Introduction
Understanding the responses of arthropod predators and parasitoids to temperature and other climatic factors can be crucial for facilitating biological control, whether using the introduction, conservation or augmentation approaches (Roy et al., Reference Roy, Brodeur and Cloutier2002). Natural enemies and their hosts or prey may differ in how temperature affects their survival, development, movement and reproduction, and this can affect the degree of population control parasitoids and predators exert on pest species of herbivores. Temperature requirements may vary among species (e.g. Frazer & McGregor, Reference Frazer and McGregor1992; Mbapila et al., Reference Mbapila, Overholt and Kayumbo2002) and populations (Gilbert, Reference Gilbert1988; Lee & Elliott, Reference Lee and Elliott1998), and can interact with other ecological factors like food source (Gilbert & Raworth, Reference Gilbert and Raworth1996) and allelochemicals (Stamp & Osier, Reference Stamp and Osier1998). Distinct thermal requirements by natural enemies and the herbivores on which they feed can cause asynchrony in periods of activity, resulting in pest outbreaks or reductions in biological control (Read, Reference Read1962). Consequently, studies on the developmental rates of natural enemies and their hosts/prey at different temperatures are instrumental for predicting the efficiencies of biological control strategies.
The diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae), is a serious pest of brassicaceous vegetable and oilseed crops and has a worldwide distribution (Talekar & Shelton, Reference Talekar and Shelton1993). Thermal effects on its survival and developmental biology from egg to adult eclosion have been well studied over a wide range of both constant (4–40°C) and fluctuating temperatures (Liu et al., Reference Liu, Chen and Zalucki2002). Throughout its range, more than 135 species of parasitoids can attack various life stages of P. xylostella (Delvare, Reference Delvare, Kirk and Bordat2004); and among these, species of the genus Diadegma (Hymenoptera: Ichneumonidae) are the most diverse and important economically (Sarfraz et al., Reference Sarfraz, Keddie and Dosdall2005). In Australia, several predator and parasitoid species can contribute to the biological control of P. xylostella, but the larval parasitoid Diadegma semiclausum (Hellén) has been dominant both in key regions of vegetable production (Furlong et al., Reference Furlong, Zu-Hua, Yin-Quan, Shi-Jian, Yao-Bin, Shu-Sheng and Zalucki2004) and in crops of canola (Furlong et al., Reference Furlong, Spafford, Ridland, Endersby, Edwards, Baker, Keller and Paull2008). Diadegma semiclausum is not native to the southern hemisphere but was introduced initially to New Zealand from the UK (Robertson, Reference Robertson1948), followed by subsequent releases and establishment in many Australasian regions, such as Malaysia (Ooi, Reference Ooi1980), Taiwan (Talekar & Yang, Reference Talekar and Yang1991), the Philippines (Poelking, Reference Poelking and Talekar1992) and Australia (Wilson, Reference Wilson1960; Furlong et al., Reference Furlong, Zu-Hua, Yin-Quan, Shi-Jian, Yao-Bin, Shu-Sheng and Zalucki2004).
Several researchers have observed that D. semiclausum is more effective for controlling populations of P. xylostella in cooler highlands in Asia-Pacific regions than in the lowlands where Cotesia vestalis (Haliday) (Hymenoptera: Braconidae) is more effective (Talekar & Yang, Reference Talekar and Yang1991; Talekar & Shelton, Reference Talekar and Shelton1993; Verkerk & Wright, Reference Verkerk and Wright1997; Liu et al., Reference Liu, Wang, Guo, He and Shi2000; Saucke et al., Reference Saucke, Dori and Schumutterer2000). The inability of D. semiclausum to adequately control populations of P. xylostella in the hotter conditions at lower elevations has been attributed to a lack of tolerance to high temperature conditions (Talekar & Yang, Reference Talekar and Yang1991; Talekar & Shelton, Reference Talekar and Shelton1993). The relationship between temperature and development of D. semiclausum on an unspecified cabbage cultivar was investigated by Yang et al. (Reference Yang, Chu and Talekar1993), who determined that proportions of individuals that hatched, pupated and eclosed as adults declined as temperatures increased from 30 to 35°C. However, developmental parameters other than survival, percent emergence and time from egg to pupation were not measured, and developmental rate below 15°C was not investigated.
