Introduction
Several whitefly species (Hemiptera: Aleyrodidae) are cosmopolitan phloem-feeding pests that cause serious problems in numerous agricultural crops. Few of these species are known to heavily damage plants through direct feeding, honeydew secretion and virus transmission. Among the many whitefly species known to date, only genus Bemisia and Trialeurodes are known to serve as virus vectors (Jones, Reference Jones2003). Whiteflies can adapt to a wide range of climates, from arid desert to tropical, subtropical and Mediterranean conditions, where prolonged freezing temperatures are rare or nonexistent (Brown, Reference Brown and Czosnek2007). Bemisia tabaci (Gennadius) represents a species complex consisting of at least 30 morphologically indistinguishable species, placed in 11 well-defined high-level groups, based on biochemical and molecular markers (Dinsdale et al., Reference Dinsdale, Cook, Riginos, Buckley and De Barro2010; Hu et al., Reference Hu, De Barro, Zhao, Wang, Nardi and Liu2011; Alemandri et al., Reference Alemandri, De Barro, Bejerman, Argüello Caro, Dumon, Mattio, Rodriguez and Truol2012). The world's two most widespread members of the B. tabaci species complex are the Middle East-Asia Minor 1 (MEAM1, known as B biotype) and Mediterranean (MED, known as Q biotype). MEAM1 and MED became global invaders and the most damaging, due to the ornamental plants trade (De Barro & Ahmed, Reference De Barro and Ahmed2011). They are known for their wide host range, high fecundity, insecticide resistance and ability to transmit plant viruses and induce plant disorders (Brown et al., Reference Brown, Frohlich and Rosell1995; Secker et al., Reference Secker, Bedford, Markham and William1998; Perring, Reference Perring2001). The greenhouse whitefly Trialeurodes vaporariorum (Westwood) and the ash whitefly Siphoninus phillyreae (Haliday) are common pests in many agrosystems worldwide and cause serious damage by direct feeding on plants and secretion of large amounts of honeydew. Although S. phillyreae is less known in agricultural crops, it is known in horticultural crops such as pomegranate, pear, olive and citrus (Nguyen & Hamon, Reference Nguyen and Hamon1990).
In Croatia, T. vaporariorum has been a serious pest in greenhouse crops since the 1970s, and it is still the predominant species, while B. tabaci MED was first recorded in 2000 (Zanic et al., Reference Zanic, Cenis, Kacic and Katalinic2005). These two pests cause significant losses on numerous horticultural crops. Similar damage is caused by both pests in the south eastern part of Bosnia and Herzegovina. The first report of B. tabaci on ornamentals from Montenegro was confirmed in 2008 (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). The control of all whitefly species that cause agricultural damage in all East Adriatic countries relies on adopting integrated pest management practices. Siphoninus phillyreae is a major pest of pomegranate in this area, and new infestations of Phillyrea spp. originate from shrubs, where the pest overwinters. Whereas T. vaporariorum and S. phillyreae can be identified based on external morphology (fig. 1), B. tabaci genetic groups can only be identified using DNA markers (Boykin et al., Reference Boykin, Shatters, Rosell, McKenzie, Bagnall, De Barro and Frohlich2007).

Fig. 1. Phenotypical differences between three whitefly species: adults of (A) B. tabaci, (C) T. vaporariorum and (E) S. phillyreae; pupal stages of (B) B. tabaci, (D) T. vaporariorum and (F) S. phillyreae.
Many insects are known to host a diverse array of bacterial symbionts which appear to interact with their hosts on several levels, ranging from parasitism to mutualism (Buchner, Reference Buchner1965; Moran & Baumann, Reference Moran and Baumann1994). All whitefly species are known to harbor the primary symbiont Portiera aleyrodidarum, which supplements their deficient phloem diet with some essential nutrients (Thao & Baumann, Reference Thao and Baumann2004a). Portiera aleyrodidarum is confined to specialized cells (bacteriocytes) and is vertically transmitted (Baumann, Reference Baumann2005). In addition, B. tabaci populations from around the world have been reported to harbor several secondary symbionts, including Hamiltonella, Arsenophonus, Cardinium, Wolbachia, Rickettsia and Fritschea, whose functions are mostly unknown in this species (Nirgianaki et al., Reference Nirgianaki, Banks, Frohlich, Veneti, Braig, Miller, Bedford, Markham, Savakis and Bourtzis2003; Baumann, Reference Baumann2005; Gottlieb et al., Reference Gottlieb, Ghanim, Chiel, Gerling, Portnoy, Steinberg, Tzuri, Horowitz, Belausov, Mozes-Daube, Kontsedalov, Gershon, Gal, Katzir and Zchori-Fein2006; Li et al., 2007). In other insects, Wolbachia, Rickettsia, Arsenophonus and Cardinium have been implicated in manipulating their host's reproduction via several mechanisms (Werren et al., Reference Werren, Skinner and Huger1986; Gherna et al., Reference Gherna, Werren, Weisburg, Cote, Woese, Mandelco and Brenner1991; Zchori-Fein & Perlman, Reference Zchori-Fein and Perlman2004; Dale & Moran, Reference Dale and Moran2006). Hamiltonella has been shown to confer resistance against parasitoids in the pea aphid Acyrthosiphon pisum (Oliver et al., Reference Oliver, Russell, Moran and Hunter2003; Ferrari et al., Reference Ferrari, Darby, Daniell, Godfray and Douglas2004; Bensadia et al., Reference Bensadia, Boudreault, Guay, Michaud and Cloutier2005) and to increase the ability of B. tabaci to be an efficient virus vector (Gottlieb et al., Reference Gottlieb, Zchori-Fein, Mozes-Daube, Kontsedalov, Skaljac, Brumin, Sobol, Czosnek, Vavre, Fleury and Ghanim2010). Rickettsia in B. tabaci has been shown to confer resistance to heat stress (Brumin et al., Reference Brumin, Kontsedalov and Ghanim2011) to increase its susceptibility to chemical insecticides (Kontsedalov et al., Reference Kontsedalov, Zchori-Fein, Chiel, Gottlieb, Inbar and Ghanim2008) and to provide the whitefly with fitness benefits (Himler et al., Reference Himler, Adachi-Hagimori, Bergen, Kozuch, Kelly, Tabashnik, Chiel, Duckworth, Dennehy, Zchori-Fein and Hunter2011).
