Introduction
Lepidopteran larvae are, perhaps, the main phytosanitary problem for many cultivated plants around the world since they attack different plant organs causing severe losses in production as well as affecting the product quality. Many of these lepidopterous insects live on a polysaccharide-rich diet and require digestive alpha-amylase to break down and utilize the starch in their food sources. These amylases play a very important role in starch digestion and in insect survival. Starch digestion by insect amylases has been demonstrated and described in several insect species: Drosophila melanogaster (Diptera: Drosophilidae) (Doane, Reference Doane1969), Prostephanus truncatus (Coleoptera: Bostrichidae) (Mendiola-Olaya et al., Reference Mendiola-Olaya, Valencia-Jiménez, Valdés-Rodríguez, Délano-Frier and Blanco-Labra2000), Sitophillus zeamais (Coleoptera: Curculionidae) (Baker, Reference Baker1983), Sitophillus orizae (Coleoptera: Curculionidae) (Yetter et al., Reference Yetter, Saunders and Boles1979; Baker, Reference Baker1987) and Zabrotes subfasciatus (Coleoptera: Bruchidae) (Lemos et al., Reference Lemos, Campos, Silva and Xavier-Filho1990).
However, it has been demonstrated that the insect amylase activity is inhibited by the presence of amylase inhibitors in plant seeds. The physiological role of amylase inhibitors in plants is unknown (Gatehouse et al., Reference Gatehouse, Fenton, Jepson and Pavey1986). There is evidence indicating that they could act as a protein reserve in seeds. Nevertheless, the inhibitors do not, generally, affect endogenous amylase except in rare cases. There are many examples where amylase inhibitors, especially those isolated from cereals, inhibit amylases from insect guts (Weselake et al., Reference Weselake, MacGregor, Hill and Duckworth1983; Shade et al., Reference Shade, Schroeder, Pueyo, Tabe, Murdock and Chrispeels1994; Chagolla-Lopez et al., Reference Chagolla-Lopez, Blanco-Labra, Patthy, Sanchez and Pongor1994; Grant et al., Reference Grant, Edwards and Pusztai1995; Valencia-Jiménez et al., Reference Valencia-Jiménez, Bustillo, Ossa and Chrispeels2000), which have led to the hypothesis that digestive enzyme inhibitors play a protective role against herbivorous. They may act as feeding deterrents (Mendiola-Olaya et al., Reference Mendiola-Olaya, Valencia-Jiménez, Valdés-Rodríguez, Délano-Frier and Blanco-Labra2000).
Amylase inhibitors are quite specific to their target enzyme; therefore, a proteinaceous inhibitor that inhibits the activity of one amylase may not have the same effect on other amylases. For example, it is well known that the amylase inhibitor present in the seeds of Amaranthus hypocondriacus (Caryophyllales: Amaranthaceae) strongly inhibits the α-amylase activity of insect larvae (Tribolium castaneum and Prostephanus truncatus) but does not inhibit mammalian alpha-amylases (Chagolla-Lopez et al., Reference Chagolla-Lopez, Blanco-Labra, Patthy, Sanchez and Pongor1994). On the basis of this specificity, genetic transformation of plants through the expression of insect digestive enzyme inhibitors requires evaluation and characterization of the digestive enzymes present in the insect gut, as well as of the effect of the inhibitors.
Tecia solanivora (Lepidoptera: Gelechiidae) is considered a serious pest of economic importance, not only in Central America (Hilje, Reference Hilje1994) but also in South America. Serious damage caused by this insect has been reported recently in Colombia, where weight losses of up to 100% have been recorded for farm-stored tubers (Botero et al., Reference Botero, Londoño, Trillos, Arias and Jaramillo Pelaez1995). The negative impact of T. solanivora on potato tubers makes this insect an important target for study, especially if transgenic potato plants expressing inhibitors of digestive enzymes are produced.
Materials and methods
Sources of plant inhibitors and insects
Tecia solanivora was obtained locally in Colombia and reared on whole potato tubers in an insectary at Corpoica in Santafé de Bogotá, Colombia. Phaseolus vulgaris L. (Fabales: Fabaceae) (cv. Radical) is a local commercial variety in Colombia. Phaseolus coccineus (Fabales: Fabaceae) accession G35619 was obtained from CIAT in Palmira, Colombia. Amaranthus sp. is a hybrid of Amaranthus hypocondriacus and A. caudatus, which was obtained in a local grocery store in Santa Barbara, CA, USA. These seed sources were selected based on their known inhibitory specificities.
Extraction procedures of insect amylases
Two different extraction procedures were evaluated. First, whole T. solanivora larvae were homogenized in five volumes of 100 mm NaCl containing 20 mm CaCl2 by using a glass homogenizer at 4°C, filtered and centrifuged at 10,000×g for 30 min at 4°C. The crude and clarified supernatant was used as a source of enzyme. Second, the guts were carefully dissected from fourth instar larvae. Ten guts were separated with the aid of a microscope and then they were immersed in 100 μl of 10 mm NaCl solution. These dissected guts with their lumen content were homogenized by using a pellet pestle. All these operations were carried out at 4°C.