Plant species on which herbivorous insects feed can affect developmental parameters of their parasitoids, due to the nutritional composition of the diet of the host, the occurrence of allelochemicals in the host that can be detrimental to the parasitoid or possible interactions between nutrients and allelochemicals (Turlings & Benrey, Reference Turlings and Benrey1998). Sarfraz et al. (Reference Sarfraz, Dosdall and Keddie2008) investigated Diadegma insulare (Cresson), a Neotropical congener of D. semiclausum, and found that pre-imaginal developmental time, pupal weight, adult longevity and wing area varied depending on the plant species and cultivar fed upon by P. xylostella hosts. However, it is not known whether developmental characteristics of D. semiclausum at a given temperature can be affected by the plant species fed upon by its host, or if interactions exist between temperature and host plant species. With few exceptions (e.g. Iheagwam, Reference Iheagwam1980; Tomkins et al., Reference Tomkins, Penman and Chapman1989; Larsen et al., Reference Larsen, Madden and Nault1990; Abdel-Baky, Reference Abdel-Baky2008), interactions between insect rearing temperature and host plant species have rarely been investigated, and never previously to our knowledge at the third trophic level. The objective of this study was to investigate effects of temperature on several developmental parameters of D. semiclausum when its P. xylostella hosts were reared on three different brassicaceous host plant species that comprise economically important crops grown within the geographical ranges of both the herbivore and parasitoid. The study was designed to test the hypotheses that, when reared on P. xylostella, developmental parameters of D. semiclausum are affected by both temperature and host plant species, and that parameters associated with species fitness indicate a decline in fitness at higher constant rearing temperatures.
Methods and materials
Insects and host plants
The study was conducted using laboratory colonies of P. xylostella and D. semiclausum and three host plant species in the Brassicaceae: Chinese cabbage (Brassica rapa L. ssp. pekinensis (Lour) Olsson) cv. Wombok, common cabbage (Brassica oleracea L. var. capitata cv. Warrior) and canola (Brassica napus L. cv. Thunder TT). Plants were grown in a glasshouse at the University of Queensland, St Lucia, QLD, Australia in 15-cm-diameter plastic pots using an organic potting medium treated with a slow release fertilizer (Osmocote [N:P:K, 16:35:10] Scotts Australia Pty Ltd, Baulkham Hills, NSW, Australia). Three- to four-week-old plants were used for all experiments.
Specimens of D. semiclausum and P. xylostella were collected initially from a commercial cabbage field near Gatton, QLD (27°33′S; 152°17′E) and maintained in a laboratory growth cabinet on cabbage and Chinese cabbage at 25±0.5°C, a relative humidity of 70%, and photoperiod of 16 h L:8 h D. Although D. semiclausum can oviposit in all four larval instars of P. xylostella (Yang et al., Reference Yang, Chu and Talekar1993), second instars were used for D. semiclausum oviposition in each of our experiments. Newly molted second-instar larvae of P. xylostella were obtained by placing a potted plant of B. rapa ssp. pekinensis in a cage with 20–30 mated females of P. xylostella within a growth cabinet (at 25±0.5°C) in darkness for 6–8 h for oviposition. Adult moths were then removed, and the plant was maintained at a constant temperature of 25°C for eight days until second-instar larvae emerged from their leaf mines. Approximately 60 to 80 second-instar P. xylostella larvae were then removed, placed into a Petri dish (9-cm-diameter) with moistened filter paper and a fresh leaf of the host plant species being evaluated. Six to eight mated females of D. semiclausum were then placed in the Petri dish for 6–8 h for parasitization. Mated females were obtained by transferring newly eclosed D. semiclausum females from the laboratory colony to small cages (approximately 30×30×30 cm) along with an equivalent number of males. Wasps were held for 2–3 days and provided ad libitum with water and honey, which was administered via cotton placed into a small test tube.
Following parasitization, the wasps were removed and larvae of P. xylostella were placed in Petri dishes (5-cm-diameter), with each dish containing a single larva and a filter paper moistened with distilled water. Fresh, excised leaf tissue of the host plant being evaluated was added and replaced daily, to enable each larval specimen to feed ad libitum.
Petri dishes with parasitized P. xylostella larvae and excised leaf tissue of B. rapa ssp. pekinensis were incubated at constant temperatures of 10, 15, 20, 23, 25, 28 and 30°C. Dishes with larvae and excised leaf tissue of B. oleracea var. capitata were incubated at 10, 15, 20, 23, 25 and 30°C, and dishes with larvae and excised leaf tissue of B. napus were incubated at 10, 15, 20, 25 and 30°C.
Parameters measured
Developmental times of D. semiclausum from egg to pupa were recorded for each replicate specimen. Pupae were removed within 12 h of pupation, weighed using a Sartorius Supermicro scale (Sartorius Inc., Edgewood, NY, USA) and returned to their respective containers until adult eclosion. Developmental times from pupa to adult emergence were recorded. Newly eclosed adults were maintained in sealed petri dishes with moistened filter paper but without food and returned to the controlled environment chambers to be held at the temperatures at which they were reared. Days from eclosion to death were recorded. After adult specimens died, they were held in labeled containers and allowed to dry; adult dry weights were recorded 7–10 days later. At this time, one forewing, a hindwing and a hind tibia were dissected from each specimen and photographed using a Dino-Lite Digital Microscope. DinoCapture software was used to determine tibia length, forewing length and hindwing length. The digital photographic images of the wings for each specimen were then imported to Image J software (National Institutes of Health, Bethesda, MD, USA) to quantify the areas of each according to the method of Sarfraz et al. (Reference Sarfraz, Dosdall and Keddie2007).