In 2008, we conducted a survey on the distribution of B. tabaci and T. vaporariorum populations and their infection status with bacterial symbionts in Croatia, and Bosnia and Herzegovina (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). In the same study, the localization of the different endosymbionts within both whitefly species was investigated using fluorescent in situ hybridizations (FISH), and new localization patterns were discovered (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). The infection status of B. tabaci differed from previously published results (Chiel et al., Reference Chiel, Gottlieb, Zchori-Fein, Mozes-Daube, Katzir, Inbar and Ghanim2007; Gottlieb et al., Reference Gottlieb, Ghanim, Gueguen, Kontsedalov, Vavre, Fleury and Zchori-Fein2008; Gueguen et al., Reference Gueguen, Vavre, Gnankine, Peterschmitt, Charif, Chiel, Gottlieb, Ghanim, Zchori-Fein and Fleury2010). Here, we surveyed more populations of T. vaporariorum, B. tabaci and an additional whitefly species, S. phillyreae, and focused on populations imported in Croatia and Montenegro. Unexpectedly, the infection statuses of the different whitefly populations differed from those obtained in 2008, and new infections were discovered. These results may suggest movement of whitefly populations within and between Eastern Adriatic neighboring countries over time. The results further confirm recent findings obtained from China which showed that, over time, infection levels in secondary symbionts of B. tabaci populations change markedly for some endosymbionts (Chu et al., Reference Chu, Gao, De Barro, Zhang, Wan and Khan2011).
Materials and methods
Whitefly populations
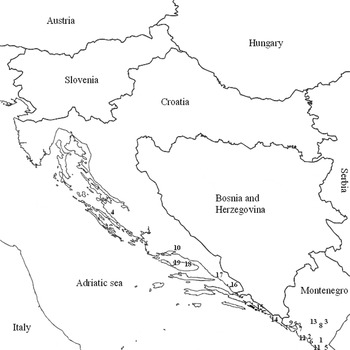
Three whitefly species were collected in summer 2011 from open fields or greenhouses in coastal Croatia and Montenegro (fig. 2). Sampling locations and host plants are shown in table 1. The sampling of B. tabaci and T. vaporariorum in Croatia included only new populations, imported on ornamental plants, and that from Montenegro contained new B. tabaci and T. vaporariorum populations collected from different locations. Collections were made in the summer when high population densities were available. Bemisia tabaci and T. vaporariorum nymphs were collected in the laboratory from leaves of different host plant samples in the field and moved into ethanol. Siphoninus phillyreae nymphs were similarly collected from leaves of pomegranate trees (Punica granatum L.) in gardens and orchards. Adults of the three whitefly species were collected using a Pasteur pipette attached to a hand-held aspirator and moved into ethanol. Scarcity in the egg stage was observed for all studied species, possibly due the very fast generation turnover in the summer season, thus eggs were not used for subsequent analysis. The collected insects were kept at −20 °C, until processing. One imported population of B. tabaci from Italy and one T. vaporariorum population from Slovenia were found in Croatia at the nymphal stage on host plants Hibiscus sp. and Euphorbia pulcherrima Willd., respectively. Imported infested plants were maintained in insect-proof cages in the laboratory under standard conditions (26 ± 2 °C, 60% RH, 14:10h of light:dark) until adult emergence.

Fig. 2. Locations of collected whitefly populations in coastal Croatia and Montenegro according to population numbers in table 1.
Table 1. Presence (+) or absence (−) of secondary symbionts (SS) in B. tabaci, T. vaporariorum and S. phillyreae populations tested in this study.

* Population imported by trade from Italy (Rome, region Lazio).