Preparation of crude inhibitors
Phaseolus vulgaris and P. coccineus seeds were washed, dried and ground in a mill to obtain a fine powder. The powder was defatted with acetone, dried and powdered again. Defatted seed powder was extracted at 4°C by stirring with five volumes of 0.1 m NaCl for 3 h. The slurry was centrifuged at 12,000×g for 1 h and the supernatant was dialyzed against distilled water to allow the globulins to precipitate. This procedure was carried out four times for 12 h each. After centrifugation (12,000×g for 30 min), the supernatant was freeze-dried. The resulting powder was used as a source of crude inhibitors for alpha-amylase inhibition assays. The Amaranthus inhibitor, which has been well characterized by Chagolla-Lopez et al. (Reference Chagolla-Lopez, Blanco-Labra, Patthy, Sanchez and Pongor1994), was purified as described by Valencia-Jiménez et al. (Reference Valencia-Jiménez, Bustillo, Ossa and Chrispeels2000). Briefly, the crude extract was heated at 70°C for 30 min and the pH of the solution was changed from 6.8 to 8.4 with 0.1 m NaOH. The resulting precipitate was removed by centrifugation; and the precipitated protein, which contained nearly pure amaranth inhibitors, was dissolved in 20 mm potassium phosphate buffer, pH 8.4.
Amylases activities assays
Amylase activity was determined using the method described by Valencia-Jiménez et al. (Reference Valencia-Jiménez, Bustillo, Ossa and Chrispeels2000). In all cases twenty, microlitres of the enzyme solution were incubated for 15 min at 30°C with 680 μl of glycine solution (0.05 M; pH 6.0 or 9.0) containing 10 mm NaCl and 20 mm CaCl2. The enzymatic reaction was started by adding 500 μl of a solution of 0.125% soluble starch (sigma) to the buffer solution and was stopped by adding 5 ml of iodine/iodide (0.5% I2 and 5% KI). The absorbance was determined at 580 nm by using a spectrophotometer Unicam UV2. Amylase inhibitor activity was assayed as above, but enzyme and inhibitor were preincubated together for 15 min prior to the addition of the starch. Each assay was performed with three replicates.
Optimum pH for activity
The optimum pH for activity of the Tecia solanivora alpha-amylases was determined using different buffer solutions. The buffer solutions used were: citrate (pH 3.0 and 5.0), succinate (pH 4.0 and 6.0), tris-HCl (pH 7.0–9.0) and phosphate (pH 11.0–12.0). Each buffer was prepared to a final concentration of 50 mm. For all buffer solutions, 10 mm NaCl and 20 mm CaCl2 were added. Each assay was performed with three replicates.
Thermal stability
