
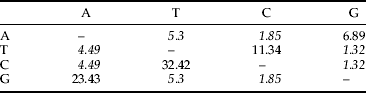
The CO-I from all the 32 aphid species (table 1) were successfully sequenced, further analyses revealing that 308 characters were variable and 270 characters were parsimony informative from the 658 bp regions investigated. No pseudogenes were amplified as indicated by the absence of stop codons within the sequences and the base composition was similar with no indels (Rebijith et al., Reference Rebijith, Asokan, Krishna Kumar, Srikumar, Ramamurthy and Shivarama Bhat2012). Reliability of the clustering pattern in the trees was determined using the bootstrap test with 1000 replications employing MEGA 5.0 (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011) (table 2). Nucleotide frequencies were 34.7% (A), 40.9% (T), 10.2% (C) and 14.3% (G). The base composition of the CO-I gene fragment was found to be biased towards Adenine and Thymine, which together constituted 75.5% of the total as expected from earlier studies on aphids (Wang et al., Reference Wang, Chen, Luo, Zhao, Zhang and Tlali2011). The overall transition (ti)/transversion (tv) bias of nucleotide sequence was R=2.2.
Table 2. Maximum composite likelihood estimate of the pattern of nucleotide substitution from 142 individuals of 32 species of aphids.
NJ analysis
The CO-I data set yielded one NJ tree representing the 32 species of aphids studied, which formed distinct clusters (fig. 1). The intraspecific COI sequence divergences ranged from 0 to 3.8% (table 3), whereas interspecific divergences ranged from 2.3 to 18.9%, with a mean of 11.6% (Supplementary material 1). Intrageneric distances ranged from 2.0 to 6.3% (table 4) and intergeneric divergences from 5.0 to 18.2% with a mean of 11.6% (Supplementary material 2). Thus, a discrete barcoding gap between the intra and interspecific distances (Hebert et al., Reference Hebert, Penton, Burns, Janzen and Hallwachs2004) was observed in the current study, except for the cotton–melon aphid, Aphis gossypii Glover and the pomegranate aphid, Aphis punicae Passerini (fig. 1). The study also revealed that aphids within the genus Toxoptera (citrus aphids) are polyphyletic as inferred from the NJ tree.

Fig. 1. NJ tree with bootstrap support (1000 replicates) showing clusters of species for COX-1 sequences. Distinct clades for 32 species of aphids can be seen in the figure, in which three species viz. B. brassicae, H. carduellinus and B. helichrysi showing two distinct groups with >90% bootstrap support. The numbers indicated in brackets represents the individuals analysed in the corresponding species.
Table 3. The intraspecific genetic divergences of 22 species that have two or more sequences of aphids with minimum, maximum and average values.

Table 4. The intrageneric divergences of 5 genus that have two or more sequences of aphids with mean distance values.

With regard to the vectoring potential of the aphids studied, we analysed all the available sequences for both A. gossypii and the peach-potato aphid, Myzus persicae (Sulzer), which revealed that these species are apparently individual cosmopolitan, polyphagous species without any obvious cryptic species or biotypes. However, the NJ tree of 46 samples of the banana aphid, Pentalonia nigronervosa revealed that samples collected from banana formed a first clade (belonging to P. nigronervosa Coquerel sensu stricto) and samples collected from cardamom, alpinia, colocasia and ginger formed a second clade (belonging to Pentalonia caladii van der Goot) as described by Foottit et al. (Reference Foottit, Maw, Pike and Miller2010). For the first time, we were also able to record the existence of cryptic species within the three species, Brevicoryne brassicae (Linnaeus), Hyperomyzus carduellinus (Theobald) and Brachycaudus helichrysi (Kalt.) from India, based on the mean intraspecific variations of CO-I within a group (10X rule) (Hebert et al., Reference Hebert, Penton, Burns, Janzen and Hallwachs2004). These findings were further supported by the NJ bootstrap values of 99, 98 and 100 for B. brassicae, H. carduellinus and B. helichrysi, respectively (fig. 1) and by the calculated intra and interspecific distances for Group 1 & 2 of these three species (fig. 2).

Fig. 2. The range of intra and interspecific distances of group 1 and 2 of three newly identified cryptic species of aphids viz. B. brassicae, H. carduellinus and B. helichrysi according to Hebert's barcoding gap of 10X intraspecific to interspecific distances.
