Introduction
The major challenge in agriculture today is increasing crop yields (Poppy et al., Reference Poppy, Jepson, Pickett and Birkett2014). An estimated 40% of the yield is compromised by arthropod pests and pathogens (Sobhy et al., Reference Sobhy, Erb, Lou and Turlings2014). Chemical control is the main method for suppressing arthropod pest populations. However, the irrational use of chemical pesticides can cause well-known problems, such as pest outbreaks resulting from the selection of resistant arthropod populations (Dutcher, Reference Dutcher, Ciancio and Mukerji2007), reduced populations of natural enemies (Roubos et al., Reference Roubos, Rodriguez-Saona and Isaacs2014), and environmental impacts. Efficient and sustainable insect-control strategies that conform to the principles of integrated pest management (IPM) (Flint, Reference Flint2012) can avoid the side-effects of chemical control, and at the same time, maintain insect populations under control.
Elicitors of plant resistance are promising IPM tools because they pose no risks of rapid evolution of pest resistance, besides enhancing biological-control efficacy (Stout et al., Reference Stout, Zehnder and Baur2002; Sobhy et al., Reference Sobhy, Erb, Lou and Turlings2014). Application of elicitors triggers the synthesis of plant defences against herbivores and pathogens (Inbar et al., Reference Inbar, Doostdar, Sonoda, Leibee and Mayer1998; Wasternack & Hause, Reference Wasternack and Hause2013), and these defences are modulated primarily by the phytohormones jasmonic acid (JA), salicylic acid (SA), and ethylene (ET) (Erb et al., Reference Erb, Meldau and Howe2012; Pieterse et al., Reference Pieterse, Van der Does, Zamioudis, Leon-Reyes and Van Wees2012). In the case of insect herbivores, elicitor-treated plants produce defences that act directly against the herbivore (Thaler et al., Reference Thaler, Stout, Karban and Duffey2001; Tierranegra-García et al., Reference Tierranegra-García, Salinas-Soto, Torres-Pacheco, Ocampo-Velázquez, Rico-García, Mendoza-Diaz, Feregrino-Pérez, Mercado-Luna, Vargas-Hernandez and Soto-Zarazúa2011) and/or indirectly by emitting volatile organic compounds (VOCs) that are attractive to natural enemies (James & Grasswitz, Reference James and Grasswitz2005; Thaler, Reference Thaler1999a; Sobhy et al., Reference Sobhy, Erb, Lou and Turlings2014).
An effective elicitor of plant resistance should possess a suitable degree of specificity, induce direct and indirect resistance at the plant growth stage susceptible to pests, and induce long-lasting and effective resistance against a broad spectrum of herbivores (Stout et al., Reference Stout, Zehnder and Baur2002). JA, the main phytohormone responsible for signal-transduction pathways in plants damaged by chewing insects (Wasternack & Hause, Reference Wasternack and Hause2013), and its catabolites (methyl jasmonate [MeJA], cis-jasmone) have been widely studied as elicitors in plants, against not only insect pests but also pathogen infections (Antico et al., Reference Antico, Colon, Banks and Ramonell2012; Meldau et al., Reference Meldau, Erb and Baldwin2012; Wasternack & Hause, Reference Wasternack and Hause2013).
For many crop plants, the exogenous application of synthetic JA induces increased levels of defensive metabolites that negatively affect herbivore preference and/or performance (Thaler et al., Reference Thaler, Stout, Karban and Duffey1996; Thaler, Reference Thaler1999b; Omer et al., Reference Omer, Granett, Karban and Villa2001; Tierranegra-García et al., Reference Tierranegra-García, Salinas-Soto, Torres-Pacheco, Ocampo-Velázquez, Rico-García, Mendoza-Diaz, Feregrino-Pérez, Mercado-Luna, Vargas-Hernandez and Soto-Zarazúa2011; Accamando & Cronin, Reference Accamando and Cronin2012) as well as the emission of terpenes that are attractive to herbivore natural enemies (Thaler, Reference Thaler1999a; Thaler et al., Reference Thaler, Farag, Paré and Dicke2002; Gols et al., Reference Gols, Roosjen, Dijkman and Dicke2003; Bruinsma et al., Reference Bruinsma, Posthumus, Mumm, Mueller, van Loon and Dicke2009). However, the potential of JA as a plant-resistance elicitor has not yet been studied in one of the currently most important crop plants, sugarcane, a source of sugar and the biofuel ethanol (Dante et al., Reference Dante, Cristofoletti and Gerhardt2010). Brazil is the main producing country worldwide and has exported over US$ 7 billion annually in sugarcane commodities in the past few years (Cheavegatti-Gianotto et al., Reference Cheavegatti-Gianotto, de Abreu, Arruda, Bespalhok Filho, Burnquist, Creste, di Ciero, Ferro, de Oliveira Figueira and de Sousa Filgueiras2011). Nevertheless, sugarcane yield can be further increased by reducing damage from the sugarcane borer Diatraea saccharalis Fabricius (Lepidoptera: Pyralidae) (Cheavegatti-Gianotto et al., Reference Cheavegatti-Gianotto, de Abreu, Arruda, Bespalhok Filho, Burnquist, Creste, di Ciero, Ferro, de Oliveira Figueira and de Sousa Filgueiras2011). This key-pest impacts yield not only directly, because the larva feeds on the sugarcane stalk; but also indirectly, by facilitating colonization by opportunistic fungi (Ogunwolu et al., Reference Ogunwolu, Reagan, Flynn and Hensley1991; Cheavegatti-Gianotto et al., Reference Cheavegatti-Gianotto, de Abreu, Arruda, Bespalhok Filho, Burnquist, Creste, di Ciero, Ferro, de Oliveira Figueira and de Sousa Filgueiras2011).
Nowadays, D. saccharalis populations are exclusively controlled by releases of the larval parasitoid Cotesia flavipes Cameron (Hymenoptera: Braconidae) in half of the sugarcane area in Brazil (Parra, Reference Parra2014). This parasitoid wasp, native to Asia, is a gregarious koinobiont endoparasitoid specialized in mid-to-late instar stemborer larvae that attack grasses (Ngi-Song et al., Reference Ngi-Song, Overholt and Ayertey1995). It was introduced in several countries in the 1970s including Brazil, where its efficacy in controlling the sugarcane borer is superior to native parasitoids (Botelho, Reference Botelho1992), and greatly reduced the host population in sugarcane, which dropped from 7 to 2% (Botelho & Macedo, Reference Botelho, Macedo and Parra2002).
The parasitism rates of sugarcane borer by C. flavipes are around 30% (Wiedenmann & Smith, Reference Wiedenmann and Smith1993) even in Brazil (Botelho & Macedo, Reference Botelho, Macedo and Parra2002). In sugarcane plantations, C. flavipes host finding is likely mediated by odours emitted by the host, such as frass (Overholt et al., Reference Overholt, Ngi-Song, Omwega, Kimani-Njogu, Mbapila, Sallam and Ofomata1997), and herbivore-induced volatiles (Setamou et al., Reference Setamou, Bernal, Legaspi and Mirkov2002; Mesquita et al., Reference Mesquita, Mendonça, Da Silva, De Oliveira Correia, Sales, Cabral-Junior and Nascimento2011). Therefore, parasitism may be incremented by adopting tactics that increase attraction of the parasitoid to host-damaged plants, such as the use of plant resistance elicitors, which not only recruit parasitoids, but also enhance their efficiency of host-finding (Sobhy et al., Reference Sobhy, Erb, Lou and Turlings2014).