Thermal stability of α-amylases in the presence of NaCl and CaCl2 was determined by pre-incubating the enzymatic extract in the assay buffer (50 mm glycine buffer pH 9.0) for 10, 25, 30, 40, 50 and 60 min at 70°C. Each assay was performed with three replicates.
Electrophoretic separation and enzyme zymogram in native and isoelectrofocusing (IEF) gels
Gels were run using the PhastSystem electrophoresis unit (Pharmacia) following the manufacturer's instructions. Zymograms for amylase activity were carried out under non-denaturing conditions on native and IEF Phastgels. For native separation of amylase, a Phastgel with 7.5% polyacrylamide resolving gel and Phastgel (3.0–9.0 pH range) for IEF electrophoresis were used. For zymograms, after electrophoresis, both native and IEF gels were placed in a 1.5% soluble starch solution in 50 mm Glycine buffer, pH 9.0, containing 10 mm NaCl and 20 mm CaCl2 for 1 h at 4°C. After one hour, the gel was carefully rinsed with distilled water and incubated for another hour in the same buffer. Amylase activity appeared on the polyacrylamide-starch matrix as clear bands on a blue-colored background after staining with a KI/I2 solution for 10 min. The gels were washed, in order to remove the excess iodine solution, and then photographed. The isoelectric points were calculated by using standard proteins with known isoelectric points using the software ImageMaster VDS.
Results
Characteristics of midgut α-amylases
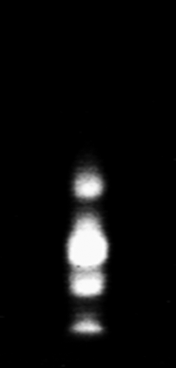
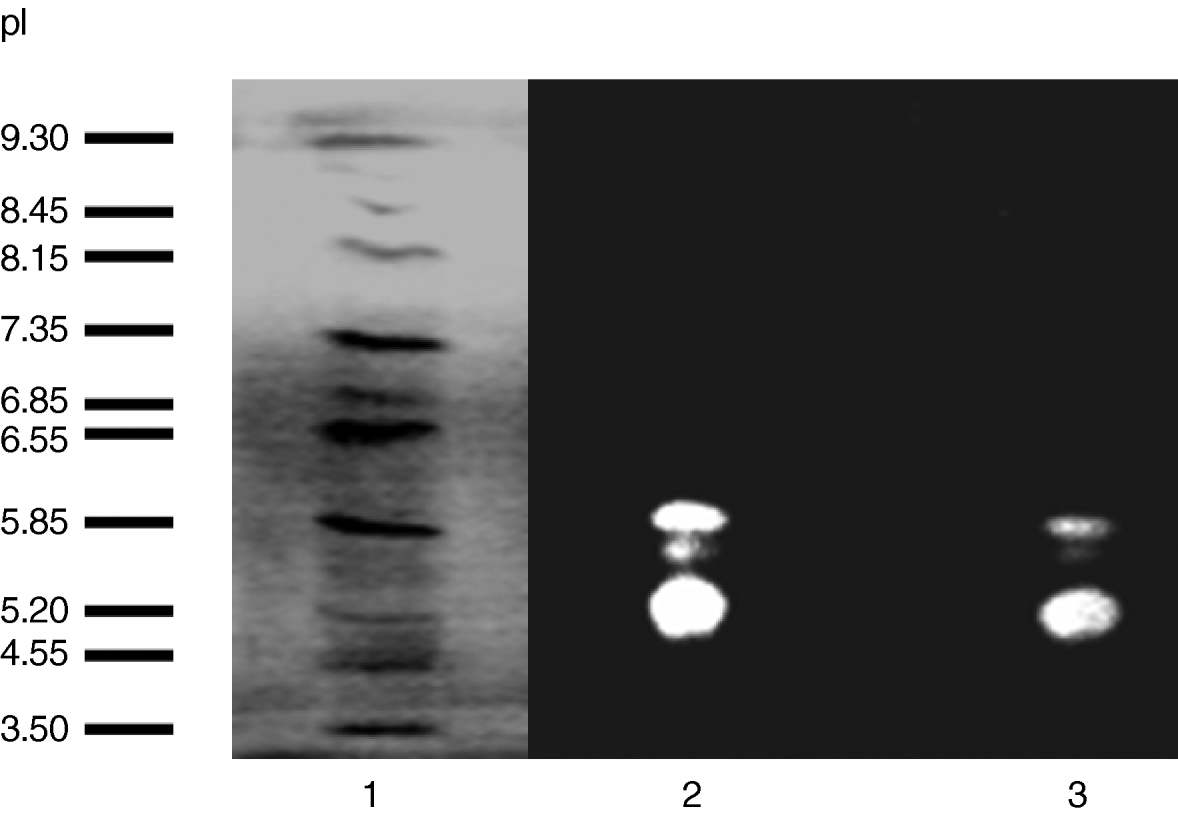
Preliminarily, to find out the possible presence of different alpha-amylase isoforms, the whole larval extracts, as well as those from the intestinal tracts, were analyzed in native 7.5% polyacrylamide and IEF gels. Four bands of amylase activity were observed in the 7.5% Phastgel zymogram of the extract from whole larvae (fig. 1), and the same pattern of alpha-amylases was also observed for the gut extract. The zymogram of the alpha-amylase activities run in an isoelectric focusing gel (fig. 2) clearly showed three bands. The isoelectric points, estimated by using pI markers, were 5.30, 5.70 and 5.98.
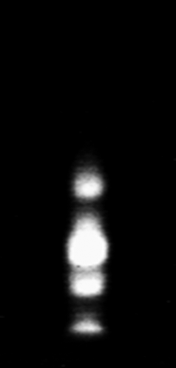
Fig. 1. Zimogram using a 7.5% native Phastgel of α-amylases present in the whole-insect (Larvae) extract of Tecia solanivora.
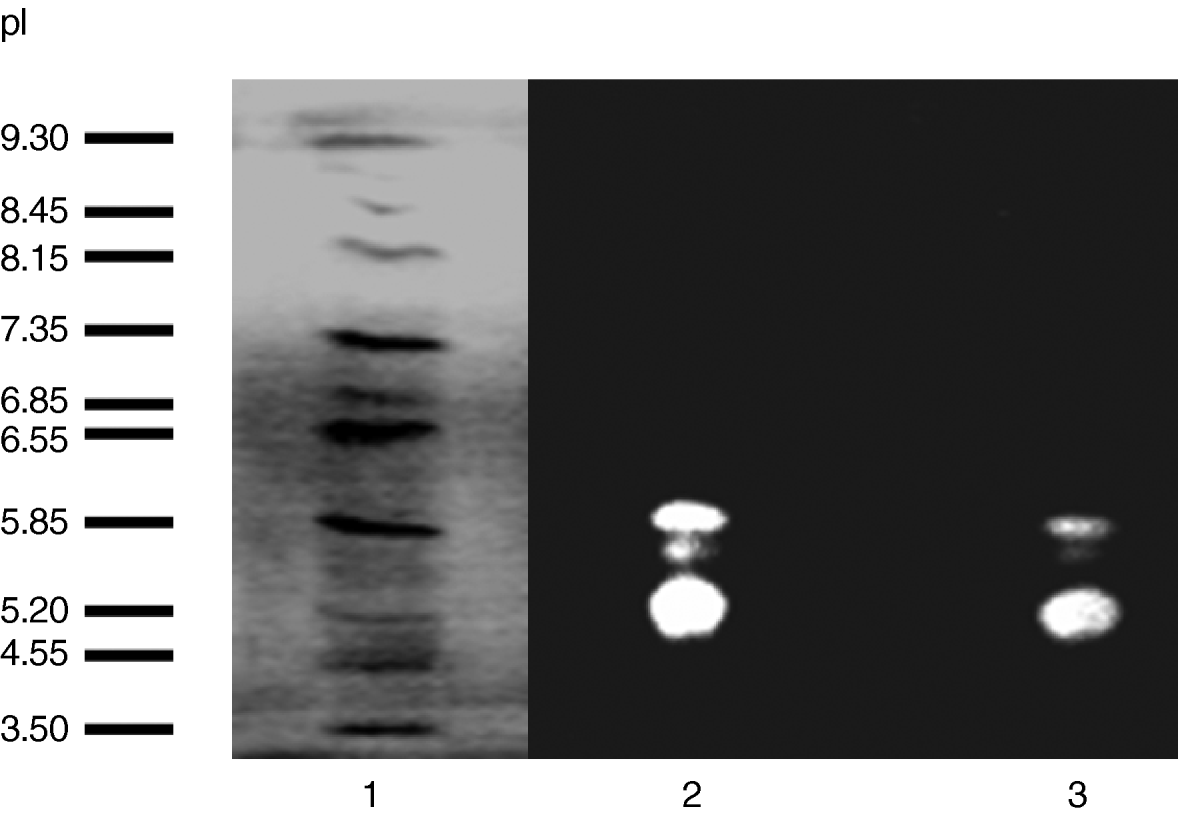
Fig. 2. Determination of the isoelectric point (pI) of the digestive amylases from Tecia solanivora by using an IEF Phastgel with a pH range from 3.0 to 9.0. Lane 1 displays the pI markers. Lane 2 represents the α-amylases from whole-insect (larvae) extract. Lane 3 represents the α-amylases from intestinal tract.