** Population imported by trade from Slovenia (Čatež ob Savi).
R, Rickettsia; H, Hamiltonella; Ars580, Arsenophonus 580bp; Ars760, Arsenophonus 760bp; W, Wolbachia; C, Cardinium
Screening of secondary bacterial symbionts and B. tabaci species
Adult females (n = 10–20 per population) from the studied whitefly species were tested for the presence of secondary bacterial symbionts, as were species (MED or MEAM1) of four B. tabaci populations. Genomic DNA of each individual was extracted in lysis buffer as previously described and used for species and secondary symbiont screening (Chiel et al., Reference Chiel, Gottlieb, Zchori-Fein, Mozes-Daube, Katzir, Inbar and Ghanim2007). Bemisia tabaci MED or MEAM1 groups were identified using microsattelite markers by polymerase chain reaction (PCR) amplification using Bem 23 primers and fragment size. The product size obtained from B biotype (MEAM1) was 200bp and from the Q biotype (MED) was 400bp. Three B. tabaci MED populations that were collected in this study were confirmed to belong to the Q1 group based on Mitochondrial Cytochrome Oxidase I (COI) sequences that were obtained following the protocol described in Gueguen et al. (Reference Gueguen, Vavre, Gnankine, Peterschmitt, Charif, Chiel, Gottlieb, Ghanim, Zchori-Fein and Fleury2010). The sequences showed 99.5–99.8% similarity (0.2–0.5% divergence) to the Q1 group, while representative Q2 and Q3 group sequences generated in Gueguen et al. (Reference Gueguen, Vavre, Gnankine, Peterschmitt, Charif, Chiel, Gottlieb, Ghanim, Zchori-Fein and Fleury2010) diverged by 1.5–2.0%. Numerous studies around the world using different molecular methods have identified six secondary symbionts in whiteflies: Rickettsia (R), Hamiltonella (H), Arsenophonus (A), Wolbachia (W), Cardinium (C) and Fritschea (F). The presence of these secondary symbionts was tested by PCR using genus-specific primers (16S or 23S rDNA) (table 2). PCR was carried out in 20μl volume containing 4μl DNA lysate, 20 pmol of each primer, 10mM dNTP mix, 10 Dream Taq buffer (+MgCl2) and 5 units μl−1 of Dream Taq polymerase (Fermentas). PCR products were visualized on a 1.5% agarose gel containing ethidium bromide. To verify the PCR products, bands were eluted and DNA was sent for sequencing (3730 xl DNA analyzer, Macrogen Europe, Amsterdam, Netherlands). The sequences were compared with those in the databases using BLAST algorithm in NCBI; 10–20 samples per set of primers were sequenced.
Table 2. List of primers used in the research.

Fluorescent in situ hybridization analysis
FISH analysis of S. phillyreae adults and nymphs and B. tabaci nymphs (for reference) was performed as previously described (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010), using short symbiont-specific 16S/23S rRNA DNA probes harboring a fluorescent Cy3/Cy5 molecule on their 5′ end (table 3 in Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). Stained samples were mounted whole and viewed using an IX81 Olympus FluoView 500 confocal microscope (Olympus, Tokyo, Japan). For each developmental stage, at least 30 specimens were viewed under the microscope to confirm reproducibility. Optical sections (0.7–1.0μm thick) were prepared from each specimen. Specificity of detection was confirmed using no probe staining and RNase digested specimen staining. In addition, each population was tested with all of the probes as controls. Thus, staining of a population known not to have a particular symbiont but harboring others was performed.
Results
General results for all whitefly species tested
Twenty individuals from each collected population were tested for the presence of the different secondary symbionts in each individual using genus-specific primers, except for two T. vaporariorum populations where only ten individuals were analyzed. Portiera aleyrodidarum, the primary symbiont of whiteflies, was detected in all individuals tested and served as a control for the quality of the extracted DNA. Fritschea was not detected in any of the whitefly individuals tested.
Bemisia tabaci infection by secondary symbionts
Bemisia tabaci populations were collected in Montenegro and Croatia (table 1 and fig. 3). Three populations were collected in Montenegro and were identified as MEAM1 and MED groups. The MEAM1 population was infected with Hamiltonella and Rickettsia. Hamiltonella was fixed in the population, while Rickettsia was highly prevalent. The MED populations collected in Bar and Ulcinj showed high levels of multiple infections with Rickettsia, Hamiltonella, Arsenophonus and Wolbachia, with some of these symbionts being fixed or close to fixation such as Rickettsia and Arsenophonus. Hamiltonella was present in 30–95% of the tested populations, Wolbachia in 30–40% and Rickettsia in about 65% of the tested individuals. Within all B. tabaci populations from Montenegro, 22% of all individuals showed single infection with secondary symbionts, 40% showed double infection, 22% were infected with three symbionts, and 13% showed infection with four symbionts. Overall, 97% (58/60) of all B. tabaci individuals from Montenegro were infected with at least one secondary symbiont; only two individuals did not contain any of the tested secondary symbionts. The B. tabaci MED population imported from Italy to Croatia (table 1) was infected with Hamiltonella, Rickettsia, Arsenophonus and Wolbachia (fig. 3), with high prevalence of mixed infections.