JA is a promising candidate as an elicitor in sugarcane for two reasons. First, JA treatment can cause side-effects on the production of flowers, fruits and seeds (Thaler, Reference Thaler1999b; Accamando & Cronin, Reference Accamando and Cronin2012), but sugarcane yield might not be affected as it depends only on the stalk, not the reproductive organs. Second, while in other crops systems MeJa promotes plant resistance against herbivores concomitant to mediating the plant susceptibility to infections (Thaler et al., Reference Thaler, Fidantsef, Duffey and Bostock1999, Reference Thaler, Agrawal and Halitschke2010), the few studies that have examined the effect of MeJA in sugarcane revealed increased defences against pathogens (Bower et al., Reference Bower, Casu, Maclean, Reverter, Chapman and Manners2005; Nogueira et al., Reference Nogueira, Menossi, Ulian and Arruda2005).
Here, we investigated the effect of JA treatment on direct and indirect defences against the specialist herbivore and main pest of sugarcane, D. saccharalis. We compared the effect of JA-induced sugarcane direct defences on the sugarcane borer with the effect on a generalist leaf herbivore, the fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), also a pest of sugarcane, to test whether herbivores with different degrees of specialization respond differently to sugarcane defences. We predicted that the specialist herbivore would not be affected by induced defences, or not as strongly affected as the generalist, because of the co-evolutionary history with the plant (van Dam & Oomen, Reference van Dam and Oomen2008; Ali & Agrawal, Reference Ali and Agrawal2012). Moreover, we examined the hypothesis that the sugarcane resistance was mediated by plant volatile emissions testing the olfactory response of only D. saccharalis, which was the target insect of our study. With regard to indirect defences, we hypothesized that the parasitoid C. flavipes would be attracted to JA-treated sugarcane, but that the JA treatment would not disrupt host location in a way that wasps can discriminate JA-treated from JA-treated and host-damaged sugarcane. As plant response to JA exogenous application can vary over time (Schmelz et al., Reference Schmelz, Alborn, Banchio and Tumlinson2003; Bruinsma et al., Reference Bruinsma, Posthumus, Mumm, Mueller, van Loon and Dicke2009), we tested the parasitoid response to plant volatiles of JA-treated sugarcane along a time course to select an interval when treated plants release attractive volatiles. To address these questions, we conducted behavioural assays with the herbivores to detect antixenosis and antibiosis types of resistance; olfactometry assays with the sugarcane borer and the wasps; and determined the plant volatile profile as well as leaf colorimetric parameters, in order to understand the underlying mechanisms of JA-induced resistance. Our results for the direct and indirect effects mediated by JA induction are discussed in both applied and ecological contexts.
Material and methods
Plants and insects
One-eyed seed sets of sugarcane Saccharum spp. cultivar ‘SP80-1842’, which is susceptible to D. saccharalis and expresses genes associated with herbivory-induced defences (Rocha et al., Reference Rocha, Papini-Terzi, Nishiyama, Vêncio, Vicentini, Duarte, de Rosa, Vinagre, Barsalobres and Medeiros2007; Medeiros et al., Reference Medeiros, Franco, Matos, de Castro, Santos-Silva, Henrique-Silva, Goldman, Moura and Silva-Filho2012), were grown in plastic pots (250 ml) with a substrate of organically grown coconut fibre (Golden Mix, Piracicaba, São Paulo, Brazil) and fertilized with Osmocote® (10N: 10P: 10 K). The plants were kept in a sealed, insect-free greenhouse under natural conditions of light, temperature and humidity (18–34°C, RH 40 ± 10%) until they developed four expanded leaves (30–35 days old), and were then used in the experiments. Diatraea saccharalis and S. frugiperda were reared under laboratory conditions (23 ± 2°C, RH 50 ± 10%, 12L:12D) and fed on artificial diet (Parra, Reference Parra2001). The parasitoid C. flavipes was fed with pure honey and multiplied in fourth-instar D. saccharalis larvae. The experiments were conducted from August 2013 to August 2014, in Piracicaba (22°42′S, 047°38′W and altitude 546 m) in the laboratory under controlled conditions (23 ± 2°C, RH 50 ± 10%) and using supplemental light for plants (12L:12D).
JA and herbivore-damage treatments
On the day before the JA treatment, four-leaf sugarcane plants were transferred from the greenhouse to a laboratory room to acclimate. Each plant was sprayed with 10 ml of an aqueous solution of 1 mM synthetic JA (Sigma-Aldrich, St. Louis, MO, USA) and 1% methanol. The JA concentration was determined in preliminary assays (fig. S1). Control plants (Mock) were sprayed similarly, but with no JA in the solution. In the assays in which we tested direct sugarcane defences, we used mock plants as controls and JA-treated plants at a 24 h time interval (designated as JA in this set of experiments). In the assays for indirect defences, we first tested JA treatments over time, at 24 h (JA 24 h), 48 h (JA 48 h) and 72 h (JA 72 h), and also performed assays with the same time periods on mock plants.
Plants were damaged by fourth-instar D. saccharalis larvae starved for 48 h. A single larva was enclosed in a cylindrical cage attached to the base of a sugarcane stalk in the morning, and was allowed to damage the plant for 24 h. Plants that were sprayed (JA or Mock) and exposed to herbivore damage, first received the application and 24 h later the larva was added. In two treatments, the plants were subjected to both spraying and herbivore damage: mock 48 h + D. saccharalis damage 24 h (Mock + DS) and JA 48 h + D. saccharalis damage 24 h (JA + DS). Thus, both treatments were tested 48 h after the spraying (either control or JA solution) and 24 h after D. saccharalis was introduced.
Herbivores performance
The effects of the sugarcane defences directly induced by JA on D. saccharalis and S. frugiperda were evaluated by performance and dual-choice preference assays. The performances of D. saccharalis and S. frugiperda larvae were assessed by weight gain when the larvae were fed on either mock or JA-treated plants. To assess D. saccharalis performance, third-instar larvae, starved for 72 h and previously weighed, were enclosed individually in a cage fixed to the base of a mock or JA-treated sugarcane stalk. Larvae were monitored every 2 h for 48 h, to evaluate if they had penetrated into the stalk. The performance of S. frugiperda was assessed in a similar experimental set-up. Third-instar larvae were also used, but placed individually on the meristem of a plant bagged with a fine-fabric bag to prevent the larva from escaping. Larvae of both species were recovered and weighed after feeding for 72 h, and their weight gain was estimated based on the difference between the final and the initial larval weights. We performed at least nine replicates for each assay.
Herbivores preference
The preference of D. saccharalis for mock or JA-treated plants was evaluated in an open arena made of Styrofoam™ (26 cm long, 11 cm wide and 22 cm high). In each trial, a third-instar sugarcane borer larva was released in the middle of the arena, with two plants, one of each treatment, placed on opposite sides of the arena. Potted plants were inserted in the arena through holes made in the base of the arena, such that the substrate was on the same level as the sugarcane base. In order to prevent the larva from escaping, the top of the arena was covered with fine-mesh fabric. The larva was considered to have made a choice if it penetrated into the stalk within 48 h.