Effect of pH and temperature on amylase activities
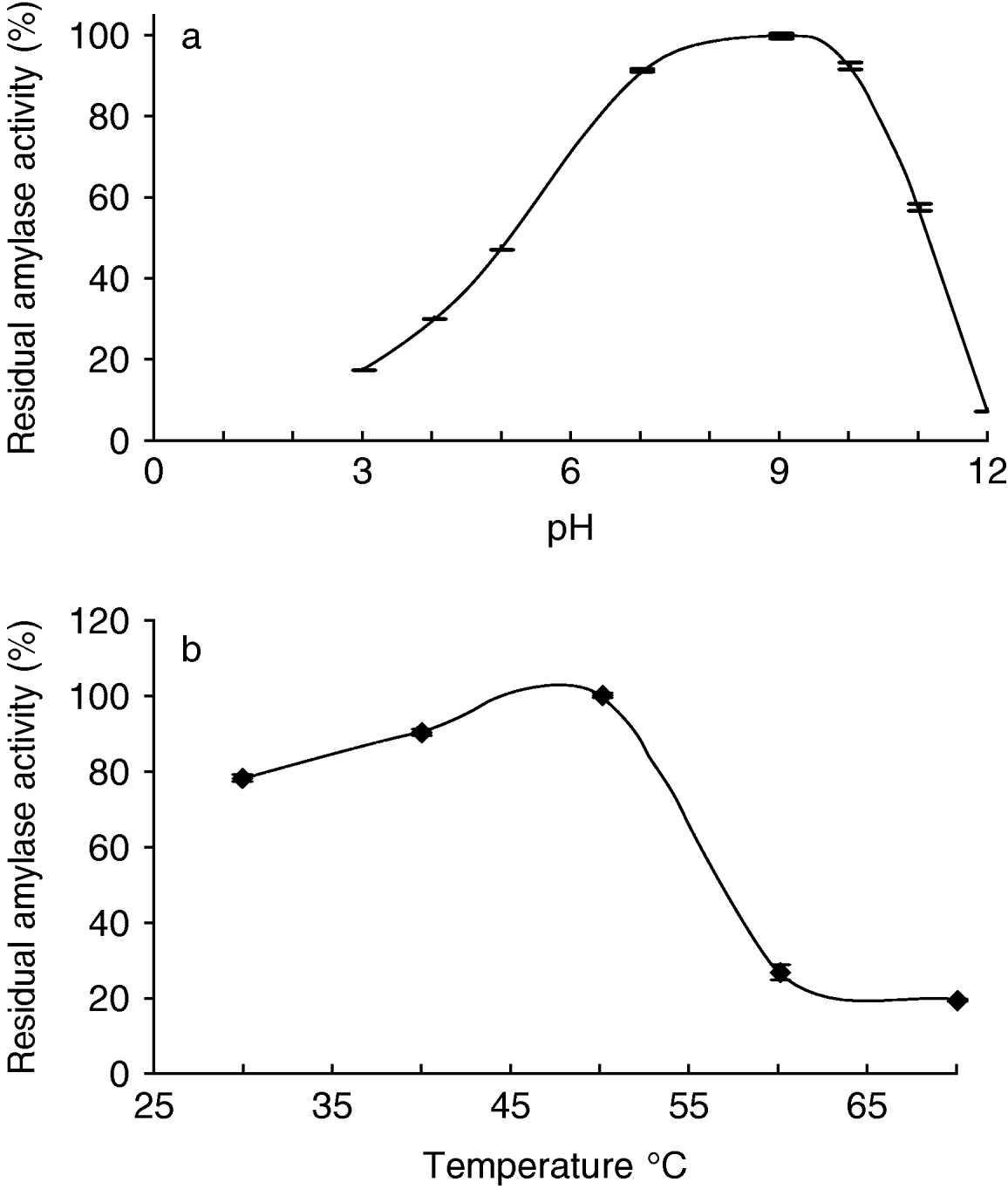
Some properties of the alpha-amylase(s) in Tecia solanivora larvae were determined in preliminary experiments. We found that the optimal pH values for starch hydrolysis by T. solanivora intestinal α-amylases were in the alkaline range of 7.0–10.0 with a peak at about pH 9.0 (fig. 3a). In addition, these enzymes were found to be still active after heating up to 50°C (fig. 3b).