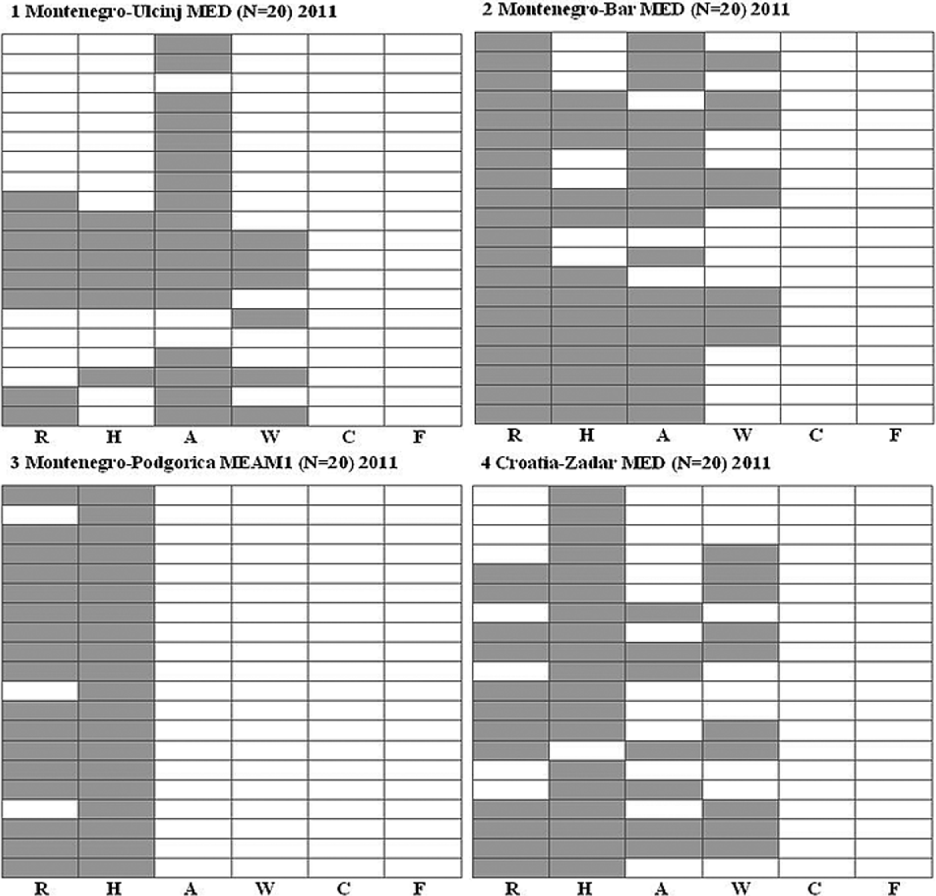
Fig. 3. Individual and multiple infections by secondary bacterial symbionts in four B. tabaci populations from Montenegro and Croatia (introduced from Italy). Each square represents one population, and each column represents one type of symbiont; the 10–20 rows per table represent the 10–20 individuals tested per population. Gray fields indicate positive infection for the tested symbiont. Population number, country and geographical location, species, number of tested individuals and year of sampling are indicated at the top of each table. Symbionts: R, Rickettsia; H, Hamiltonella; A, Arsenophonus; W, Wolbachia; C, Cardinium; F, Fritschea.
Trialeurodes vaporariorum infection by secondary symbionts
Five T. vaporariorum populations were collected in Montenegro and one in Croatia (fig. 4). Trialeurodes vaporariorum was more prevalent than B. tabaci in all examined locations of Montenegro, as is the case in Croatia. Arsenophonus was the most prevalent symbiont and was fixed or close to fixation in the populations collected in Montenegro (fig. 4). In addition, 20–45% infection rates with Hamiltonella were detected, whereas Cardinium infected about 30% of the individuals. Wolbachia and Rickettsia were present in only 3% of all individuals tested. Since almost all T. vaporariorum individuals harbored Arsenophonus, double infections were prevalent and included, besides Arsenophonus, one of the other detected symbionts, such as Cardinium, Wolbachia and Hamiltonella. Only three cases of triple infections were found. Two of the cases included Arsenophonus, Cardinium and Hamiltonella, and the third case included Arsenophonus, Wolbachia and Rickettsia. Overall, 99% (89/90) of all T. vaporariorum individuals from Montenegro were infected with at least one secondary symbiont, while only one individual did not contain any of the tested secondary symbionts. One T. vaporariorum population imported from Slovenia to Croatia (Split) showed infection with Arsenophonus and Hamiltonella. Arsenophonus was more prevalent with an 80% infection rate in all individuals examined, while Hamiltonella infected 50% of the tested individuals. Double infections with both symbionts, as well as individuals that did not contain any secondary symbiont, were also detected.

Fig. 4. Individual and multiple infections by secondary bacterial symbionts in the six T. vaporariorum populations collected from Montenegro and Croatia (introduced from Slovenia). For more information see the legend of fig. 3.