To evaluate the feeding preference of S. frugiperda, we used a Petri dish (14 cm diameter) with two rectangular holes in the bottom (5 cm × 1 cm) and suspended on a platform (16.5 cm high) as the experimental arena. The second leaf of a mock or JA-treated plant was positioned under the bottom of the Petri dish so that the rectangular holes delimited the foliar surface, without excising the leaf from the plant. A single S. frugiperda third-instar larva was released in the middle of the arena in each trial. After 48 h, the leaves were removed and their images were scanned and imported into the software ImageJ 1.44p (National Institutes of Health, Bethesda, MD, USA) to estimate the consumed leaf area (cm2). We performed 14 replicates for both herbivore-preference assays.
Sugarcane borer olfactometer assays
The olfactory preference of D. saccharalis for mock or JA-treated plants was evaluated in a Y-tube olfactometer (side and main arms: 18.5 cm long and 1.5 cm internal diameter), during the day. The olfactometer was connected to an ARS Volatile Collection System (ARS, Gainesville, FL, USA), coupled to charcoal filters, humidifiers and flow-meters, which pushed filtered and humidified air through PTFE (polytetrafluoroethylene) hoses into sealed glass chambers containing the plants as odour sources, and thence into the side arms of the olfactometer. The air flow was adjusted to 1.0 l min−1 arm−1. The olfactometer was positioned horizontally and the side arms were rotated in every replicate to prevent side bias. Five third-instar D. saccharalis larvae were introduced into the olfactometer main arm, and their choice was recorded after 1 h, only for the larvae that crossed the distal third of the side arms. We conducted a total of eight trials.
Parasitoid olfactometer assays
The olfactory preference of 2-day-old naïve C. flavipes females was tested in a Y-tube olfactometer (side and main arms: 15 cm long and 4 cm internal diameter) following a method similar to that described above. The assays were set up during the photoperiod, from 08:00 to 16:00 h, when the wasps are more active (Setamou et al., Reference Setamou, Bernal, Legaspi and Mirkov2002). The olfactometer was positioned vertically and the device was rotated every two replicates to prevent side bias. A single female was introduced into the main arm, and was considered to have made a choice if it crossed the distal third of a side arm within 5 min. Wasps were discarded after the tests. Each replicate consisted of the olfactory preference of a single female, and for each assay we conducted 10 trials per pair of plants. We first evaluated the preference of C. flavipes exposed to the following combinations: (i) Mock vs. clean air (CA) and (ii) Mock vs. JA 24, 48 and 72 h. Based on these results, we performed a second set of experiments with JA 48 h, which was the first time interval in which the plants emitted attractive volatiles to wasps, to examine whether the wasps were able to discriminate JA-treated from host-damaged sugarcane. These assays included testing the combinations: (iii) Mock vs. Mock + DS; (iv) Mock + DS vs. JA; and (v) JA vs. JA + DS.
Plant colorimetric parameters
Plant visual cues were measured by colorimetric analyses. Colorimetric data of the adaxial surface of the second leaf of six mock and JA-treated sugarcane plants were obtained with a Chromameter CR-400 (Konika Minolta, Japan) that uses a D65 illuminant that resembles standard daylight. The parameters were expressed in CIE (Commission Internationale de l’Éclairage) system values for lightness level (*L), green (*a) and yellow colour (*b) (Konica Minolta, 1998).
Volatile collection and analysis
Sugarcane plants were placed individually in sealed glass chambers (10 cm diameter and 25 cm high) connected to the ARS Volatile Collection System. The airflow pushed into the chambers was adjusted to 1.0 l−1 min−1 and a vacuum pump connected to filters with the adsorbent polymer Haysep® (30 mg, Supelco, PA, USA) pulled air through the chambers at the same rate. We collected volatiles from six plants of each treatment (Mock and JA after 24, 48, and 72 h, Mock + DS and JA + DS) for 12 h during the photoperiod (08:00–20:00 h). Thereafter, polymer filters were washed with 150 µl of dichloromethane and the resultant extracts were stored in sealed glass vials, which were kept in a freezer at −30°C until the analysis. We added 2 µl of a 40 ng µl−1 nonyl acetate solution as internal standard to each sample. Samples were analyzed in a Varian 3800 gas chromatograph coupled to a Varian 4000 mass spectrometer (CG-MS) using helium as the carrier gas. We adopted Electron Impact (EI) in full scan as ionization method. Detector was calibrated to mass range analysis from 35 to 250 m z−1 and 25 ms of maximum ion time. We injected 2 µl of each sample into a nonpolar capillary column HP5-MS (JeW Scientific, Folsom, CA, USA; 30 m × 0.25 mm × 0.25 µm). The column temperature was kept at 40°C for 5 min, and then was raised at 5°C min−1 until it reached 150°C and then raised at 20°C min−1 until it reached 250°C. Each compound was quantified based on the compound peak area relative to nonyl acetate in the GC-MS, and corrected for the plant dry weight. It is important to note that this relative quantification does not provide real ratios between compounds in the blend. Compounds were identified by calculating the Kovats index as well as comparing their mass spectrum to the NIST 08 Mass Spectral Library and, when available, to those of the synthetic standards (table S1 for synthetic standards description).
Statistical analyses
The normality and heteroscedasticity of the data were confirmed with the Levene and Shapiro–Wilk tests. The data for larval weight gain obtained in the performance assays were analyzed with non-paired t tests, and the data on S. frugiperda consumed leaf area and D. saccharalis preference in the olfactometer assays were analyzed with paired t tests. The choices of C. flavipes in the olfactometer assays and the D. saccharalis preference in the arena tests were compared with a chi-square test. Colorimetric parameters of sugarcane leaves were tested with a MANOVA. Relative quantifications of volatile chemical classes over the time course were analyzed with a MANOVA, considering treatments as fixed effects and time intervals as random effects. As the MANOVA results were significant, it was followed by univariate ANOVA and Tukey's test for pairwise comparisons. The total amount of plant volatiles and quantification of volatile chemical groups were analyzed with a generalized linear model (GLM) followed by Tukey's test. Volatile composition was analyzed with a principal components analysis (PCA). Statistical analyses of behavioural assays (Y-tube assays, herbivore performance and preference) were conducted in R software version 3.1.1 (www.R-project.org), while analyses for volatile and colorimetric data were run in Minitab® Release 14 (Minitab Inc., State College, PA, USA) using the significance levels of 5 and 1%.
Results
Herbivores behaviour
The D. saccharalis and S. frugiperda larvae showed similar weight gains when fed on mock and JA-treated plants (fig. 1, non-paired t test, D. saccharalis: t = −0.11 P = 0.91; S. frugiperda: t = −0.91 P = 0.36). In the preference assay, both larvae preferred to feed on mock over JA-treated plants (fig. 1, chi-square test, D. saccharalis: χ2 = 4.57 P = 0.03; paired t test, S. frugiperda: t = 2.32 P = 0.03). However, in the olfactometer assays, D. saccharalis showed a different response by orienting preferentially to odours of JA-treated over mock plants (fig. 2, paired t test, t = −2.02 P = 0.04).