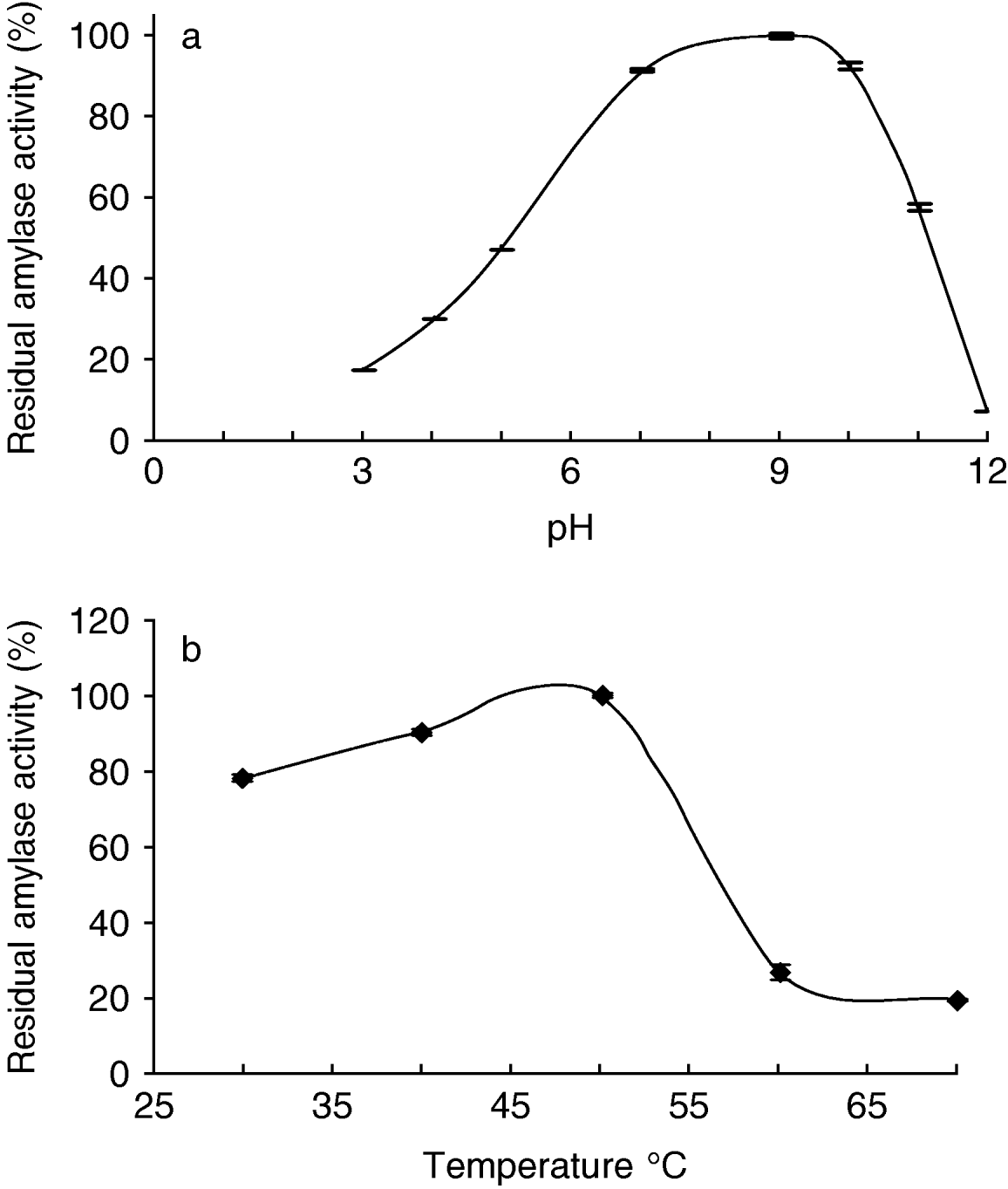
Fig. 3. Effect of the pH on the alpha-amylases activities of Tecia solanivora larvae (whole insect extract). The α-amylase activity was determined using different buffer solutions. (a) Effect of the temperature on the stability of the Tecia solanivora alpha-amylases. (b) Thermal stability was determined by measuring the residual α-amylase activity remaining after 10 min at each temperature. Each point is the average of three measurements. Error bars indicate standard error.
Effect of amylase inhibitors
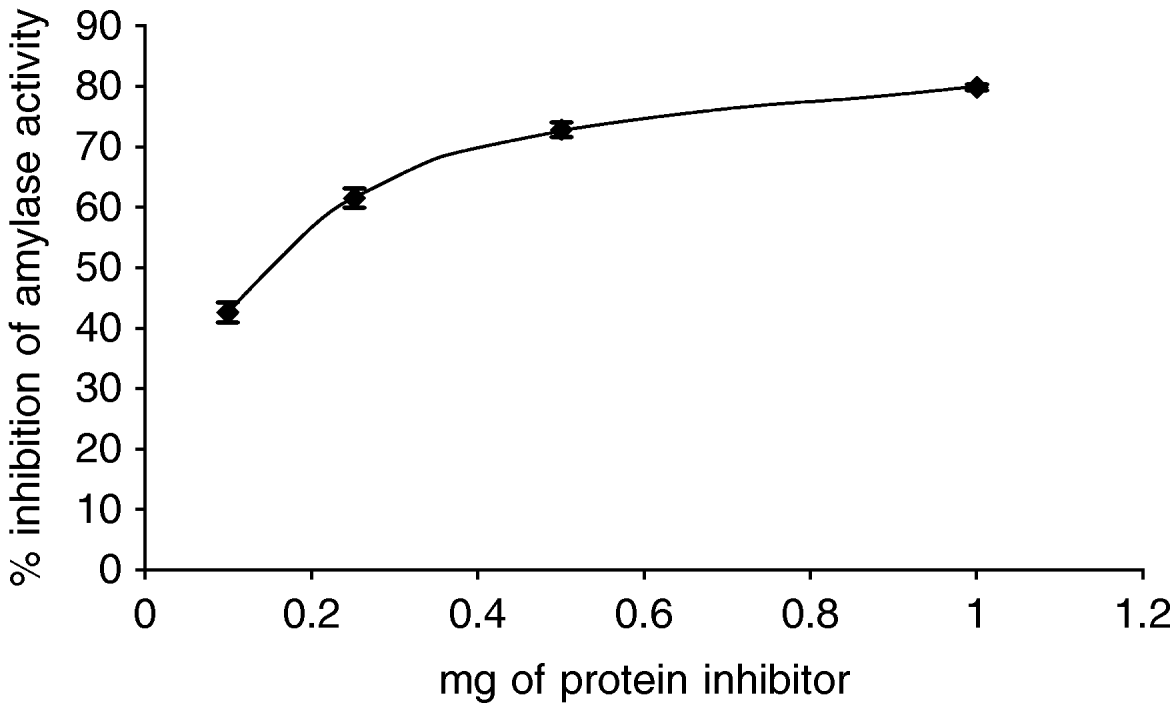
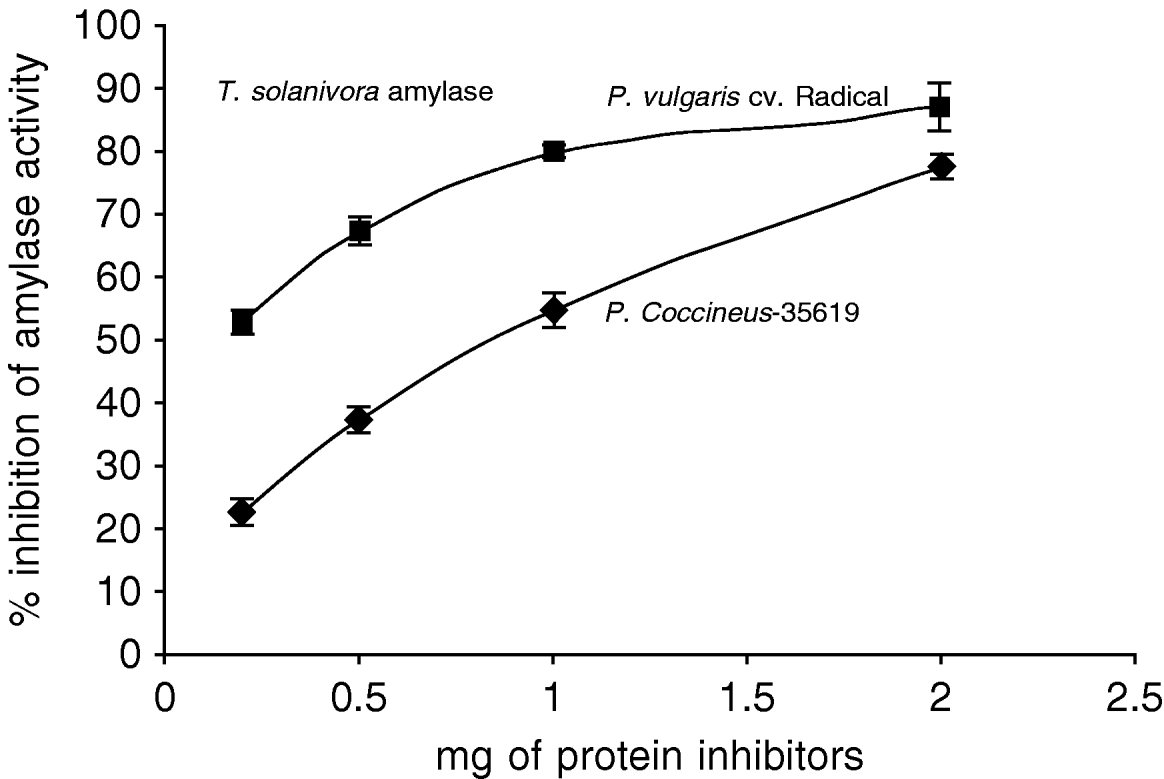
The effect of the inhibitor from an Amaranthus hybrid on the amylase activities from Tecia solanivora was measured. The results indicate that the purified inhibitor had no effect on the amylolytic activity at pH 6.0 (data not shown) but showed high inhibitory activity at pH 9.0, which is the optimum pH for the activity of this enzyme in vitro. The maximum inhibition obtained with the purified inhibitor from Amaranthus hybrid was 80% (fig. 4). To explore if the different Phaseolus species differ in inhibitory activity, we checked the effect of proteins from P. coccineus 35,619 and from P. vulgaris cv. Radical on the alpha-amylase activity of T. solanivora. The results (fig. 5) showed that the P. vulgaris inhibitor caused 87% inhibition of alpha-amylase activity at pH 6.0. Under the conditions of this experiment, the inhibitor from P. coccineus 35 619 blocks the enzyme activity up to 78% at pH 6.0. Thus, the insect enzyme is not as readily inhibited at pH 9.0 when compared to the observed inhibition at pH 6.0 (data not shown).