Discussion
Rapid and timely identification of invasive insects such as aphids is important and challenging worldwide, as these particular pests outnumber all other insects in terms of both number and diversity (Footit et al., Reference Foottit, Maw, von Dohlen and Hebert2008). In this regard, while classical taxonomy has its own strengths, molecular identification employing CO-I barcoding has the added advantage of not being limited by polymorphism, sexual form (asexual/sexual) and life stages of the target species (Asokan et al., Reference Asokan, Rebijith, Singh, Sidhu, Siddharthan, Karanth, Ellango and Ramamurthy2011). All the aphid species employed in the present study were differentiated clearly on the basis of DNA barcodes, which proved to be a valuable tool for the identification of these serious insect pests, an approach complementing classical taxonomy.
1. DNA barcoding and current taxonomy of aphids
Morphological identification of aphids poses a serious problem due to the smaller size, polymorphism, insufficient discerning morphological characters, and the complex association with multiple hosts (Miller & Foottit, Reference Miller, Foottit, Foottit and Adler2009; Lee et al., Reference Lee, Kim, Lim, Choi, Kim, Ji, Foottit and Lee2010). Because of this, DNA barcoding employing the CO-I gene sequence (Hebert et al., Reference Hebert, Penton, Burns, Janzen and Hallwachs2004) has become an alternative and effective tool for species identification (Foottit et al., Reference Foottit, Maw, von Dohlen and Hebert2008; Glover et al., Reference Glover, Collins, Walsh and Boonham2010; Lee et al., Reference Lee, Kim, Lim, Choi, Kim, Ji, Foottit and Lee2010). In addition, CO-I may be suitably employed to elucidate the prevalence of biotypes (Shufran et al., Reference Shufran, Burd, Anstead and Lushai2000) and for the discovery of new species within the Aphididae (Foottit, Reference Foottit, Claridge, Dawah and Wilson1997). Recently, Foottit et al. (Reference Foottit, Maw, Pike and Miller2010) examined P. nigronervosa using integrated taxonomic approaches and designated P. nigronervosa form typica as P. nigronervosa (infesting banana) and P. nigronervosa form caladii as P. caladii (infesting plants belonging to the families Zingiberaceae (ginger) and Araceae (Arums). Our present study based on CO-I supports this classification. In yet another study, the genus Toxoptera raised by Koch in Reference Koch1856, comprising six species namely T oxoptera aurantii (Boyer de Fonscolombe), Toxoptera celtis Shinji, Toxoptera citricida (Kirkaldy), Toxoptera odinae (van der Goot), Toxoptera victoriae Martin and Toxoptera chaetosiphon Qiao, Wang & Zhang, has a lot of morphological similarity with the genus Aphis, except for the presence or absence of stridulatory apparatus. In this respect, our study showed that T. aurantii and T. odinae form a clade with members of the genus Aphis, supporting recent COI barcoding and phylogenetic studies by Foottit et al. (Reference Foottit, Maw, von Dohlen and Hebert2008), Lee et al. (Reference Lee, Kim, Lim, Choi, Kim, Ji, Foottit and Lee2010) and Kim et al. (Reference Kim, Lee and Jang2011). Recently, Blackman et al. (Reference Blackman, Sorin and Miyazaki2011) studied the sexual morphs and colour variants of T. odinae and placed it again in the genus Aphis. Similarly based on the present molecular studies, we propose that T. aurantii also be placed in the genus Aphis.
Our studies showed the possible existence of cryptic species in three aphid species, namely, B. brassicae, H. carduellinus and B. helichrysi. Two biotypes – NZ-1 and 2 – of B. brassicae were previously reported by Lammerink (Reference Lammerink1968) based on field experiments. This contention was supported by our molecular data, even though clades corresponding to host plants were unclear. Our observations of the existence of sibling species of B. helichrysi have been well supported by the recent studies of Madjdzadeh et al. (Reference Madjdzadeh, Mehrparvar and Abolhasanzadeh2009) and Piffaretti et al. (Reference Piffaretti, Vanlerberghe-Masutti, Tayeh, Clamens, Coeur d'acier and Jousselin2012) employing morphometrics and molecular methods, respectively.
2. Host-associated genetic differentiation
Host association in aphids is likely to influence reproductive isolation when migration occurs from one host to other. This could be due to pre-mating or post-mating selection against migrants and hybrid progeny (Liou & Price, Reference Liou and Price1994; Brunner et al., Reference Brunner, Chatzivassilious, Katis and Frey2004). Even though some aphid species may, at a population level, appear to be polyphagous over large spatial scales, they tend to be monophagous at the colony level due to the availability of suitable host at this much smaller spatial scale (Eastop, Reference Eastop1979). This might have cascading effects on evolution of biotypes and cryptic species favouring host adaptation (Wang & Qiao, Reference Wang and Qiao2009), which is evident in the greenbug aphid, Schizaphis graminum (Rondani) (Shufran et al., Reference Shufran, Burd, Anstead and Lushai2000). However in our study, none of the species showed host-associated genetic differences as previously reported by Wang et al. (Reference Wang, Chen, Luo, Zhao, Zhang and Tlali2011) in the cowpea aphid, Aphis craccivora, although Foottit et al. (Reference Foottit, Maw, Pike and Miller2010) did show that P. nigronervosa, which feeds only on banana and P. caladii that infests several hosts, including cardamom, ginger and alpinia, are host specific and our study indicates the same too.