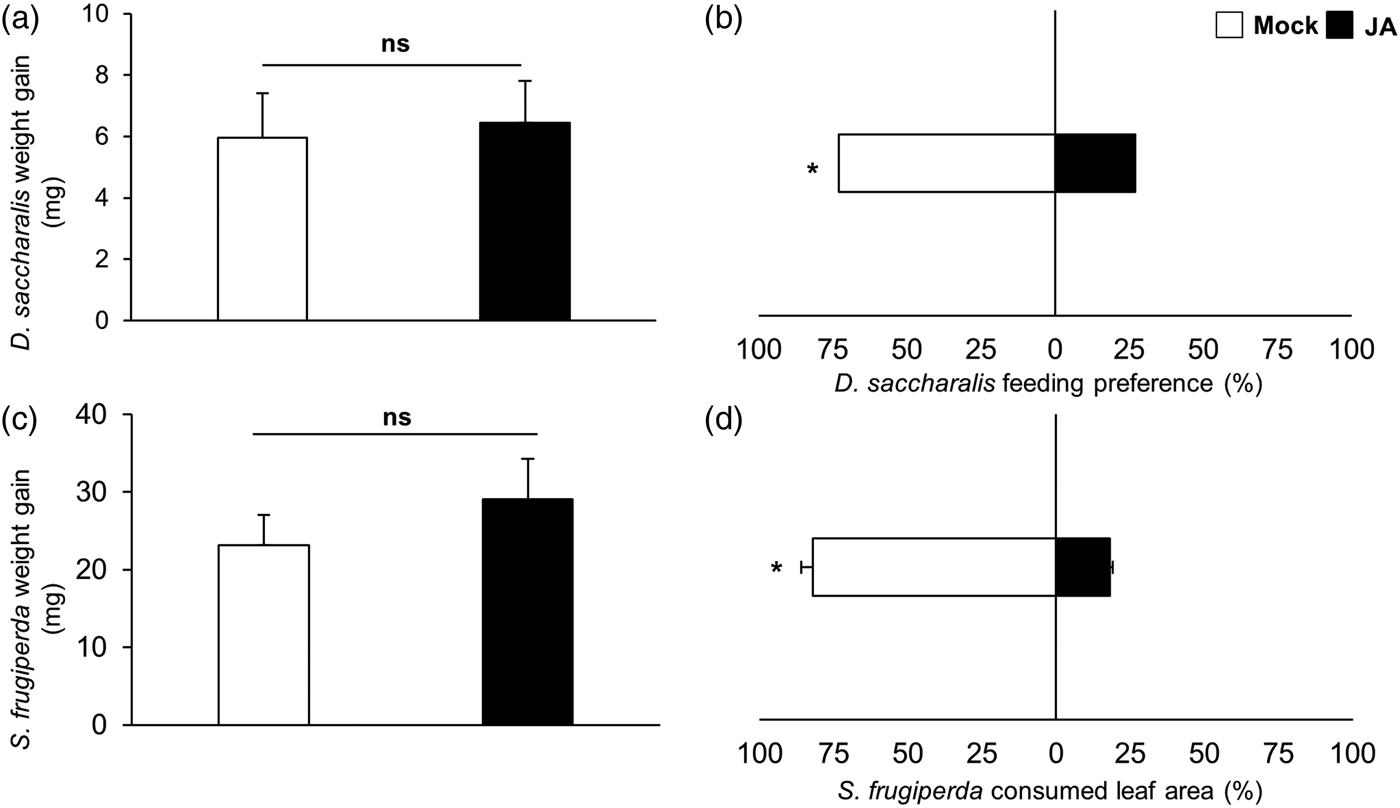
Fig. 1. Direct defence assay in mock and jasmonic acid-treated plants after 24 h (JA). Feeding performance of Diatraea saccharalis (a) and Spodoptera frugiperda (c); Feeding-preference assay of D. saccharalis (b) and S. frugiperda (d). * designates significant difference at 5% according to non-paired t-test (a and c), χ2 test (b) and paired t-test (d); ns, no significant difference.
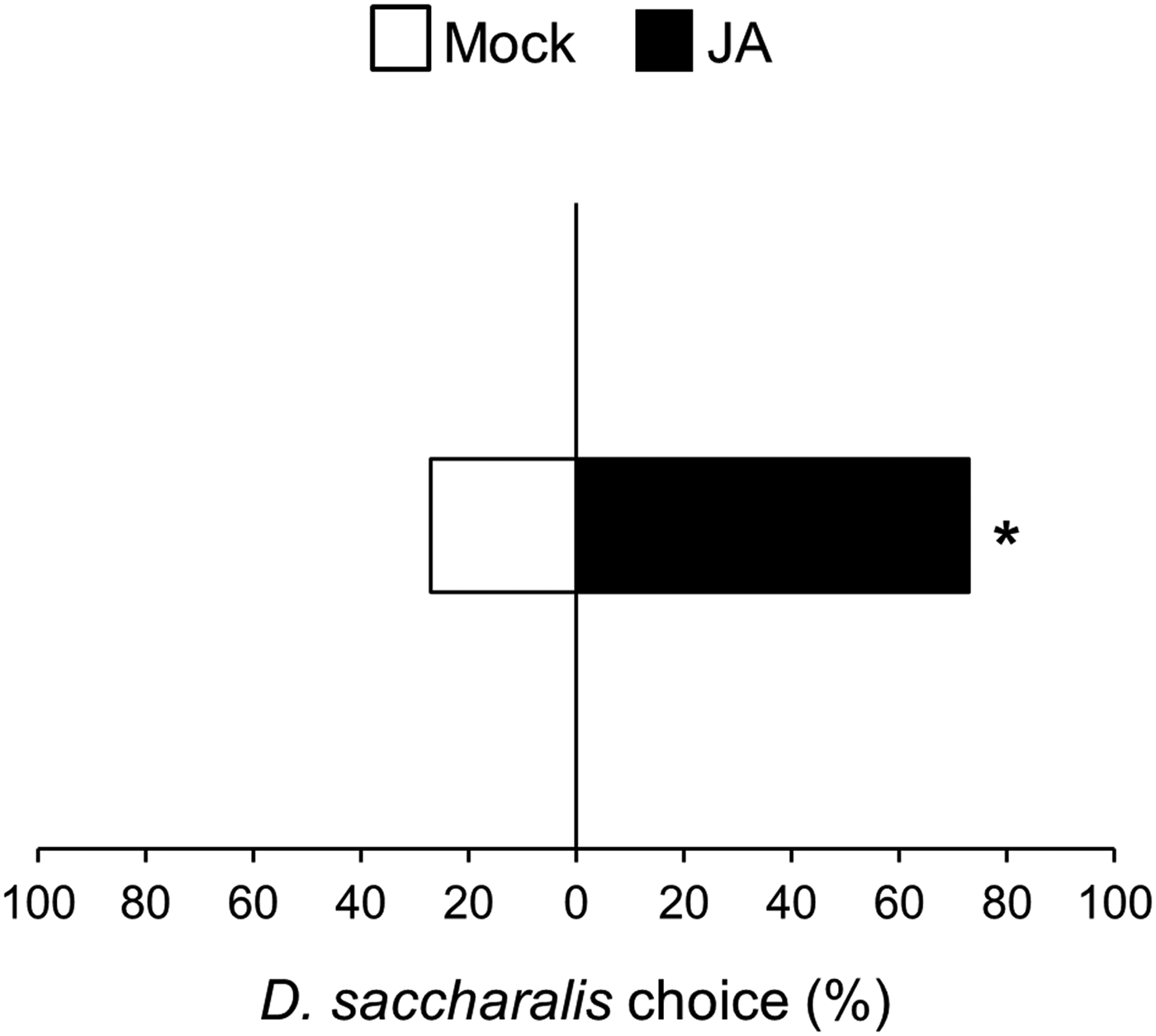
Fig. 2. Dual-choice preference of the herbivore Diatraea saccharalis for mock and JA-treated (JA) sugarcane volatiles in olfactometry assays. *Designates significant difference at 5% according to paired t-test.