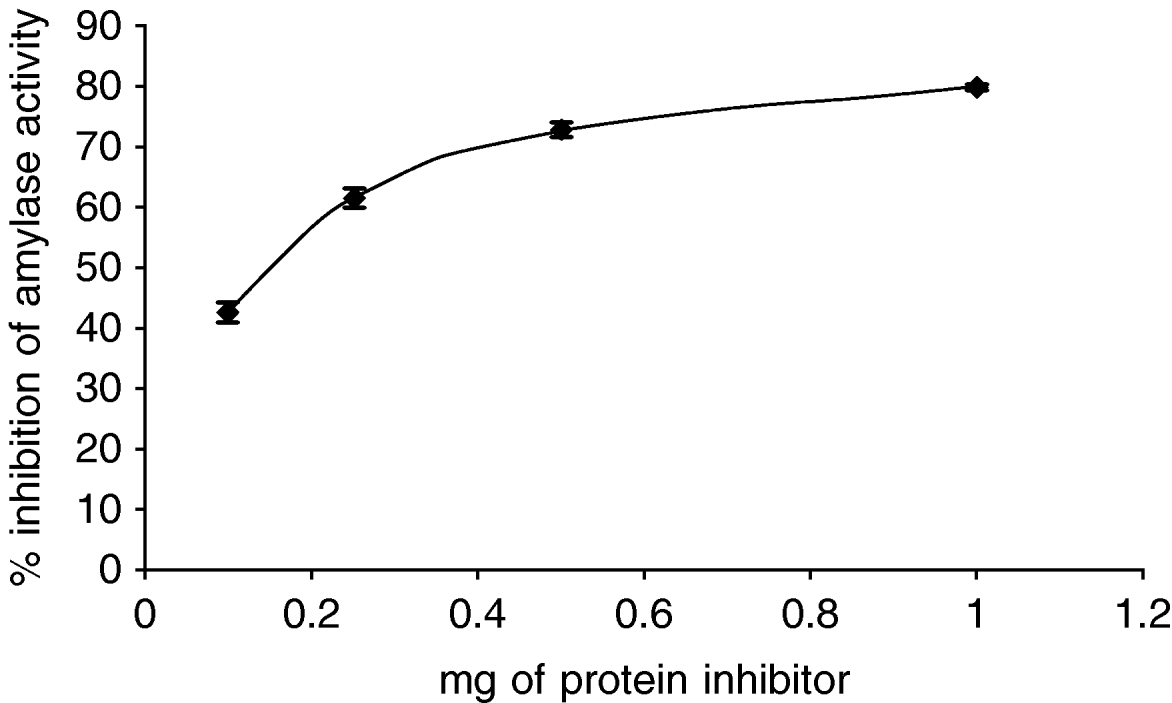
Fig. 4. Inhibition of Tecia solanivora α-amylases by inhibitor preparation of Amaranthus sp. The inhibition assay was done at pH 9.0. Each point is the average of three measurements. Error bars indicate standard error.
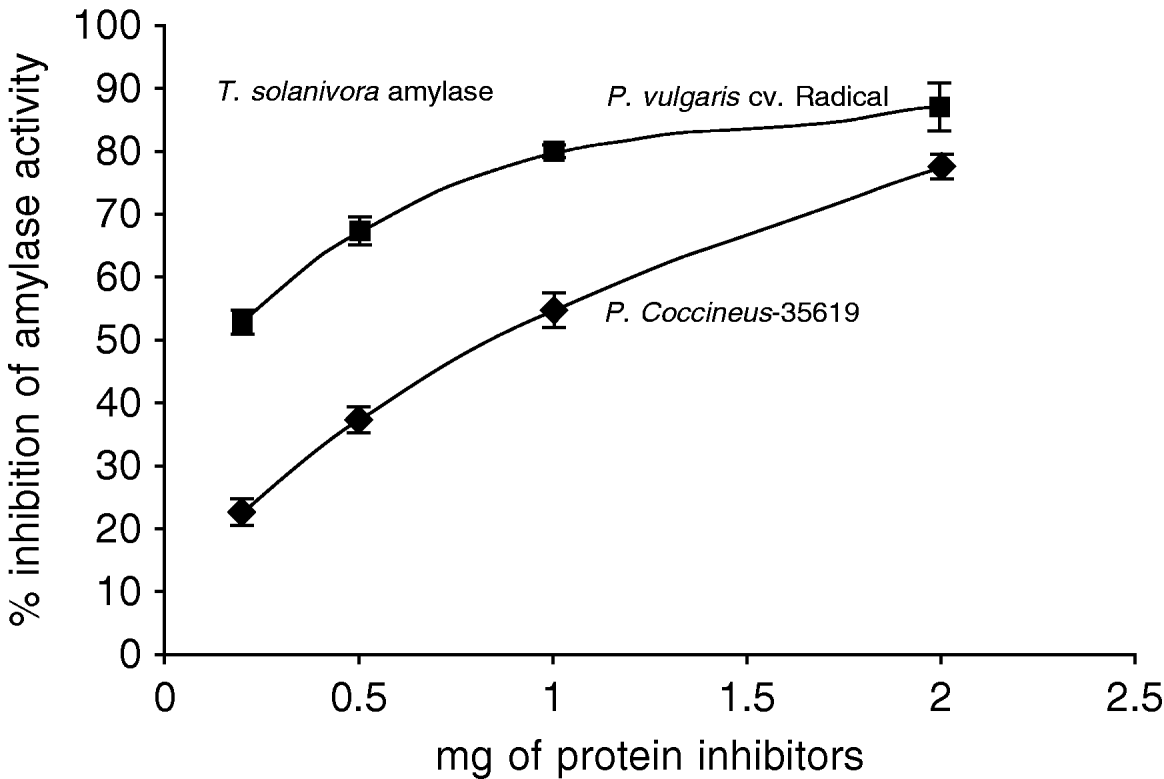
Fig. 5. Inhibition of Tecia solanivora α-amylases by crude inhibitor preparations of Phaseolus vulgaris cv. Radical and Phaseolus coccineus 35,619 at pH 6.0. Each point is the average of three measurements. Error bars indicate standard error.