Siphoninus phillyreae infection by secondary symbionts
Nine populations of S. phillyreae were collected across coastal Croatia and Montenegro (fig. 2) in summer 2011, when heavy ash whitefly infestations of pomegranate were observed in both countries. These populations were tested for individual and multiple infections by secondary symbionts (fig. 5). All populations showed high heterogeneity in secondary symbiont composition. Hamiltonella showed the highest prevalence in tested populations, detected in 85% of the tested individuals and was fixed or close to fixation in the populations collected in Croatia. Wolbachia appeared with infection rate ranging from 15% to 70% of individuals from the populations collected both in Croatia and Montenegro. Infection rates with Cardinium ranged from 5% to 70% of all individuals in the Croatian populations but were not detected in any S. phillyreae population collected in Montenegro. Arsenophonus was present in all populations tested, and infection rates ranged from 20% to about 100%. In most of the S. phillyreae populations tested, Arsenophonus exhibited two alleles, a 580bp (Ars580) and a 760bp (Ars760) PCR products. Both products showed close to 100% similarity to the 23S rDNA of the Arsenophonus symbiont of S. phillyreae. Overall, about 98% (177/180) of all S. phillyreae individuals were infected with at least one secondary symbiont.

Fig. 5. Individual and multiple infections by secondary bacterial symbionts in the nine S. phillyreae populations from coastal Croatia and Montenegro. For more information, see the legend of fig. 3. Gray fields for (A) Arsenophonus represent the Ars580 allele and dark gray fields represent Arsenophonus with the Ars760 allele.
Localization of secondary symbionts in S. phillyreae
Since localization of secondary symbionts in B. tabaci and T. vaporariorum was previously studied (Gottlieb et al., Reference Gottlieb, Ghanim, Gueguen, Kontsedalov, Vavre, Fleury and Zchori-Fein2008; Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010), we focused our FISH experiments on S. phillyreae. Only adults and nymphs were tested due to the scarcity of eggs in the collected locations and difficulties in collecting enough eggs for the analyses. Bemisia tabaci nymphs were used in the FISH experiments for reference. In the developmental stages tested, symbionts were either randomly scattered outside the bacteriocyte or confined to the bacteriocyte with the primary symbiont. Interestingly, localization patterns of some of the symbionts differed from the previously reported ones. The localization of Portiera, the primary symbiont, revealed an interesting structure of the bacterisome, which had not been seen previously in other whitefly species. In the nymphal stages of B. tabaci and T. vaporariorum, the bacteriosomes always appear as two separate structures (for example figs 6H–I, 7H–I and 8H–I); whereas, in S. phillyreae, they are always connected (figs 6E–F and 7E–F). Surprisingly, Hamiltonella was localized outside the bacteriosome and showed a random and scattered localization pattern in adults (fig. 6A–C) and nymphs (fig. 6D–F), compared with the confined localization of Hamiltonella in B. tabaci (fig. 6G–I). Hamiltonella was observed in the circumference of bacteriocytes in adults or nymphs, where co-localized with Portiera. It was localized in other tissues as well, mainly in the abdomen and adult head, and was sometimes observed in the thorax (fig. 6A–C). This is the first time such a localization pattern has been observed in a whitefly species. Previously, only confined localization had been observed in B. tabaci and T. vaporariorum (Gottlieb et al., Reference Gottlieb, Ghanim, Gueguen, Kontsedalov, Vavre, Fleury and Zchori-Fein2008; Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010).

Fig. 6. Portiera and Hamiltonella FISH of S. phillyreae (A–C) adults and (D–F) nymphs and B. tabaci (G–I) nymphs. Portiera-specific probe (red) and Hamiltonella-specific probe (green) were used. (A, D and G) FISH of Hamiltonella alone, (B, E and H) double FISH of Hamiltonella and Portiera under dark field, and (C, F and I) double FISH of Hamiltonella and Portiera under bright field are shown.
Arsenophonus co-localized with Portiera, inside the bacteriosome in S. phillyreae adults (fig. 7A–F), as was previously shown for Arsenophonus from B. tabaci MED and T. vaporariorum (Gottlieb et al., Reference Gottlieb, Ghanim, Gueguen, Kontsedalov, Vavre, Fleury and Zchori-Fein2008; Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010), and was also confirmed here (fig. 7G–I). Arsenophonus was observed to be rod-shaped in TEM and light microscopy results of cell lines infected with this bacterium (Szklarzewicz & Moskal, Reference Szklarzewicz and Moskal2001) and appears to have a similar localization pattern in S. phillyreae.

Fig. 7. (A–F) Portiera and Arsenophonus FISH of S. phillyreae adults and (G–I) B. tabaci nymphs. Portiera-specific probe (red) and Arsenophonus-specific probe (yellow) were used. (A, D and G) FISH of Arsenophonus alone, (B, E and H) double FISH of Arsenophonus and Portiera under dark field, and (C, F and I) double FISH of Arsenophonus and Portiera under bright field. D, E and F are zoom-in images of the FISH signals in A, B and C, respectively.
