Introduction
Aedes aegypti is identified as the main vector for dengue transmission, which has been ranked as one of the top most invasive species worldwide (Gratz, Reference Gratz2004; Invasive Species Specialist Group, 2009; Lambrechts et al., Reference Lambrechts, Scott and Gubler2010; Medlock et al., Reference Medlock, Hansford, Schaffner, Versteirt, Hendrickx, Zeller and Bortel2012). Over the past several decades, the range of Ae. aegypti has extended into new geographic regions mainly due to the international tire trade (Reiter & Sprenger, Reference Reiter and Sprenger1987; Benedict et al., Reference Benedict, Levine, Hawley and Lounibos2007; Enserink, Reference Enserink2008). This vector has gained a new importance, since it may be responsible for the transmission of Dengue, Chikungunya, and Zika in the world (Nadeeka et al., Reference Nadeeka, Gunathilaka and Amarasinghe2014; Center for Disease Control, 2016).
In Sri Lanka, dengue is the most rapidly spreading mosquito-borne viral disease, which is mainly transmitted by Ae. aegypti and Ae. albopictus mosquitoes (Nadeeka et al., Reference Nadeeka, Gunathilaka and Amarasinghe2014). Often the density of Ae. aegypti act as the determining factor of dengue epidemics within Sri Lanka. From January to 14 July in 2017, 89, 885 suspected dengue cases have been reported to the Epidemiology Unit, Sri Lanka from all over the island indicating the highest ever number of cases per year (Epidemiology Unit, 2017).
In the absence of an efficient vaccine to protect human population from mosquito-transmitted diseases such as dengue, the difficult and complex task of mosquito control is one of the few proven ways to reduce the risk of transmission (Benedict & Robinson, Reference Benedict and Robinson2003; Townson et al., Reference Townson, Nathan, Zaim, Guillet, Manga, Bos and Kindhauser2005; Alphey et al., Reference Alphey, Benedict, Bellini, Clark, Dame, Service and Dobson2010; Bonizzoni et al., Reference Bonizzoni, Gasperi, Chen and James2013; Wong et al., Reference Wong, Li, Chong, Ng and Tan2013; Rainey et al., Reference Rainey, Shah, Kohl and Dietrich2014). Until now, conventional pest control methods have failed to keep this species below sustainable and safe levels (Carrieri et al., Reference Carrieri, Colonna, Gentile and Bellini2006), probably because of the eco-biology of Aedes larvae, that exploits a variety of water collecting containers found in private gardens, backyards, and vegetated areas (Bellini et al., Reference Bellini, Albieri, Balestrino, Carrieri, Porretta, Urbanelli, Calvitti, Moretti and Maini2010).
When used with well-integrated conventional control methods, the Sterile Insect Technique (SIT) has proven to be an effective tool to control various insect species of agricultural importance (Dyck et al., Reference Dyck, Hendrichs and Robinson2005). The SIT is a biological method of suppressing the insect population using sustained releases of large numbers of mass-reared and sexually sterilized males to reduce the fertility of a field population of the same species. Effective control can be accomplished when the sterile insects are used systematically, in an area-wide integrated pest management (AW-IPM) programme (Knipling, Reference Knipling1955; Coleman & Alphey, Reference Coleman and Alphey2004; Hendrichs & Robinson, Reference Hendrichs, Robinson, Resh and Carde2009).
However, application of the SIT involves colonization of the target species and large-scale rearing of viable and competitive males for release, which must be able to locate and mate with wild females. Therefore, the colonization processes should enhance the characters necessary for these functions (Benedict et al., Reference Benedict, Knols, Bossin, Howell, Mialhe, Caceres and Robinson2009). In the mass rearing phase, larval diet quality and rearing conditions have a direct and often irreversible effect on adult traits (Briegel & Timmermann, Reference Briegel and Timmermann2001; Cohen, Reference Cohen2003; Benedict et al., Reference Benedict, Knols, Bossin, Howell, Mialhe, Caceres and Robinson2009), especially on the size of the laboratory-reared adults and other physiological functions associated with their nutrition. If the laboratory-reared colony is not compatible with the existing wild population, expected outcomes of the SIT programmes would not become a reality.