The measured parameters were selected for their known or predicted importance for indicating fitness: more rapid developmental times can enable parasitoids to avoid high mortality levels when fluctuations occur in host densities (Hackett-Jones et al., Reference Hackett-Jones, White and Cobbold2011), greater pupal and adult mass and longer-lived adults often indicate greater reproductive potential (e.g. Honek, Reference Honek1993; Godfray, Reference Godfray1994; Schmid-Hempel & Schmid-Hempel, Reference Schmid-Hempel and Schmid-Hempel1996; West et al., Reference West, Flanagan and Godfray1996), and larger wing areas and tibiae are generally positively correlated with fitness (Bethke et al., Reference Bethke, Nuessly, Paine and Redak1991; Sagarra et al., Reference Sagarra, Vincent and Stewart2001; Salvo & Valladares, Reference Salvo and Valladares2002).
Data analyses
Because D. semiclausum frequently has a male-biased sex ratio (Yang et al., Reference Yang, Chu and Talekar1993; Khatri et al., Reference Khatri, Wang and He2008), comparatively small numbers of replicate females were obtained, especially at temperature extremes; consequently, only data from males were used in our analyses. The effect of temperature for each developmental parameter was determined for each constant rearing temperature for each of the three host plant species using PROC MIXED of SAS (SAS Institute, 2005). All factors were considered fixed, and differences between temperatures for each host plant species were assessed using the LSMEANS statement with the PDIFF option in PROC MIXED.
Effects of host plant species, temperature, and interactions between host plant species and temperature were determined for all constant rearing temperatures common to all plant species and for which survival of D. semiclausum from egg to pupa occurred: 10, 15, 20 and 25°C. Data were subjected to analysis of variance using PROC MIXED of SAS, with all factors considered as fixed effects (SAS Institute, 2005). PROC MIXED was selected because it can better accommodate unbalanced data than PROC GLM (Yang, Reference Yang2010). Differences between temperatures for each host plant species were assessed using the LSMEANS statement with the PDIFF option in PROC MIXED. Correlations were assessed among forewing area, hindwing area, tibia length and adult and pupal mass using PROC CORR of SAS. A Bonferonni correction was performed for pair-wise comparisons (the ADJUST=BON associated with the LSMEANS statement) to address potential correlations among adult and pupal mass. Data were distributed normally for all parameters except development time from egg to pupa, development time from egg to adult eclosion and longevity without food. Rank transformations were performed for data for those parameters not normally distributed according to Canover & Iman (Reference Conover and Iman1981), and data were subsequently analyzed with PROC MIXED. There are some concerns regarding inflation of Type I errors using this technique, when strong correlation exists between pre-test and post-test scores (Blair & Higgins, Reference Blair and Higgins1985). To address these concerns, a Bonferonni correction was performed for pair-wise comparisons.
Stepwise multiple regression analysis was performed for pupal and adult mass at each constant rearing temperature for each of the three host plant species using SAS PROC REG with the stepwise selection option (SAS Institute, 2005). Temperature and a variable created to represent the quadratic of temperature were evaluated as the independent variables with a developmental parameter as the dependent variable. A value of α≤0.05 was required to retain parameters in the model. The main effect of thermal regime was decomposed to linear and quadratic effects to assess the trends of D. semiclausum development at various constant temperatures. This analysis helped facilitate identification of optimal and suboptimal temperature requirements for D. semiclausum because arthropod developmental rate is generally linear at moderate or optimal temperatures but curvilinear near the temperature extremes for a species (Wagner et al., Reference Wagner, Wu, Sharpe, Schoolfield and Coulson1984).
Days from oviposition to pupation, oviposition to eclosion and days to death following eclosion were evaluated for each temperature and host plant combination using the logistic regression model
(as per McClay & Hughes, Reference McClay and Hughes2007) where P represents the proportion of D. semiclausum pupating, eclosing or dying, T is time in days, and a and b are temperature-dependent parameters. The parameters a and b were fitted using the logistic regression procedure, specifying the described model, using Statistix 9.0 (Analytical Software, Tallahassee, FL, USA). These parameters were used to develop estimates of time (days) for 25% (T 0.25), 50% (T 0.50), 75% (T 0.75) and 100% (T 1.00) of D. semiclausum to pupate, eclose and die at each temperature and associated with each host plant. Death was restricted to a two-day period on Chinese cabbage at 15 and 25°C; therefore, days to death were transformed from 4.0 to 4.1 days for one of the 15 D. semiclausum specimens that died on the fourth day and from 2.0 to 2.1 days for one of the nine D. semiclausum that died on the second day to allow fitting of logistic models to these data.
Results
Hindwing and forewing length were highly correlated (R=0.890; df=260; P<0.001) as were hindwing length and area (R=0.879; df=261; P<0.001) and forewing length and area (R=0.897; df=267; P<0.001). Pupal and adult mass and hindwing length were also highly correlated (R=0.654; df=255; P<0.001 and R=0.776; df=268; P<0.001, respectively). Pupal and adult mass and tibial length were highly correlated (R=0.587; df=265; P<0.001 and R=0.633; df=272; P<0.001, respectively). Because forewing area, hindwing area, tibia length and adult mass are measures of the same fitness measure, body size, only effects of host plant species and temperature on pupal and adult masses were assessed and presented.