Parasitoid behaviour
The parasitic wasp C. flavipes did not discriminate odours from mock and JA 24 h plants (fig. 3a, chi-square test, χ2 = 0.22 P = 0.63). However, the wasps preferred volatiles emitted from JA 48 h (χ2 = 8.06 P < 0.01) and JA 72 h (χ2 = 20.82 P < 0.01) over control plants (Mock).
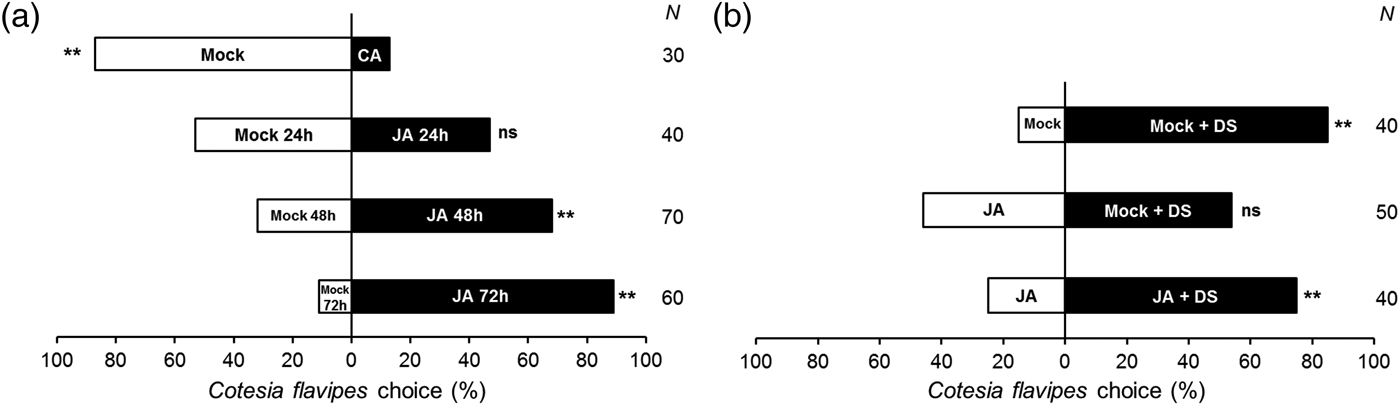
Fig. 3. Dual-choice preference of the larval parasitoid Cotesia flavipes for sugarcane volatiles in olfactometry assays. (a) jasmonic acid-induced (JA) and mock plant along time course; (b) damaged by the host Diatraea saccharalis and/or jasmonic acid. Bars indicate percentage of total choices for treatments. The number of replicates (N) is given on the right side of the figure. CA, clean air; Mock, mock plant after 24, 48 and 72 h; JA, jasmonic acid-treated plant after 24, 48 and 72 h; Mock + DS, mock plant followed by D. saccharalis damage; JA + DS, jasmonic acid-treated plant followed by D. saccharalis damage. **designates significant difference at 1% according to χ2 test; ns, no significant difference.
When C. flavipes was given a choice between Mock vs. Mock + DS, the wasp preferred volatiles from Mock + DS (fig. 3b, χ2 = 19.60 P < 0.01). However, the parasitoid did not differentiate volatile emissions of Mock + DS from JA (χ2 = 0.32 P = 0.57). C. flavipes preferred the odours released by JA + DS plants over JA plants (χ2 = 10.00 P < 0.01).
Plant visual and olfactory cues
None of the colorimetric parameters differed between JA-treated and mock sugarcane (fig. S2, MANOVA, Wilk's Criterion, F = 3.41, P = 0.073). In contrast, JA treatment in sugarcane significantly altered the volatile composition (MANOVA, Wilk's Criterion, treatment effect: F = 15.82, P < 0.001) by inducing qualitative and quantitative differences in the volatile blend compared to mock plants over time (fig. 4, fig. S3 and table S2). The benzenoid class of compounds was found in higher concentrations in the blend emitted by JA-treated plants after 24 h, and the content of sesquiterpenes was highest in JA-treated plants after 24 h, followed by 48 and 72 h; while the ester content did not change over time (MANOVA followed by univariate ANOVA, benzenoid: F 3;32 = 10.0, P = 0.003, Tukey P < 0.01; sesquiterpenes: F 3;32 = 41.8, P = 0.001, Tukey P < 0.01; ester: F 3;32 = 1.3, Tukey P = 0.29). Analyses of the individual volatiles revealed that the mock sugarcane blend contained a single ester compound, methyl anthranilate (fig. 4, table S2). JA-treated plants emitted higher total amounts of volatiles at 24 h relative to the later time intervals (table S2, GLM, Tukey's test T 24h;48h = – 3.17 P < 0.01, T 24;72h = – 4.12 P < 0.01), whereas no differences were detected in the total amounts released at 48 and 72 h (Tukey T 48h;72h = – 0.94 P = 0.61).
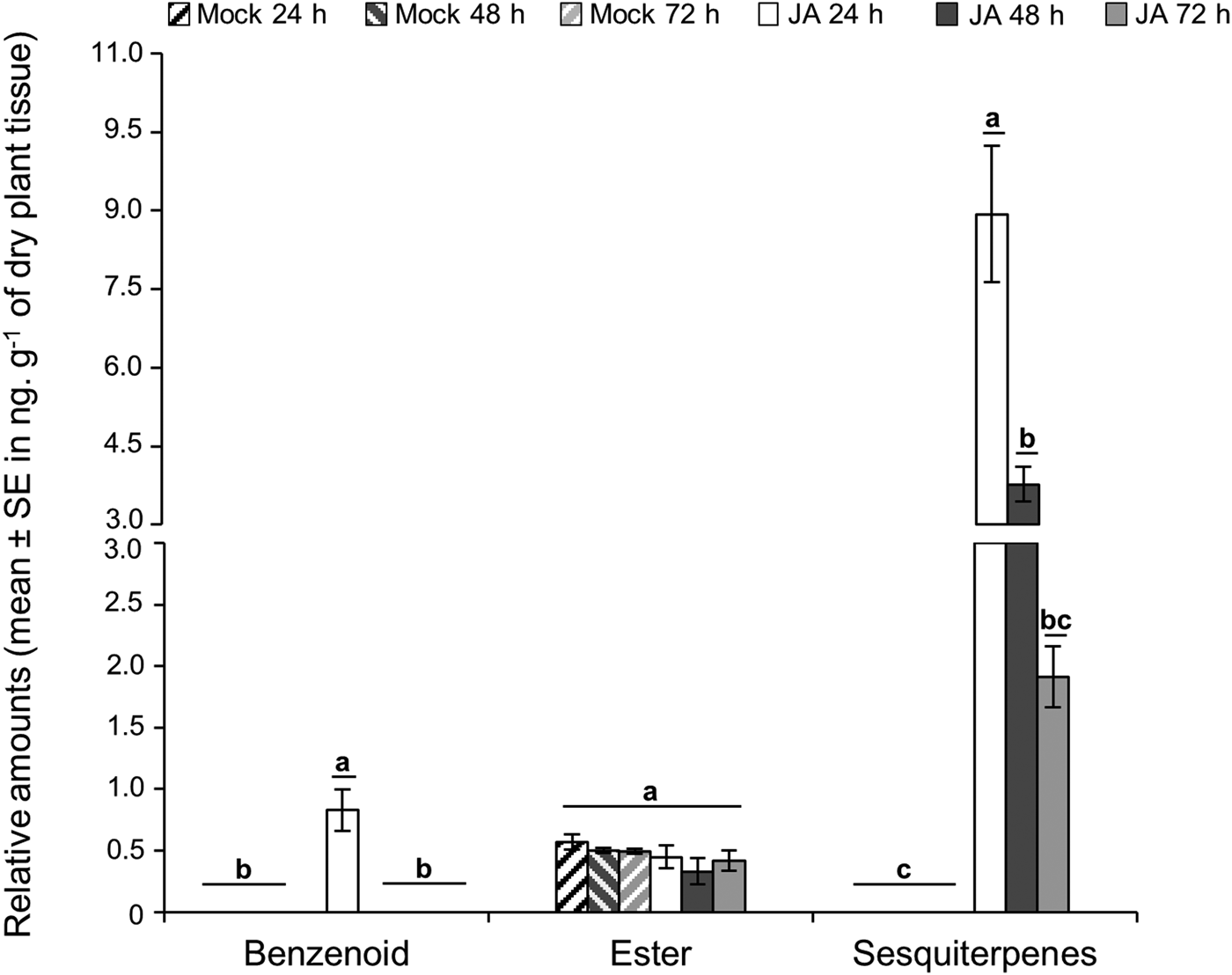
Fig. 4. Sugarcane volatile composition according to volatile chemical classes released by mock and jasmonic acid-treated plants (JA) after 24, 48 and 72 h. Relative amounts (mean ± SE in ng. g−1 of dry plant tissue) were estimated based on the internal standard. Same letters do not differ at 5% significance according to MANOVA followed by ANOVA and Tukey's test for pairwise comparisons.