Wolbachia has been previously shown to localize at the circumference of and inside the bacteriocytes of B. tabaci and was also seen in the abdomen outside the bacteriosome (Gottlieb et al., Reference Gottlieb, Ghanim, Gueguen, Kontsedalov, Vavre, Fleury and Zchori-Fein2008). Further FISH analysis on B. tabaci populations from Croatia have shown that Wolbachia could only be detected inside the bacteriocytes with the primary symbiont, and was not detected in any other organ at any developmental stage (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). In the current study, Wolbachia was confined to the bacteriosome in the adult stage of S. phillyreae (fig. 8A–C) but was not detected in the nymphal stage (fig. 8D–F), possibly due to low amounts of the bacterium. This localization pattern is similar to the confined pattern that was previously observed in B. tabaci and confirmed in the current study (fig. 8G–I). The localization of Wolbachia in other insects is diverse and was shown to localize to several organs, including the salivary glands, gut, Malpighian tubules, fat body and brain (Min & Benzer, Reference Min and Benzer1997; Ijichi et al., Reference Ijichi, Kondo, Matsumoto, Shimada, Ishikawa and Fukatsu2002; Mitsuhashi et al., Reference Mitsuhashi, Saiki, Wei, Kawakita and Sato2002).
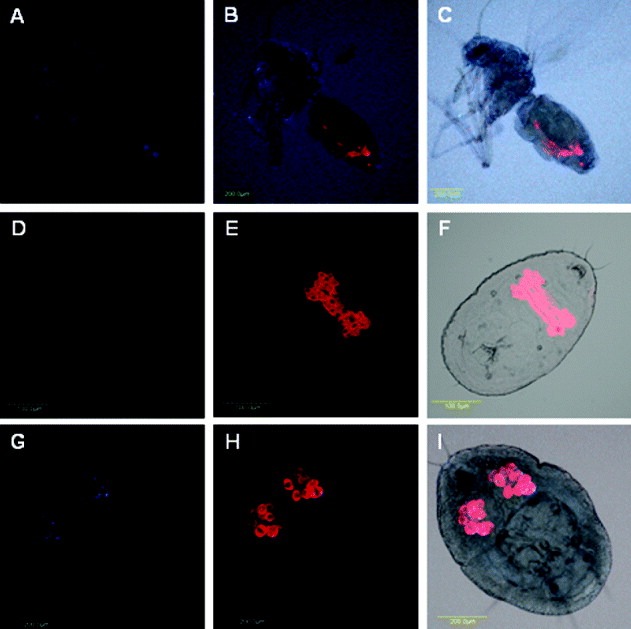
Fig. 8. Portiera and Wolbachia FISH of S. phillyreae adults (A–C) and nymphs (D–F) and B. tabaci nymphs (G–I). Portiera-specific probe (red) and Wolbachia-specific probe (blue) were used. FISH of Wolbachia alone (A, D and G), double FISH of Hamiltonella and Portiera under dark field (B, E and H), and double FISH of Hamiltonella and Portiera under bright field (C, F and I) are shown.
Cardinium was the only endosymbionts not detected in any of the developmental stages of S. phillyreae using FISH, possibly due to low amounts of the bacterium which are below detection levels. Previous research has shown that Cardinium localizes both inside and outside the bacteriosome of B. tabaci; however, it was not detected in T. vaporariorum (Gottlieb et al., Reference Gottlieb, Ghanim, Gueguen, Kontsedalov, Vavre, Fleury and Zchori-Fein2008; Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). Rickettsia was not detected by PCR, suggesting that this bacterium does not infect S. phillyreae.
Discussion
This study reports on a detailed screening of secondary symbionts of B. tabaci and T. vaporariorum in Montenegro, extending a previous screening of these whiteflies in Croatia, and provides a first such screen of S. phillyreae populations in the two countries. A first report of Arsenophonus in a Croatian B. tabaci MED population, introduced via the plant trade, further clarifies the previously observed diversity in secondary symbionts of Croatian whitefly populations. The MED populations found in Croatia and Montenegro were confirmed to belong to the Q1 group, as assessed by sequencing the COI gene based on the work conducted by Gueguen et al. (Reference Gueguen, Vavre, Gnankine, Peterschmitt, Charif, Chiel, Gottlieb, Ghanim, Zchori-Fein and Fleury2010). This fact is particularly important and relevant to the current study because the Q1 group reported here, the populations that were previously described (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010) from Croatia and the Q1 populations described by Gueguen et al. (Reference Gueguen, Vavre, Gnankine, Peterschmitt, Charif, Chiel, Gottlieb, Ghanim, Zchori-Fein and Fleury2010) harbor similar secondary symbiont loads. These results confirm the Q1 identity of the populations tested in the current study. A B. tabaci population from Montenegro, identified as MEAM1, was found to harbor Rickettsia and Hamiltonella, while the MED group from Montenegro harbored Rickettsia, Hamiltonella, Arsenophonus and Wolbachia. According to our previous data, a MEAM1 B. tabaci population collected in Montenegro (Podgorica) in 2008 was similar in symbiont composition to a MED B. tabaci population collected in Croatia and another MED population collected in Bosnia and Herzegovina, carrying only Hamiltonella and Wolbachia (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). The B. tabaci MEAM1 population collected in the same location in Montenegro, three years later (in 2011) showed infection status similar to that of a B. tabaci MEAM1 from Israel, harboring only Rickettsia and Hamiltonella (Chiel et al., Reference Chiel, Gottlieb, Zchori-Fein, Mozes-Daube, Katzir, Inbar and Ghanim2007). In the present survey, two B. tabaci populations from Montenegro, identified as the MED group, contained Arsenophonus and Hamiltonella, among other symbionts. Interestingly, in neighboring Croatia and in Bosnia and Herzegovina, only the MED Q1 was found among the tested B. tabaci populations and none of them carried Arsenophonus, unlike the MED group in Israel that belongs to the Q2 group, which has never been infected with Hamiltonella (Chiel et al., Reference Chiel, Gottlieb, Zchori-Fein, Mozes-Daube, Katzir, Inbar and