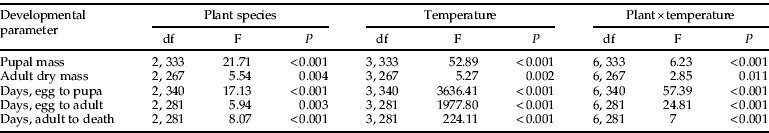
Host plant species fed upon by P. xylostella significantly affected several developmental parameters of D. semiclausum, including pupal weight, adult dry weight, developmental time from egg to pupa and from egg to adult, and longevity of adults held without food (P<0.01 for all parameters) (table 1). Rearing temperature significantly affected all developmental parameters measured (P<0.01 for all comparisons), and significant interactions between host plant species and constant rearing temperature were detected for all developmental parameters (P<0.05) (table 1).
Table 1. Results of analysis of variance for several developmental parameters of the parasitoid Diadegma semiclausum when its Plutella xylostella hosts were reared on different brassicaceous host plants at various constant temperatures. Analysis was performed for combined data of all host plant species (Brassica rapa ssp. pekinensis, Brassica oleracea var. capitata, and Brassica napus) when larvae were reared at temperatures of 10, 15, 20 and 25°C.
Pupal weights of D. semiclausum reared on P. xylostella larvae that consumed B. oleracea var. capitata differed significantly between 20 and 23°C (F 1, 110=3.27; P=0.021) but not between other incremental temperature increases (P>0.05 for all comparisons) (fig. 1). Specimens of D. semiclausum reared on B. rapa had significant reductions in pupal weight between 10 and 15°C (F 1, 222=3.31; P=0.023), and between 20 and 23°C (F 1, 222=3.99; P=0.002). Specimens reared on B. napus had significant reductions in pupal weight between 15 and 20°C (F 1, 132=5.79; P<0.001) but not between other incremental temperature increases (P>0.05 for all comparisons) (fig. 1).

Fig. 1. Mean (±SE) pupal and adult dry weights of Diadegma semiclausum male specimens reared on reared on Plutella xylostella hosts at various constant temperatures on leaf tissue of Brassica rapa, Brassica oleracea and Brassica napus (![]() , Brassica rapa;
, Brassica rapa; ![]() , Brassica oleracea;
, Brassica oleracea; ![]() , Brassica napus).
, Brassica napus).
Adult dry weights of D. semiclausum when its P. xylostella hosts were reared on B. oleracea did not differ significantly between any of the incremental temperature increases (P>0.05 for all comparisons) (fig. 1). However, adult dry weights differed significantly between constant rearing temperatures of 20 and 30°C (F 1, 89=3.96; P=0.002). On B. rapa, adult dry weights of D. semiclausum differed significantly between 10 and 15°C (F 1, 142=2.09; P=0.0385) and between 20 and 23°C (F 1, 142=2.20; P=0.0294). Adult dry weights of D. semiclausum when P. xylostella fed upon B. napus differed significantly between 15 and 20°C (F 1, 110=3.22; P=0.010) but not between other incremental temperature increases (P>0.05 for all comparisons) (fig. 1).
Development time of D. semiclausum from egg to pupation when its P. xylostella hosts were reared on B. oleracea declined significantly between all incremental rearing temperatures: 10–15°C (F 1, 113=7.68; P<0.0001), 15–20°C (F 1, 113=7.39; P<0.0001), 20–23°C (F 1, 113=8.43; P<0.0001), 23–25°C (F 1, 113=3.12; P=0.0034) and 25–30°C (F 1, 113=4.59; P=0.002) (fig. 2). When host larvae were reared on B. rapa, reductions in time from egg to pupation were observed for temperatures between 10 and 15°C (F 1, 232=5.40; P<0.001), 15 and 20°C (F 1, 232=5.29; P<0.001), 20 and 23°C (F 1, 232=11.11; P<0.001), and 25 and 28°C (F 1, 232=3.95; P=0.002). When reared on B. napus, development time to pupation declined between 10 and 15°C (F 1, 133=16.62; P<0.001), 15 and 20°C (F 1, 133=12.97; P<0.001), and 20 and 25°C (F 1, 133=12.02; P<0.001) (fig. 2).

Fig. 2. Mean (±SE) development times from egg to pupa, egg to adult, and eclosion to death for Diadegma semiclausum male specimens reared on Plutella xylostella hosts at various constant temperatures on leaf tissue of Brassica rapa, Brassica oleracea and Brassica napus (![]() , Brassica rapa;
, Brassica rapa; ![]() , Brassica oleracea;
, Brassica oleracea; ![]() , Brassica napus).
, Brassica napus).