As reported by Timmermann & Briegel (Reference Timmermann and Briegel1999), the larval diet should provide a wide range of nutrients to avoid the risk of deficiencies that could negatively affect both the rearing productivity and the fitness of the males produced. Therefore, the effects of diet quality, quantity, and insect handling during mass rearing process need further investigation.
Another aspect that should be considered when selecting a diet for mass rearing purposes is the composition of the ingredients. Components such as wheat germ, soy flour, ground beef, and chicken eggs are relatively inexpensive and many are highly nutritious. It would be expensive and challenging to rear many successive generations of fecund insects on a chemically defined diet (Cohen, Reference Cohen2001). In addition, such diets may be expensive and time consuming to prepare. In order to remedy this, the Food and Agricultural Organization (FAO)/International Atomic Energy agency (IAEA) laboratory, Austria has formulated two diets specifically for mosquito mass rearing, which comprise tuna meal, bovine liver powder, and brewer's yeast. However, optimization of larval diet is essential in mass rearing of mosquitoes, in order to obtain a compatible adult population to compete with the wild types of the similar species in SIT-based applications.
Therefore, the objective of the present study was to evaluate the effect of different larval diet concentrations on the growth and development of Ae. aegypti, in order to determine optimum conditions for larval development, which is a critical aspect for developing standardized rearing procedures in SIT-based strategies.
Method
Mosquito rearing
Egg papers of Aedes aegypti were obtained from the existing insectary at the Molecular Medicine Unit, Faculty of Medicine University of Kelaniya, Sri Lanka and kept for one generation. Adults were housed in adult mosquito rearing cages (24 × 24 × 24 cm3) with mesh screening on top, provided with a 10% sugar solution and water ad libitum under the standard conditions (27 ± 2 °C, 75 ± 5% relative humidity [RH], and a photoperiod of 12 : 12 [L:D] h). Females were allowed to feed on cattle blood using an artificial membrane feeding system. The mosquitoes were starved of sucrose solution for 24 h prior to blood inoculation. Females were allowed to lay eggs on white filter papers contained in plastic cylindrical containers (diameter 9.5 cm, height 5 cm) filled with de-chlorinated water. Egg papers were removed from the cages and left to dry under the standard conditions for 24 h. For hatching, eggs were put into a closed, one liter capacity jar filled with deionized water. First instar larvae emerged from above eggs were taken for the experiment.
Formulation of larval diet
Larval diet was prepared according to the recommended compositions of the International Atomic Energy Agency (Puggioli et al., Reference Puggioli, Carrieri, Dindo, Medici, Lees, Gilles and Bellini2016) by dissolving 50% tuna meal (12.5 g), 36% bovine liver powder (9.0 g), and 14% brewer's yeast (3.5 g) in 100 ml of distilled water. The homogenized stock slurry was stored at −20 °C to prevent the degradation of diet components and microbial proliferation. The initial concentration of the prepared stock slurry was considered as 100% throughout the study.
Experimental design
Five batches of 750 first instar larvae (L1) of Ae. aegypti were counted manually and added to larval rearing trays (25 × 25 × 7 cm3) filled with deionized water. The total volume in each experimental setup was retained at 500 ml. Larval diet concentrations were maintained as 2% (3.33 × 10−2 mg per larvae), 4% (6.66 × 10−2 mg per larvae), 6% (9.99 × 10−2 mg per larvae), 8% (1.33 × 10−2 mg per larvae) and 10% (1.65 × 10−2 mg per larvae) of the above prepared stock slurry concentration, by adding adequate amounts (10, 20, 30, 40, and 50 ml, respectively) of the stock slurry (100%) for respective concentration.