Ghanim2007; Gueguen et al., Reference Gueguen, Vavre, Gnankine, Peterschmitt, Charif, Chiel, Gottlieb, Ghanim, Zchori-Fein and Fleury2010; Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). In B. tabaci MED populations from Montenegro, Arsenophonus and Hamiltonella were found together in 43% of the tested individuals, similar to Q1 populations described from Burkina, while this was never recorded in the Q2 or Q3 B. tabaci groups described from several countries (Gueguen et al., Reference Gueguen, Vavre, Gnankine, Peterschmitt, Charif, Chiel, Gottlieb, Ghanim, Zchori-Fein and Fleury2010). Co-infection of Arsenophonus and Hamiltonella was also frequent in T. vaporariorum populations from Croatia and from Bosnia and Herzegovina, whereas in the present survey, these two symbionts were found together in 28% of the individuals in T. vaporariorum populations from Montenegro (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). Based on our previous and current study, and the study of Gueguen et al. (Reference Gueguen, Vavre, Gnankine, Peterschmitt, Charif, Chiel, Gottlieb, Ghanim, Zchori-Fein and Fleury2010), the composition of secondary symbionts in B. tabaci populations from Croatia, Bosnia and Herzegovina, and Montenegro reveals high diversity and heterogeneity among the different species and populations (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). This can be attributed to the existence of the MED and MEAM1 groups, and the Q1, Q2 and Q3 sub-groups of MED.
In summer 2011, a B. tabaci population was found on Hibiscus sp. imported from Italy into Croatia. Infection of this B. tabaci MED population with Arsenophonus represented the first finding of this secondary symbiont in Croatia since 2008. This further clarifies the unique co-infection pattern in recently tested Croatian B. tabaci populations, which is suggested to be due to horizontal symbiont transfer, introduction of new whitefly populations via the plant trade or whitefly populations with new infections that exist in some niches and were not sampled in the survey conducted in 2010. Chu et al. (Reference Chu, Gao, De Barro, Zhang, Wan and Khan2011) showed that the symbiotic composition in the MED and MEAM1 groups tested in China markedly changed over time, suggesting that other unknown factors may influence this composition.
Trialeurodes vaporariorum is much more prevalent than B. tabaci in Croatia and Montenegro, most likely due to climate conditions. In a previous survey, Arsenophonus and Hamiltonella were the only two symbionts detected in T. vaporariorum populations collected in Croatia, and Bosnia and Herzegovina (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010). In the current study, a more diverse composition of secondary symbionts was recorded in T. vaporariorum populations from Montenegro, where five tested populations harbored Rickettsia, Hamiltonella, Arsenophonus, Wolbachia and Cardinium. Arsenophonus was prevalent in all of them, showing a pattern of fixation or near fixation. The present Arsenophonus infections are similar to those reported from T. vaporariorum populations collected in Croatia, and Bosnia and Herzegovina. Such infection rates may indicate a mutualistic or obligatory association with the insect host and indicate either a functional advantage for the host or manipulation of its reproduction (Gottlieb et al., Reference Gottlieb, Ghanim, Gueguen, Kontsedalov, Vavre, Fleury and Zchori-Fein2008). Indeed, Arsenophonus was shown to be a reproductive manipulator in other insects (Werren et al., Reference Werren, Skinner and Huger1986), and further investigation is needed to confirm or refute this hypothesis in T. vaporariorum.
Heavy S. phillyreae infestation of pomegranate led us to initiate a first survey of its secondary symbionts in Croatia and Montenegro. We found S. phillyreae to harbor Hamiltonella, Arsenophonus, Wolbachia and Cardinium. Hamiltonella showed the highest prevalence, infecting 85% of all tested individuals. In most of the populations tested, Hamiltonella was fixed or close to fixation, whereas Arsenophonus showed similar infection status in all S. phillyreae populations from Montenegro and two Croatian populations. Interestingly, Arsenophonus appeared in two alleles of the 23S rDNA amplification. Close Arsenophonus relatives, such as Proteus, Yersinia, Providencia and Salmonella, and other whitefly species such as Aleurodicus disperses and Aleuroplantus gelatinosus besides S. phillyreae have been suggested to have an intervening sequence (IVS) inserted in their 23S rDNA which results in a longer PCR (Miller et al., Reference Miller, Pabbaraju and Sanderson2000; Thao & Baumann, Reference Thao and Baumann2004b). It appears that possession of an IVS is not a distinct species characteristic and is unevenly allocated within bacterial genera (Miller et al., Reference Miller, Pabbaraju and Sanderson2000). This would clearly explain why several individuals in the tested S. phillyreae populations appear to have an IVS while others do not.