Development time of D. semiclausum from egg to adult eclosion when its P. xylostella hosts were reared on B. oleracea declined for all incremental rearing temperatures: 10–15°C (F 1, 95=11.15; P<0.001), 15–20°C (F 1, 95=9.91; P<0.001), 20–23°C (F 1, 95=8.56; P<0.001), 23–25°C (F 1, 95=4.41; P<0.001) and 25–30°C (F 1, 95=6.81; P<0.0001) (fig. 2). Similarly, on B. rapa, development time from egg to adult eclosion decreased from 10 to 15°C (F 1, 151=9.75; P<0.001), 15 to 20°C (F 1, 151=10.47; P<0.001), 20 to 23°C (F 1, 151=10.89; P<0.001), 25 to 28°C (F 1, 151=3.79; P=0.0005) and 28 to 30°C (F 1, 151=3.40; P=0.018). For P. xylostella hosts reared on B. napus, days from egg to eclosion of D. semiclausum declined from 10 to 15°C (F 1, 111=17.20; P<0.001), 15 to 20°C (F 1, 111=11.80; P<0.001) and from 20 to 25°C (F 1, 111=7.94; P<0.001) (fig. 2).
Days from adult eclosion to death of D. semiclausum when its P. xylostella hosts were reared on B. oleracea declined from 15 to 20°C (F 1, 95=4.24; P<0.001) and 20 to 23°C (F 1, 95=4.36; P<0.001) (fig. 2). When parasitized P. xylostella were reared on B. rapa, days from eclosion to death of D. semiclausum declined from 10 to 15°C (F 1, 151=9.75; P<0.1001), 15 to 20°C (F 1, 151=10.47; P<0.001), 20 to 23°C (F 1, 151=10.89; P<0.001), 25 to 28°C (F 1, 151=3.79; P=0.005) and from 28 to 30°C (F 1, 151=3.40; P=0.018). Days from adult eclosion to death when P. xylostella hosts were reared on B. napus declined from 10 to 15°C (F 1, 111=6.74; P<0.001) and 15 to 20°C (F 1, 111=7.27; P<0.001) (fig. 2).
The significant interaction between plant species and temperature on pupal mass (F 6, 333=6.23; P<0.001) (table 1) occurred between low and moderate temperatures. At 10°C the order of pupal mass was B. rapa>B. oleracea=B. napus, but at 20°C this changed to B. oleracea>B. rapa>B. napus (fig. 1). The interaction between plant species and temperature on adult dry weight (F 6, 267=2.85; P=0.011) (table 1) was also detected between low and moderate temperatures with a change in dry weight from B. rapa=B. napus=B. oleracea at 10°C to B. oleracea=B. rapa>B. napus at 20°C (fig. 1).
The significant interaction between plant species and temperature on developmental time from egg to pupa (F 6, 340=57.39; P<0.001) (table 1) was evident between 10 and 15°C, and between 15 and 20°C (fig. 2). At 10°C the order of plant species for time to pupation was B. oleracea>B. rapa>B. napus, but at 15°C this changed to B. napus>B. oleracea=B. rapa. At 20°C the order was B. rapa>B. oleracea>B. napus. Similarly, developmental time from egg to adult also had significant interactions (F 6, 281=24.81; P<0.001) (table 1) between 10 and 15°C and between 15 and 20°C. At 10°C developmental time of D. semiclausum reared on P. xylostella larvae that consumed B. oleracea was similar to that of B. napus and B. rapa, but at 15°C the order of days from egg to adult eclosion was B. napus>B. oleracea>B. rapa. At 20°C the order was B. rapa and B. oleracea>B. napus (fig. 2).
Days from adult eclosion to death of D. semiclausum also had a significant interaction of plant species by temperature (F 6, 281=7.46; P<0.001) (table 1) (fig. 2). At 15°C days to death for wasp specimens reared on P. xylostella hosts that fed on B. rapa significantly exceeded mortality times for B. napus and B. oleracea. At 20°C this order had changed to B. oleracea>B. rapa=B. napus, and at 25°C days to death for parasitoids reared on larvae that consumed B. rapa, B. oleracea and B. napus were similar (fig. 2).
Stepwise multiple regression analysis of the various developmental parameters for D. semiclausum when its P. xylostella hosts were reared on leaf tissue of B. rapa identified significant linear decreases in pupal mass (M P) with temperature (T): M P=−1.29E−4(T)+0.008. A decrease in adult mass (M A) with the quadratic of temperature was also detected for this host plant: M A=−1.26E−7(T 2)+6.28E−4. A peak in pupal mass near 14°C was detected for parasitoids from P. xylostella reared on B. oleracea: M P=2.17E−4(T)+−7.99E−6(T 2) +0.005. Adult parasitoids from P. xylostella reared on this host plant also demonstrated a peak in mass near 18°C: M A=2.38E−5(T)+−6.96E−7(T 2)+3.68E−4. A linear decrease in pupal mass with temperature, and a decrease in adult mass with the quadratic of temperature were detected for parasitoids from P. xylostella reared on B. napus: M P=−8.70E−4(T)+0.007 and M A=−1.39E−7(T 2)+6.02E−4, respectively (table 2). For B. rapa, the model explained little of the variation in the data for adult dry weight (R 2=0.11; df=148), but explained much of the variation for pupal weight (R 2=0.56; df=228). For B. oleracea and B. napus, the models developed for the relationship of temperature and pupal mass also accounted for more variation (R 2=0.40; df=115 and R 2=0.28; df=135, respectively) than the models developed for adult mass (R 2=0.14; df=94; and R 2=0.06; df=113, respectively) (table 2).