In regard to the second set of experiments testing JA application and herbivore damage, the blend from herbivore-damaged sugarcane (Mock + DS) comprised mainly of sesquiterpenes and small amounts of benzenoids (fig. 5, fig. S4 and table S3), which included the benzenoid methyl salicylate, found only in this treatment, and the sesquiterpene β-caryophyllene (table S3). In contrast to the first volatile collection, mock and JA plants did not release the ester methyl anthranilate (figs 4 and 5, tables S1 and S2). Furthermore, mock plants released none of the 16 major plant volatiles found in the volatile collection during the time course. We also observed that JA released the benzenoid 1H-indole and a wider diversity of sesquiterpenes than in the first volatile collection. Doubly induced plants, JA + DS, released only alcohols (fig. 5, glm F 3;20 = 1506.93 P < 0.001, Tukey P < 0.001) and methyl anthranilate (glm F 3;20 = 592.71 P < 0.001, Tukey P < 0.001), and emitted the highest concentrations of 1H-indole (glm F 3;20 = 13.90 P < 0.001, Tukey P < 0.01) as well as sesquiterpenes (glm F 3;20 = 5.84 P < 0.01,Tukey P < 0.001).
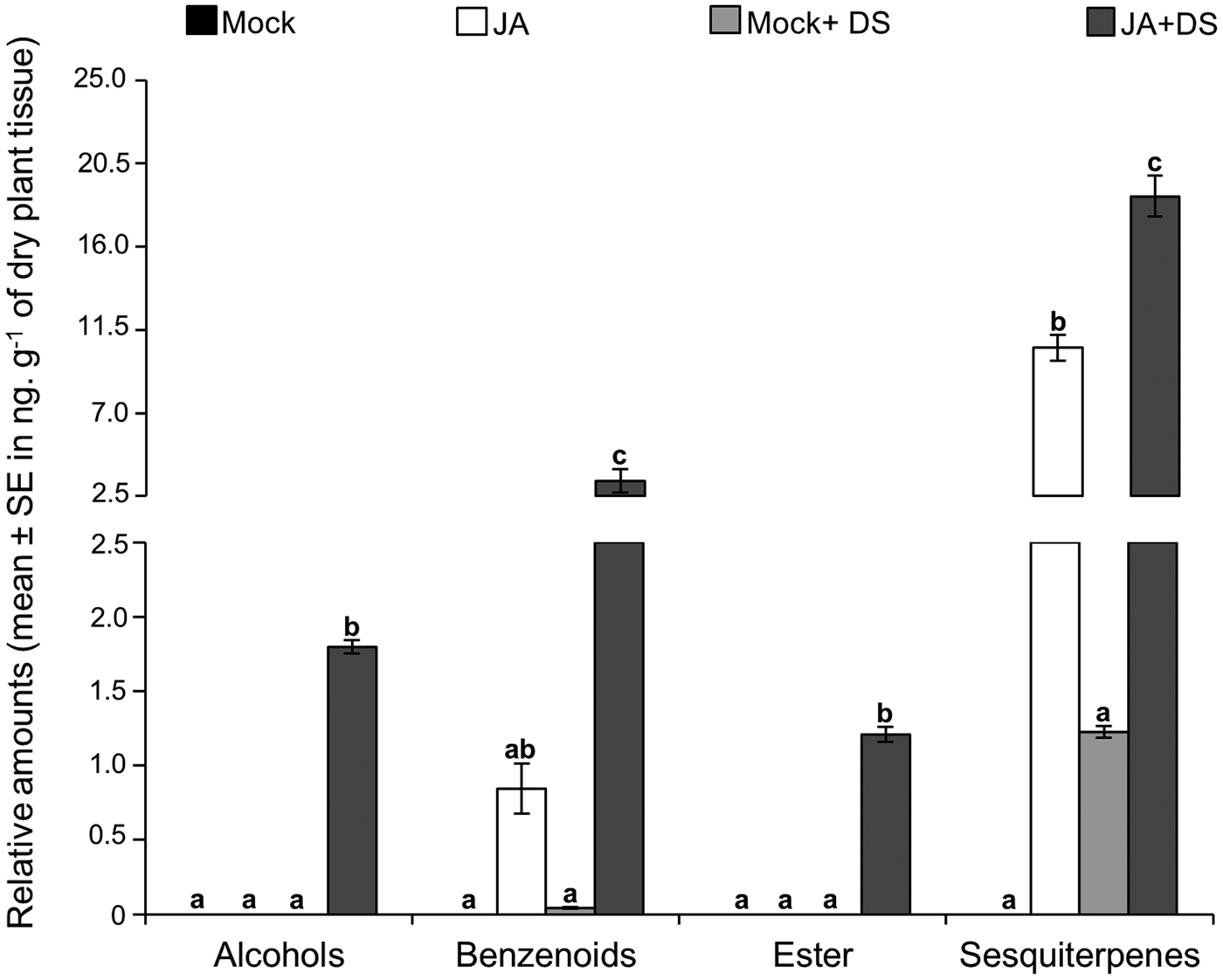
Fig. 5. Sugarcane volatile composition according to volatile chemical classes released by mock plants, jasmonic acid-treated plants after 48 h (JA), mock plant followed by Diatraea saccharalis damage (Mock + DS) and jasmonic acid-treated plant followed by D. saccharalis damage (JA + DS). Relative amounts (mean ± SE in ng. g−1 of dry plant tissue) were estimated based on the internal standard. Same letters do not differ at 5% significance according to general linear model test followed by Tukey's test for pairwise comparisons.