Our study supports the hypothesis that closely related heritable bacteria are often distributed across distantly related insect hosts, due to possible horizontal transfer or host switching (Aksoy et al., Reference Aksoy, Chen and Hypša1997; Moran et al., Reference Moran, McCutcheon and Nakabachi2008). In this report, three different whitefly genera and species were found to share similar secondary symbionts, which also suggest that symbionts can survive, reproduce and undergo efficient colonization in new arthropod hosts. Arsenophonus and Hamiltonella have been shown to share and persist in new insect hosts, A. pisum, after transfer via microinjection from their natural aphid hosts (Russell & Moran, Reference Russell and Moran2005). The butterfly Acraea encedon (L.) from Tanzania is distantly related to the butterfly Hypolimnas bolina (L.) from the Fiji Islands, but they share an identical male-killing Wolbachia, which strongly implicates horizontal transmission of the male killing element (Dyson et al., Reference Dyson, Kamath and Hurst2002). Recently, Rickettsia was shown to be horizontally transferred between B. tabaci individuals via the plant host, explaining the presence of this secondary symbiont in distantly related B. tabaci species (Caspi-Fluger et al., Reference Caspi-Fluger, Inbar, Mozes-Daube, Katzir, Portnoy, Belausov, Hunter and Zchori-Fein2011). Moran et al. (Reference Moran, McCutcheon and Nakabachi2008) showed another example for the existence of closely related symbionts in evolutionary distant hosts, which may suggest inter- and intra-specific mechanisms for horizontal transmission.
A few of the populations surveyed here, particularly of T. vaporariorum from Montenegro, showed low infection rates with some secondary symbionts (Rickettsia, Wolbachia and Cardinium), suggesting recent introduction through horizontal transfer, the aforementioned plant trade or unknown factors that may influence the symbiotic composition over time, as was recently shown in Chinese populations of B. tabaci (Chu et al., Reference Chu, Gao, De Barro, Zhang, Wan and Khan2011). Zchori-Fein & Perlman (Reference Zchori-Fein and Perlman2004) presented a phylogenetic analysis of 16S rDNA of Cardinium, which revealed that distantly related arthropods can harbor closely related symbionts, and closely related Cardinium were found to cluster among closely related hosts. This pattern suggests host specialization and horizontal transmission, which is particularly likely between B. tabaci and T. vaporariorum, since they are known to share plant hosts (Skaljac et al., Reference Skaljac, Zanic, Goreta Ban, Kontsedalov and Ghanim2010).
Although the populations of the different whitefly species tested in this study were collected from different host plants, we did not detect significant correlation between host plant and specific symbiotic content in any of the three species. The domination of S. phillyreae on pomegranate may indicate the adaptation of this specific species to the plant; however, other factors may affect this adaptation, such as the ability of the insect to manipulate plant secondary metabolites and toxic materials, and climate conditions. Further investigations are required to obtain conclusions regarding the host-insect adaptation, and whether secondary symbionts play a role in these interactions. Such investigations under controlled conditions may shed light on the contribution of the host plant to the composition of secondary symbionts in each whitefly species.
Finally, our study revealed unique co-infection patterns in B. tabaci, T. vaporariorum and S. phillyreae from Croatia and Montenegro. Our previous and present study of secondary symbiont co-infection in B. tabaci suggest that it is difficult to associate genetic groups (species) with secondary symbiont composition. The observation of Rickettsia, Wolbachia and Cardinium in T. vaporariorum, as well as the high diversity of secondary symbionts in S. phillyreae, suggest horizontal transfer of secondary symbionts between whitefly species. This study and recent studies from China (Chu et al., Reference Chu, Gao, De Barro, Zhang, Wan and Khan2011) indicate that the symbiotic composition within whitefly populations is subject to change over time and space. These changes are possibly influences by a diversity of factors which are not fully known, but might be related to climate conditions, host plants, genetic background and other factors that are yet to be discovered. Recorded compositions of secondary symbionts contribute to a better understanding of their ecology and evolution within the assessed whitefly species and, subsequently, to designing research for discovering the functional role of secondary symbionts in their insect hosts.
Acknowledgements
The authors thank Milorad Raičević from the Biotechnical Faculty in Podgorica for his valuable help. This study was partially funded by Croatian Ministry of Science Education and Sports grant no. 091-0910468-0281, the grant ‘Whiteflies (Aleyrodidae), viruses that they transmit and Mediterranean fruit fly (Tephritidae) in horticulture of Croatia and Montenegro’ (Bilateral research collaboration, Croatia: Montenegro) and the grant ‘Newly introduced invasive pests in the plant production of Montenegro’ (Ministry of Science, Montenegro).