Table 2. Results of stepwise multiple regression analysis to determine pupal and adult mass (dependent variables) of the parasitoid Diadegma semiclausum when its Plutella xylostella hosts were reared on different brassicaceous host plants at various constant temperatures.

Modeling of times to pupation, eclosion and death associated with different plant species and temperatures by logistic regression indicated that the sigmoidal patterns effectively described the developmental data; R 2 values ranged between 0.98 and 1.00 (fig. 3). At 10°C estimated requirements for development from egg to pupation ranged from 21.9 (B. napus) to 25.0 (B. oleracea) days for 25% of the population and from 28.6 to 44.5 days for 100% of the population on B. napus and B. oleracea, respectively (table 3). At 25°C days to pupation ranged from 5.2 (B. napus) to 5.7 (B. oleracea) for 25% of the population and from 9.9 (B. rapa) to 13.8 (B. napus) days for 100% of individuals (table 3).

Fig. 3. Proportions of Diadegma semiclausum specimens pupating, eclosing and dead predicted with logistic analysis for various constant temperatures on leaf tissues of Brassica rapa, Brassica oleracea and Brassica napus (◊, B. oleracea 10°C; ▵, B. napus 10°C; □, B. rapa 10°C; ![]() , B. oleracea 15°C;
, B. oleracea 15°C; ![]() , B. napus 15°C;
, B. napus 15°C; ![]() , B. rapa 15°C; ⧫, B. oleracea 20°C; ▲, B. napus 20°C; ▪, B. rapa 20°C;
, B. rapa 15°C; ⧫, B. oleracea 20°C; ▲, B. napus 20°C; ▪, B. rapa 20°C; ![]() , B. oleracea 25°C;
, B. oleracea 25°C; ![]() , B. napus 25°C;
, B. napus 25°C; ![]() , B. rapa 25°C;
, B. rapa 25°C; ![]() , B. oleracea;
, B. oleracea; ![]() , B. napus;
, B. napus; ![]() , B. rapa).
, B. rapa).
Table 3. Logistic model parameters a and b (see text for details) used to develop estimates of time (days) for 25% (T 25%), 50% (T 50%), 75% (T 75%) and 100% (T 100%) of D. semiclausum to pupate.

Days from egg to adult eclosion at 10°C required 49.7 (B. rapa) to 50.5 (B. napus) days for 25% of the population and 60.7 (B. napus) to 73.8 (B. oleracea) days for 100% of D. semiclausum individuals (table 4). At 25°C this range declined to 12.3 (B. napus) to 12.4 (B. rapa) days for 25% of the population and 16.3 (B. rapa) to 26.8 (B. oleracea) days for 100% (table 4).
Table 4. Logistic model parameters a and b (see text for details) used to develop estimates of time (days) for 25% (T 25%), 50% (T 50%), 75% (T 75%) and 100% (T 100%) of D. semiclausum to develop from egg to eclosion.

Days from adult eclosion to death for specimens reared at 10°C ranged from 4.6 (B. rapa) to 5.5 (B. napus) days for 25% of the population to 12.9 (B. rapa) to 14.8 (B. oleracea) days for 100% (table 5). At 25°C this range declined to 1.0 (B. napus) to 1.6 (B. rapa) days for 25% of individuals to 3.7 (B. rapa) to 8.0 (B. oleracea) days for 100% (table 5).
Table 5. Logistic model parameters a and b (see text for details) used to develop estimates of time (days) for 25% (T 25%), 50% (T 50%), 75% (T 75%) and 100% (T 100%) of D. semiclausum to die following adult eclosion.

Discussion
Sustainable production of canola in Australia and brassicaceous vegetables in Australia and other Asia-Pacific regions requires control of diamondback moth with its natural enemies, especially D. semiclausum. We determined that temperature and host plant species differentially affected various developmental parameters of D. semiclausum and that interactions between temperature and host plant species occurred for all developmental parameters measured. Our hypothesis that parameters associated with species fitness decline at higher temperatures was validated for pupal and adult dry weights, but developmental times to pupa and adult eclosion were most rapid at higher rearing temperatures (figs 1 and 2). The ability of P. xylostella to complete development at a wider range of temperatures than its principal parasitoid could explain the greater effectiveness of D. semiclausum as a biological control agent at cooler, higher elevations of Asia-Pacific regions. Our results, indicating no complete development of D. semiclausum at 30°C when larvae of its host consumed B. napus but not B. rapa and B. oleracea, suggest that D. semiclausum may be a more effective biocontrol agent for crops of common cabbage and Chinese cabbage than canola.