Analyses of the volatile composition using PCA for volatile collection over time (fig. 6a) showed that the first component, which comprised 61% of the variance, separated JA 24 and JA 48 h from the JA 72 h and all the mock plants. The PCA analyses regarding the second volatile-collection experiments (fig. 6b) revealed that the first component, which comprised 76% of the variance, clearly separated Mock, JA and Mock + DS from JA + DS.
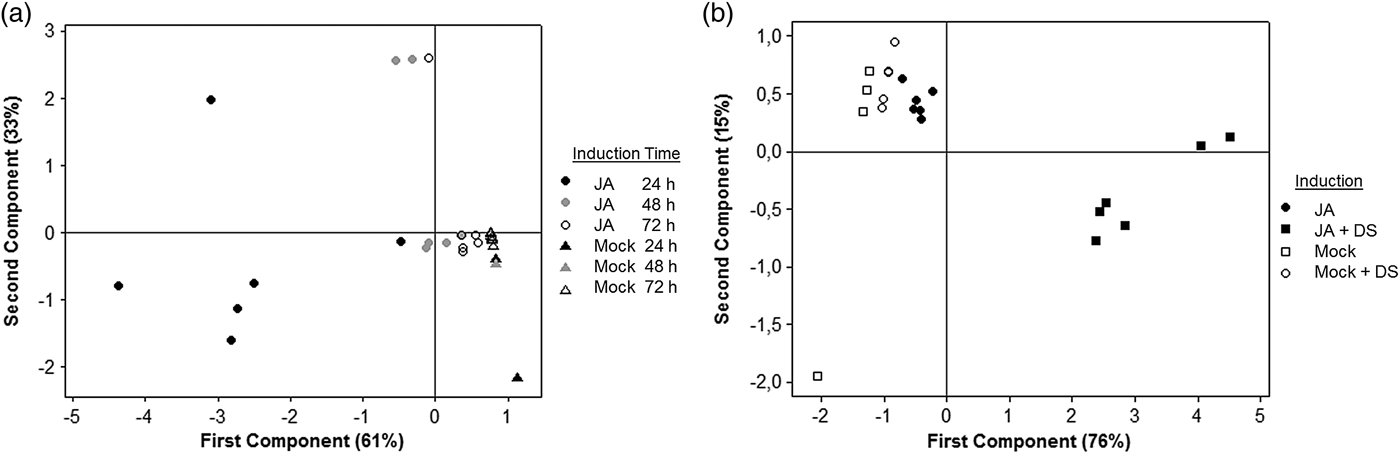
Fig. 6. Loading plots for principal components analysis (PCA) with plant volatile compounds as variables. (a) PCA of volatile composition emitted by mock plants and jasmonic acid-treated plants (JA) after 24, 48 and 72 h (b) PCA of volatile composition emitted by mock plants, jasmonic acid-treated plants (JA), mock plant followed by Diatraea saccharalis damage (Mock + DS) and after 48 h jasmonic acid-treated plant followed by D. saccharalis damage (JA + DS).
Discussion
Our results showed that the JA treatment did not induce sugarcane defences associated with antibiosis, but induced the antixenosis type of resistance against both specialist and generalist herbivores. We cannot discard the possibility, though, that JA-treated plants generate secondary metabolites that mediate a late antibiosis (Harvey et al., Reference Harvey, Van Nouhuys and Biere2005), affecting parameters such as development time and mortality (Thaler, Reference Thaler1999b; Accamando & Cronin, Reference Accamando and Cronin2012; Hegde et al., Reference Hegde, Oliveira, da Costa, Loza-Reyes, Bleicher, Santana, Caulfield, Mayon, Dewhirst and Bruce2012), which were not assessed in our study.
The antixenosis type of resistance can be mediated by plant visual, olfactory and/or gustatory cues (Cruz & Eizaguirre, Reference Cruz and Eizaguirre2015). To verify if the herbivore non-preference to JA-treated plants was mediated by olfactory cues, such as repellent plant volatiles (De Moraes et al., Reference De Moraes, Mescher and Tumlinson2001), we conducted olfactometer assays only with D. saccharalis (but not S. frugiperda), which was part of our main study system. Nevertheless, we did not find that plant volatiles are involved in the JA-induced plant resistance against the sugarcane borer, as the olfactometer assays showed contrasting results compared with the arena preference tests. Apparently, visual cues are also not involved in the JA-induced plant resistance to sugarcane borer, because the colorimetric parameters of mock and JA-treated leaves were similar. Therefore, discarding the effect of odour and visual cues, the antixenotic effect of JA-treated sugarcane on the lepidopteran herbivores is, at least, not solely mediated by plant volatiles, and gustative and/or contact cues are likely involved.
Our results refute our initial hypothesis that the generalist herbivore would be more affected by JA-mediated defences than the specialist. We attribute these findings to the many years of breeding and genetic manipulation of the plant (Cipollini & Heil, Reference Cipollini and Heil2010; Cheavegatti-Gianotto et al., Reference Cheavegatti-Gianotto, de Abreu, Arruda, Bespalhok Filho, Burnquist, Creste, di Ciero, Ferro, de Oliveira Figueira and de Sousa Filgueiras2011) that probably modified the interaction involving D. saccharalis and sugarcane, rather than to the outcome of the co-evolutionary history between the two organisms.
We observed that JA also induced indirect defences against D. saccharalis, by emitting attractive volatiles to the parasitoid C. flavipes. Although the sugarcane plants emitted the blend with the highest amounts of all compounds 24 h after JA treatment, C. flavipes did not discriminate this blend from that emitted by the control plants (Mock). Indeed, the quality of the blend can be more important than elevated quantities of herbivore-induced plant volatiles for attracting parasitic wasps (Bruce et al., Reference Bruce, Midega, Birkett, Pickett and Khan2009). Moreover, increased quantities of some compounds may act as repellents or mask the effect of attractants to wasps (D'Alessandro et al., Reference D'Alessandro, Held, Triponez and Turlings2006).
Attraction of JA-induced volatiles to C. flavipes wasps was observed only after 48 and 72 h, when an overall reduction of volatiles was detected compared with 24 h. The peak of JA endogenous levels in JA-treated sugarcane likely occurs at 24 h, translating in the highest amounts of volatile emission (Schmelz et al., Reference Schmelz, Alborn, Banchio and Tumlinson2003). Plants quickly detect JA exogenous application by elevating JA endogenous levels (Baldwin et al., Reference Baldwin, Zhang, Diab, Ohnmeiss, McCloud, Lynds and Schmelz1997; Glauser et al., Reference Glauser, Dubugnon, Mousavi, Rudaz, Wolfender and Farmer2009). However, in the absence of a new stimulus, the activity of plant metabolic pathways is attenuated, reflecting in changing composition of the volatile blend over time (Schmelz et al., Reference Schmelz, Alborn, Banchio and Tumlinson2003; Bruinsma et al., Reference Bruinsma, Posthumus, Mumm, Mueller, van Loon and Dicke2009). We observed that the volatile composition of JA-treated 72 h was different from the composition at time point 48 h, not only quantitatively, but also qualitatively, as the blend at 72 h lacked trans-β-farnesene and β-sesquiphellandrene. The attractive effect of trans-β-farnesene on C. flavipes females has been reported previously (Ngumbi et al., Reference Ngumbi, Ngi-Song, Njagi, Torto, Wadhams, Birkett, Pickett, Overholt and Torto2005), but our results did not indicate that the presence of this compound in the blend is necessary for attracting the parasitoids. Bruinsma et al. (Reference Bruinsma, Posthumus, Mumm, Mueller, van Loon and Dicke2009) found that Cotesia glomerata (L.) (Hymenoptera: Braconidae) wasps were attracted earlier to JA-treated plants using elevated concentrations of JA. In our system, this effect likely did not occur, as elevated JA concentrations can lead to a stronger volatile emission (Lou et al., Reference Lou, Du, Turlings, Cheng and Shan2005), which is not necessarily attractive to C. flavipes.