Discussion
The presence of different alpha-amylase isoforms in the whole larval extract, as well as in the intestinal tract, represent a strong evidence of the digestive nature of these alpha-amylase isoforms, suggesting that a whole larval extract could be used as a source of digestive amylases of this insect pest. The isoenzymatic analysis carried out for the digestive amylases from Tecia solanivora indicates that this insect contains several alpha-amylases isoforms, similar to what has been found in others insects (Baker, Reference Baker1983, Reference Baker1987; Campos et al., Reference Campos, Xavier-Filho, Silva and Ary1989; Mendiola-Olaya et al., Reference Mendiola-Olaya, Valencia-Jiménez, Valdés-Rodríguez, Délano-Frier and Blanco-Labra2000; Valencia-Jiménez et al., Reference Valencia-Jiménez, Bustillo, Ossa and Chrispeels2000). The isoelectric points (pI), determined by using native and IEF Phastgels, were generally different from those reported for other insect amylases. For example, the three reported alpha-amylases of Hypothenemus hampei have more acidic isoelectric points (pIs) (Valencia-Jiménez et al., Reference Valencia-Jiménez, Bustillo, Ossa and Chrispeels2000). Similarly, the isoelectric point, reported for the main alpha-amylase isolated from Phaseolus truncates, was 4.7 (Mendiola-Olaya et al., Reference Mendiola-Olaya, Valencia-Jiménez, Valdés-Rodríguez, Délano-Frier and Blanco-Labra2000).
It is important to point out that the thermal stability observed in these insect amylases is similar to that of most insect alpha-amylases, which show a rapid decrease in amylolytic activity above 40°C (Mendiola-Olaya et al., Reference Mendiola-Olaya, Valencia-Jiménez, Valdés-Rodríguez, Délano-Frier and Blanco-Labra2000; Valencia-Jiménez et al., Reference Valencia-Jiménez, Bustillo, Ossa and Chrispeels2000). Moreover, our results show that the alpha-amylase(s) in Tecia solanivora larvae have an alkaline optimal pH, which is consistent with the optimal pH that has been reported for other lepidopteran species (Abraham et al., Reference Abraham, Nagaraju and Datta1992; Markwick et al., Reference Markwick, Laing, Christeller, Reid and Newton1996). On the contrary, the digestive amylases of several insects of the Coleoptera order are very active at pH ranging from slightly acid to neutral. For example, Hypothenemus hampei shows an optimal activity at pH 5.0 (Valencia-Jiménez et al., Reference Valencia-Jiménez, Bustillo, Ossa and Chrispeels2000); the optima pHs were found at 5.2 and 5.4 for Callosobrucus chinensis (Podoler & Applebaum, Reference Podoler and Applebaum1971) and Tribolium castaneum (Applebaum & Konijn, Reference Applebaum and Konijn1965), respectively, at pH 6.0 for Prostephanus truncatus alpha-amylase (Mendiola-Olaya et al., Reference Mendiola-Olaya, Valencia-Jiménez, Valdés-Rodríguez, Délano-Frier and Blanco-Labra2000) and at pH 4.5 and 5.5 for Sitophilus zeamais and S. granarius, respectively (Baker, Reference Baker1983).
In this context, some authors, such as Biggs & McGregor (Reference Biggs and McGregor1996), point out the importance of knowing the pH of the insect gut, so as to determine the pH optimum for the activity of a digestive amylase. In this context, a good alpha-amylase inhibitor against a digestive amylase(s) of T. solanivora should inhibit the enzyme substantially, not only at the pH found in the digestive tract but also and at low inhibitor concentration.
Amaranth was used as a source of amylase inhibitors because it has been reported that seeds of this plant species contain an inhibitor of insect alpha-amylases (Chagolla-Lopez et al., Reference Chagolla-Lopez, Blanco-Labra, Patthy, Sanchez and Pongor1994), which is active against Phaseolus truncatus (Mendiola-Olaya et al., Reference Mendiola-Olaya, Valencia-Jiménez, Valdés-Rodríguez, Délano-Frier and Blanco-Labra2000) but is less active against Hypothenemus hampei (Valencia-Jiménez et al., Reference Valencia-Jiménez, Bustillo, Ossa and Chrispeels2000). Recent work done by our group, in collaboration with the M.J. Chrispeels' group (California University, San Diego, USA), shows that the bean inhibitor αAI-1 is a more potent inhibitor of amylase(s) from Hypothenemus hampei than the Amaranthus hybrid inhibitor, probably because the enzyme-inhibitor complex has a lower dissociation constant, Kd (Valencia-Jiménez et al., Reference Valencia-Jiménez, Bustillo, Ossa and Chrispeels2000). In our opinion, this is an indication of the high degree of specificity of the inhibitors, which recognize specific alpha-amylases from insects. It is of particular interest for us to determine the exact optimum pH for complex formation of Amaranthus inhibitor with T. solanivora α-amylase(s), but we also need to know if all these inhibitors are resistant to the attack of the insect gut proteases. In this way, we will know which is the best amylase inhibitor that could be used for the transformation of potato to protect against the damage caused by T. solanivora.
Based not only on the fact that the amaranth inhibitor shows high inhibition at alkaline pH but also on the fact that the maximum complex formation between insect alpha-amylases and common bean inhibitors is pH dependent with an optimum inhibition around pH 4.0 to 5.5 (Powers & Whitaker, Reference Powers and Whitaker1977; Lajolo & Finardi-Filho, Reference Lajolo and Finardi-Filho1985) and little complex formation occurs at pH 7.0 or higher, we believe that the inhibitors from amaranth seeds are more promising candidates for potato transformation than inhibitors from Phaseolus vulgaris and P. coccineus seeds. The gene for the amaranth alpha-amylase inhibitor, which has shown to be an effective inhibitor of alpha amylases from this insect, could be used as an important tool to produce transgenic potato plants with increased resistance to the insect attack.
Acknowledgements
This work was supported by a grant from Caldas University, Manizales, to A.V.J. We thank the Entomology laboratory of Corpoica-Colombia for their assistance with the insects. The authors also thank Cenicafé-Colombia for technical assistance.