3. DNA barcoding implications in pest management
In recent decades, aphids continue to pose a major threat to agriculture, horticulture and forestry, including Bt-transgenic plants (e.g., Faria et al., Reference Faria, Wäckers, Pritchard, Barrett and Turlings2007), more especially due to the evolution of pesticide resistance in some pest species infesting crops treated with conventional pesticides, including organophosphates, carbamates and pyrethroids (Foster et al., Reference Foster, Devine, Devonshire, van Emden and Harrington2007). Although many aphid species are damaging in their own right due to the physical injury they inflict on plants, their potential as disease vectors transmitting pathogenic plant viruses of one sort or another has field level implications. In plant disease management, it is advisable to control the vectors (e.g., aphids) rather than the viruses. However, it is difficult to control aphids using insecticides due to their parthenogenetic mode of reproduction (i.e., high rate of reproduction), life cycles (including alternation from crop to non-crop and hence chemically untreated plants), and apparent polyphagy in some species (e.g., M. persicae); yet, many farmers still use the chemical approaches as their primary control measure, which may well ultimately lead to the development of resistance, as has indeed occurred in many species of aphids (Devonshire, Reference Devonshire, Loxdale and den Hollander1989; Foster et al., Reference Foster, Devine, Devonshire, van Emden and Harrington2007).
Insect pest management approaches require a clear understanding on the pest species in question in terms of their particular biology, ecology and population structure/genetics. In this respect, the identification of P. nigronervosa, which infests banana transmitting Banana Bunchy Top virus (BBTV) demands quick control measures using insecticides in order to limit the spread of BBTV, whereas in the case of P. caladii, the aphid can probably be managed by employing biological agents such as ladybird beetles (Coleoptera: Coccinellidae) and hoverfly larvae (Diptera: Syrphidae) which can reduce pesticide usage and hence slow – and may be even prevent – the developement of insecticide resistance, as well as reducing the polluting impact of these poisons in the environment.
Among the known 376 species of Liriomyza flies (Diptera: Agromyzidae), four are difficult to diagnose morphologically and are significant pests globally (EPPO, 2005). Biological differences in susceptibility to pesticides and fecundity (Gao et al., Reference Gao, Reitz, Wei, Yu and Lei2012) led to the displacement of L. sativae (Blanchard) by L. trifolii (Burgess) in China and vice versa in Japan (Gao et al., Reference Gao, Lei, Abe and Reitz2011). However, use of DNA barcoding was able to readily discriminate among these four polymorphic Liriomyza species (Scheffer et al., Reference Scheffer, Lewis and Joshi2006) and has proved highly useful in pest management programmes involving biocontrol. In a nutshell, DNA barcoding can play an important role in pest management when polymorphic pest species have potential impact on the agroeconomy (i.e., direct feeding/vectoring diseases), phenology and susceptibility to specific management practices.
Conclusion
In this study, we generated CO-I barcoding sequences for 142 individual specimens representing 32 aphid species from India. We trust that our work will serve as a rapid, precise, independent identification approach for the discrimination of aphid species of different life stages and colour morphs, both for the species presently studied, and in the future, for other pest species of agricultural, horticultural and forestry interest and importance. This will in turn help in further elucidation of the epidemiology of viruses, their management and serve as a potentially valuable tool in quarantine at the port of entry. Moreover, as our study has revealed, the prevalence of three cryptic aphid species, namely, B. brassicae, H. carduellinus and B. helichrysi, shows that further studies on the evolution of these particular species (and doubtless others too) are required before we can collectively be sure that we are looking at individual species (sensu stricto) rather than complexes of cryptic species (sensu lato), perhaps of differing disease vectoring capability. Here, as we show, DNA barcoding is proving an effective tool that can be employed for species identification, elucidation of cryptic species, biotypes and also in the discovery of new species.
The supplementary materials for this article can be found at http://www.journals.cambridge.org/ber
Acknowledgements
Our sincere thanks to Dr Sunil Joshi, NBAII, Bangalore for morphological identification of aphids used in the current study and Professor Hugh Loxdale for his helpful comments on the manuscript. We acknowledge ICAR, New Delhi for funding the project on ‘Out Reach Program on Management of Sucking Pests On Horticultural Crops’ under XIth Plan. This work is a part of the PhD Thesis of the senior author.