Global temperatures have increased 0.7°C in the last century (Rosenzweig et al., Reference Rosenzweig, Iglesias, Yang, Epstein and Chivian2000), and conservative scenarios of current and future greenhouse gas emissions suggest that temperatures will increase by 1.4 to 5.8°C over the next century (Cohen & Miller, Reference Cohen, Miller, McCarthy, Canziani, Leary, Dokken and White2001; Karl & Trenbeth, Reference Karl and Trenbeth2003). Increases in global temperatures may affect insect populations by lengthening the growing season, altering emergence patterns, increasing rates of growth and development, and altering geographical distribution patterns (Porter et al., Reference Porter, Parry and Carter1991; Olfert & Weiss, Reference Olfert and Weiss2006; Sutherst et al., Reference Sutherst, Constable, Finlay, Harrington, Luck and Zalucki2011). In Australia, climate change projections for 2050 suggest that low planetary greenhouse gas emissions will result in an average temperature increase of 1.2°C relative to 1990 temperatures, but with high emissions this increase could average 2.2°C (CSIRO, 2007). Precipitation is predicted to change little in northern areas of the country, but in the south precipitation is predicted to decrease along a gradient that ultimately reaches 5–7% (CSIRO, 2007). With more degree-days, multivoltine insects like P. xylostella and D. semiclausum could produce more generations annually, as has been predicted for diamondback moth and D. insulare in North America (Dosdall et al., Reference Dosdall, Weiss, Olfert, Mason, Soroka, Shelton, Collins, Zhang and Wu2008). Perhaps more importantly, the synchrony between herbivores and their parasitoids could become uncoupled if temperature drives species phenologies in different directions (van den Putten et al., Reference van den Putten, de Ruiterb, Bezemera, Harvey, Wassen and Wolters2004; Hance et al., Reference Hance, van Baaren, Vernon and Boivin2007). In the case of P. xylostella and D. semiclausum, increases in ambient temperatures appear more likely to negatively affect the parasitoid than its host. Liu et al. (Reference Liu, Chen and Zalucki2002) determined that P. xylostella developed successfully from egg to adult eclosion at constant temperatures as high as 32°C and at alternating temperatures as high as 38°C. However, this study and previous research by Yang et al. (Reference Yang, Chu and Talekar1993) indicate that survival and development of D. semiclausum decline dramatically at constant temperatures near 30°C. It is possible, therefore, that one impact of climate change could be decoupling of the phenological synchrony of P. xylostella with D. semiclausum in some regions of Australia, leading to greater reliance on population control with insecticide applications. This scenario appears more likely in regions of canola production because of the inability of D. semiclausum to persist on this host at constant temperatures of 30°C.
Interactions between temperature and the host plant species fed upon by the P. xylostella hosts of D. semiclausum occurred most often at the low (10–15°C) and high (25–30°C) constant temperature extremes. For instance, pupal weight was highest for specimens reared on hosts feeding on B. rapa at 10°C, but at 20°C pupal weight was greater for B. oleracea (fig. 1). At 10°C development time from egg to pupation was slowest on B. oleracea than on other host plant species, but at 20°C and higher this development was most prolonged on B. rapa (fig. 2). Among the host plant species we investigated, pupal weight, adult dry weight and days from eclosion to death were greater on B. rapa at the lowest temperature (10°C) than for B. oleracea and B. napus. By contrast, some developmental parameters for B. napus were lower than for the other two host plants at 10°C (pupal weight, days from egg to pupation); moderate temperatures appeared to favor development on B. oleracea and B. rapa. These results indicate that developmental parameters of D. semiclausum at the thermal extremes cannot be considered indicative of performance at moderate temperatures on all host plant species.
The three host plants investigated in this study have distinct structural characteristics: B. rapa ssp. pekinensis and B. oleracea var. capitata are comparatively low to the ground with a compact growth habit, but B. napus is erect, more branched and much taller. Gols et al. (Reference Gols, Bukovinszky, Hemerik, Harvey, van Lenteren and Vet2005) found that the structure of vegetation on which P. xylostella larvae were feeding affected the host-finding efficiency of D. semiclausum. Foraging parasitoid females located host larvae more rapidly on small plants of Sinapis alba L. than on large plants, and Gols et al. (Reference Gols, Bukovinszky, Hemerik, Harvey, van Lenteren and Vet2005) proposed that this may be attributed to differences in the composition or quantity of volatile chemical compounds (especially glucosinolates) that affected attractiveness. Alternatively, this difference in host-finding efficiency may have resulted from plant architectural characteristics, since geometry can influence parasitoid movement, depending on the spatial scale at which foraging occurs (Andow & Prokrym, Reference Andow and Prokrym1990; Coll & Bottrell, Reference Coll and Bottrell1996). If the host-finding efficiency of D. semiclausum is reduced in taller, erect B. napus plants relative to more compact B. oleracea var. capitata or B. rapa ssp. pekinensis plants, as suggested by Gols et al. (Reference Gols, Bukovinszky, Hemerik, Harvey, van Lenteren and Vet2005), it may further contribute to reduced biocontrol of P. xylostella in canola crops.