Thus, our results indicated that exogenous application of JA in sugarcane can be used as an effective tool for recruiting C. flavipes. However, the recruitment of natural enemies by elicitor-treated plots does not assure the success of biological control (Sobhy et al., Reference Sobhy, Erb, Lou and Turlings2014), particularly if elicitor-induced plant volatile emissions are equally or more attractive than host-damaged plants to natural enemies. We therefore tested the effect of sugarcane-borer damage, combined or not with JA treatment in sugarcane, on the olfactory preference of C. flavipes. Even though the JA treatment induced a more complex blend than produced by the herbivore-damaged plants, with larger amounts of terpenes and 1-H-indole, the parasitoid C. flavipes was equally attracted to herbivore-damaged and JA-treated plants. Comparing the volatile profiles of these two treatments, one would speculate whether the parasitoid's attraction is mediated by β-caryophyllene, which was the only compound present in both mixtures. However, as noted above, JA-treated sugarcane 72 h was attractive to the wasps and its volatile emission containing no (or undetectable amounts of) β-caryophyllene, suggesting that more than one compound (singly or in mixture) is involved in the parasitoid attraction. As a generalist parasitoid that parasitizes a wide range of stemborer larvae that feed on different grasses (Potting et al., Reference Potting, Otten and Vet1997a, Reference Potting, Overholt, Danso and Takasub), it is plausible that C. flavipes uses multiple VOCs to locate its host.
Interestingly, in addition to β-caryophyllene, herbivore-damaged plants released methyl salicylate, a volatile derivative from the SA-mediated pathway and commonly related to defences against pathogen infection (Glazebrook, Reference Glazebrook2005; Pieterse et al., Reference Pieterse, Van der Does, Zamioudis, Leon-Reyes and Van Wees2012). Little is known about the defence pathways activated by D. saccharalis herbivory in sugarcane; however, we expected that the attack of the lepidopteran induced a stronger volatile emission, but no methyl salicylate in the blend, as herbivory by chewing insects does not usually activate the SA-defence pathway (Horiuchi et al., Reference Horiuchi, Arimura, Ozawa, Shimoda, Takabayashi and Nishioka2003). This somewhat unexpected result can be explained by the fact that feeding by D. saccharalis on sugarcane induces a defence pathway against pathogen infections that are closely associated with damage by the sugarcane borer (Medeiros et al., Reference Medeiros, Franco, Matos, de Castro, Santos-Silva, Henrique-Silva, Goldman, Moura and Silva-Filho2012). In addition, we cannot discard the hypothesis that specialist herbivores may attempt to down-regulate plant defences (Govind et al., Reference Govind, Mittapalli, Griebel, Allmann, Böcker and Baldwin2010) through the activation of the SA-signalling pathway, which interacts antagonistically with JA (Cipollini et al., Reference Cipollini, Enright, Traw and Bergelson2004; Chung et al., Reference Chung, Rosa, Scully, Peiffer, Tooker, Hoover, Luthe and Felton2013).
The assay testing JA-treated against herbivore-damaged sugarcane plants for C. flavipes represents a scenario where part of the plants would be sprayed, while others would not be. In this context, using JA could hamper host-finding by the sugarcane parasitoid. However, our results suggest that, in a condition where all plants in the field are sprayed, JA treatment in sugarcane likely does not disrupt host-finding by C. flavipes parasitoids. When the wasps were given a choice between JA-treated (JA) and D. saccharalis-damaged plants (JA + DS), C. flavipes was able to discriminate the plant infested with the host, similarly to other systems where induction by an elicitor combined with host damage makes plants more attractive to natural enemies than herbivore damage alone (Gols et al., Reference Gols, Roosjen, Dijkman and Dicke2003).
The volatile blend emitted by the JA + DS sugarcane was the most complex one, containing high quantities of all compounds and exclusive terpenes and alcohols and, according to the PCA, its composition was singular compared with the other treatments. We found it surprising that this blend was particularly attractive to C. flavipes, while the complex blend containing high amounts of volatiles released by JA-treated plants 24 h, was not. It is not possible to compare the composition of these volatile emissions, as the collections were performed in different experiments with plants that had grown during different seasons of the year in the greenhouse (summer and winter), which has a direct influence on plant volatile emission (Blank et al., Reference Blank, Costa, Arrigoni-Blank, Cavalcanti, Alves, Innecco, Ehlert and Sousa2007).
Interestingly, C. flavipes was attracted to the different compositions of plant volatiles emitted from JA + DS, JA-treated and herbivore-damaged sugarcane plants, probably because C. flavipes is a generalist parasitoid of stem-borer larvae and it responds to volatile emissions from a wide range of grasses (Obonyo et al., Reference Obonyo, Schulthess, Gerald, Wanyama, Le Rü and Calatayud2008). The stronger attraction of the JA + DS volatile blend from sugarcane could indicate a plant infested by multiple hosts for the parasitic wasps, or simply a highly attractive volatile composition. According to our results for the sugarcane borer performance, the C. flavipes preference and the parasitism of a host feeding on JA-treated plants does not seem to affect parasitoid fitness, as the hosts develop normally.
In summary, our study showed that JA treatment in sugarcane confers direct and indirect resistance against lepidopteran pests, without side-effects on host-finding by species in the third trophic level. Thus, there is good potential for using JA as a plant resistance elicitor to control sugarcane borer populations, although field studies are required to demonstrate that JA application can significantly suppress lepidopteran pest populations in sugarcane, while considering the economic viability. If all sugarcane plants are sprayed with JA early in the season, the antixenosis type of resistance induced by JA can reduce the sugarcane borer population in the field, as young larvae may spend a longer time searching for a suitable host, leading to higher mortality from biotic and abiotic factors (Russell, Reference Russell1989). Alternatively, JA can be sprayed on some sugarcane plots, while others are left mock to serve as trap plants for sugar-cane borer and fall armyworm larvae, and later sprayed with selective insecticides. However, based on our results, the latter strategy may reduce the efficacy of sugarcane borer biological control by C. flavipes.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485317000372
Acknowledgements
We thank the Center of Sugarcane Technology (Centro de Tecnologia Canavieira, Piracicaba, São Paulo, Brazil) for providing sugarcane one-eyed seeds, and Arodí Prado Fávaris for technical assistance. This study was supported by the National Institute of Science and Technology (INCT) Semiochemicals in Agriculture, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Process 573761/2008-6) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Processes 2008/57701-2, 2012/12252-1 and 2013/05367-0).