In addition to structural/morphological influences, host plants can affect parasitoid development and fitness through differences in quality and genotype. Sarfraz et al. (Reference Sarfraz, Dosdall and Keddie2009) found that, when D. insulare were reared on P. xylostella hosts that fed upon high-quality well fertilized plants of B. napus, developmental rate was significantly faster than on plants grown without fertilizer or with fertilizer applied at low to moderate rates. Plant genotype can also affect parasitoid development. For example, developmental time from egg to adult of D. insulare was significantly faster when parasitized P. xylostella larvae were reared on two B. napus cultivars than on B. oleracea, Brassica carinata L. and B. rapa (Sarfraz et al., Reference Sarfraz, Dosdall and Keddie2008). Glucosinolate content of host plants likely contributes to these differences. Gols et al. (Reference Gols, Bukovinszky, van Dam, Dicke, Bullock and Harvey2008) found that glucosinolate contents in leaves can vary substantially among various plant species and populations, as well as for the same plant species during different times of the year (Gols et al., Reference Gols, Raaijmakers, van Dam, Dicke, Bukovinszky and Harvey2007); this could explain, in part, differences in developmental times of parasitoids when their hosts are reared on certain host plants. Pereira et al. (Reference Pereira, Rosa, Fahey, Stephenson, Carvalho and Aires2002) reported that glucosinolate content is influenced by cultivation temperature for B. oleracea var. italica; greatest total glucosinolate content occurred at 10°C and 30 to 35°C. The influence on our results of temperature on production of plant glucosinolates is unknown but suggests directions for further study. Further, our studies were conducted with excised leaf tissue, and developmental parameters on intact tissues should be investigated.
Plant genotype and morphology can affect not only developmental rate but also parasitism success. Talekar & Yang (Reference Talekar and Yang1991) found that level of parasitism by D. semiclausum was greater when P. xylostella larvae were feeding on common cabbage than on cauliflower (B. oleracea var. italica), broccoli (B. oleracea var. botrytis) or Chinese cabbage. In our study, pupal weight of D. semiclausum, which is an important fitness correlate of parasitoids (Schmid-Hempel & Schmid-Hempel, Reference Schmid-Hempel and Schmid-Hempel1996), was greater at most constant temperatures when its P. xylostella hosts were reared on cabbage than on Chinese cabbage, and lowest on canola (fig. 1). Because D. semiclausum prefers to conduct host-seeking activity on plants on which its offspring are largest and develop most rapidly (Gols et al., Reference Gols, van Dam, Raaijmakers, Dicke and Harvey2009), the fitness advantage gained by D. semiclausum when its P. xylostella hosts feed on cabbage in terms of pupal weight may be linked to the increased parasitism success reported by Talekar & Yang (Reference Talekar and Yang1991).
Sex ratio of D. semiclausum was strongly male-biased in our study; when rearing data for all temperatures and host plants were combined, the ratio was 0.85 M:0.15 F. Male-biased sex ratios of this and other species of Diadegma have been reported previously in the literature. Yang et al. (Reference Yang, Chu and Talekar1993) investigated temperature effects in a population of D. semiclausum from Taiwan and found that sex ratio remained near 1:1 when specimens were reared from 15 to 25°C; but at higher rearing temperatures, the ratio became strongly skewed toward males. In addition to temperature, plant species on which host insects feed affects sex ratio in D. semiclausum (Rossbach, Reference Rossbach2005). However, parasitoid genotype, the presence of a bacterial symbiont and single-locus complementary sex determination could also be involved. Kenji et al. (Reference Kenji, Graham and Mitsutaka2005) determined that sex ratios D. semiclausum varied depending on genotype of the parasitoid, and van Wilgenburg et al. (Reference van Wilgenburg, Driessen and Beukeboom2006) noted that the presence of Wolbachia bacteria can affect sex determination in the Hymenoptera. Butcher et al. (Reference Butcher, Whitfield and Hubbard2000) found that single-locus complementary sex determination occurs in the congeneric species, Diadegma chrysostictos (Gmelin), wherein diploid males develop from fertilized eggs homozygous at the sex locus. The occurrence of diploid males through this process, as well as by development of haploid males from unfertilized eggs, can cause male-biased sex ratios after several generations.
In summary, we have shown that temperature and host plant species differentially affect developmental parameters of the parasitoid D. semiclausum. The frequent interactions between temperature and host plant species have implications for biological control because parasitization of herbivore hosts should presumably increase for the parasitoids with greatest fitness; depending on temperature, this can vary among crop plants. The ability of its P. xylostella hosts to complete development at a wider range of temperatures than its principal parasitoid could explain the greater effectiveness of D. semiclausum as a biological control agent at cooler, higher elevations of Asia-Pacific regions. In Australia, the potential consequences of these results for biological control appear more serious for crops of canola grown in regions subjected to high temperatures during crop production. However, the ability of other important members of the P. xylostella parasitoid fauna to develop at higher temperatures should be investigated to better understand the potential consequences for crop production associated with climate change.
Acknowledgements
Funding for this project was provided by the Natural Sciences and Engineering Research Council of Canada, the University of Alberta, Edmonton, AB, Canada and the University of Queensland, St Lucia, QLD, Australia. We are grateful to K. Renwick for technical assistance.