The larval diet concentration at each trial was maintained daily until all larvae reached the pupal stage. Excessive food, fecal matter, and debris in the larval trays were removed daily using pasture pipettes, in order to maintain satisfactory water quality levels for larval development. All the larval trays were examined daily at 09 : 00 and 15 : 00 h and pupae that had formed were manually collected. The pupae were counted and placed in transparent plastic vials (50 ml capacity) filled with 25 ml of deionized water and covered with a sponge plug. Adult emergence was also recorded daily at 09 : 00 and 15 : 00 h and sex was determined. The experiment was repeated five times for each diet concentration.
Pupation time and pupation success
The pupation time, which is defined as the developmental time from egg submersion to pupation onset and the pupation success (percentage of larvae that pupated) were investigated at each dietary concentration.
Determination of the adult success and sex ratio
Collected mosquito pupae were placed in separate mosquito cages for each dietary concentration and maintained for the emergence of adults. The adult success (percentage of pupae that emerged as adults) were calculated at each dietary concentration. The gender of the adults was determined to calculate the proportion of male and female pupae (sex ratios) at each concentration.
Body size of larvae and pupae at different diet concentration
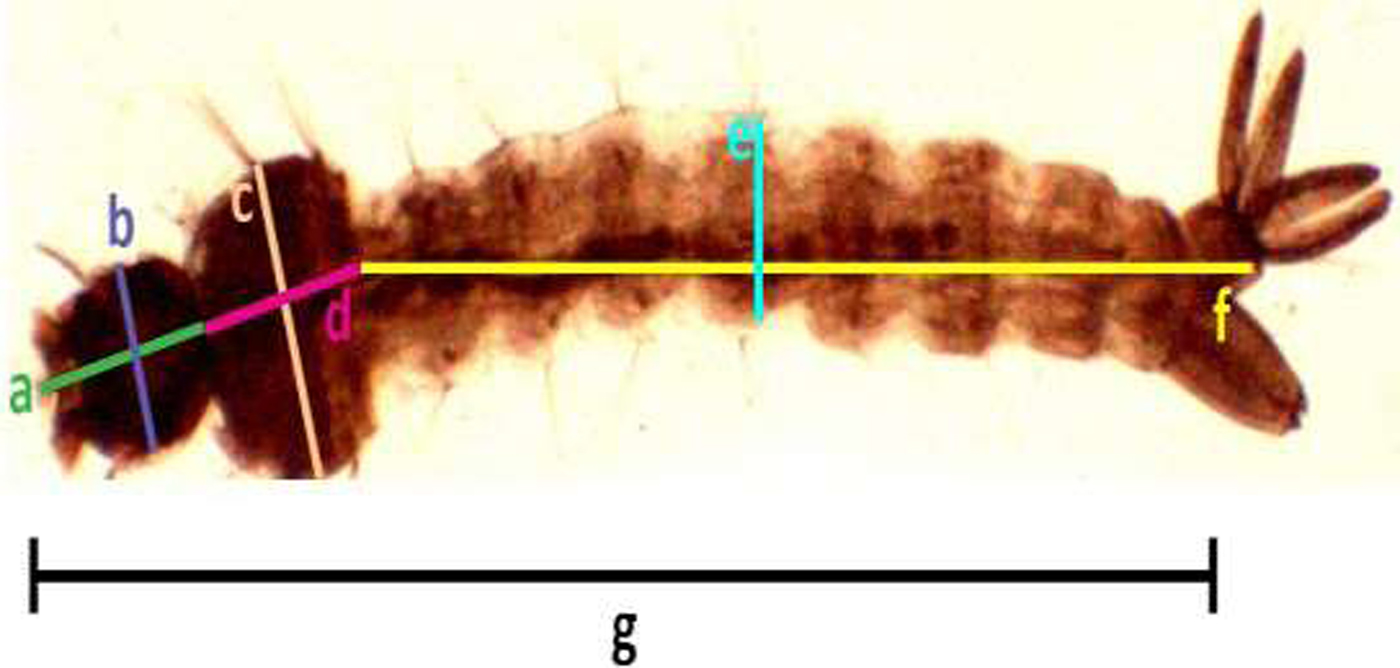
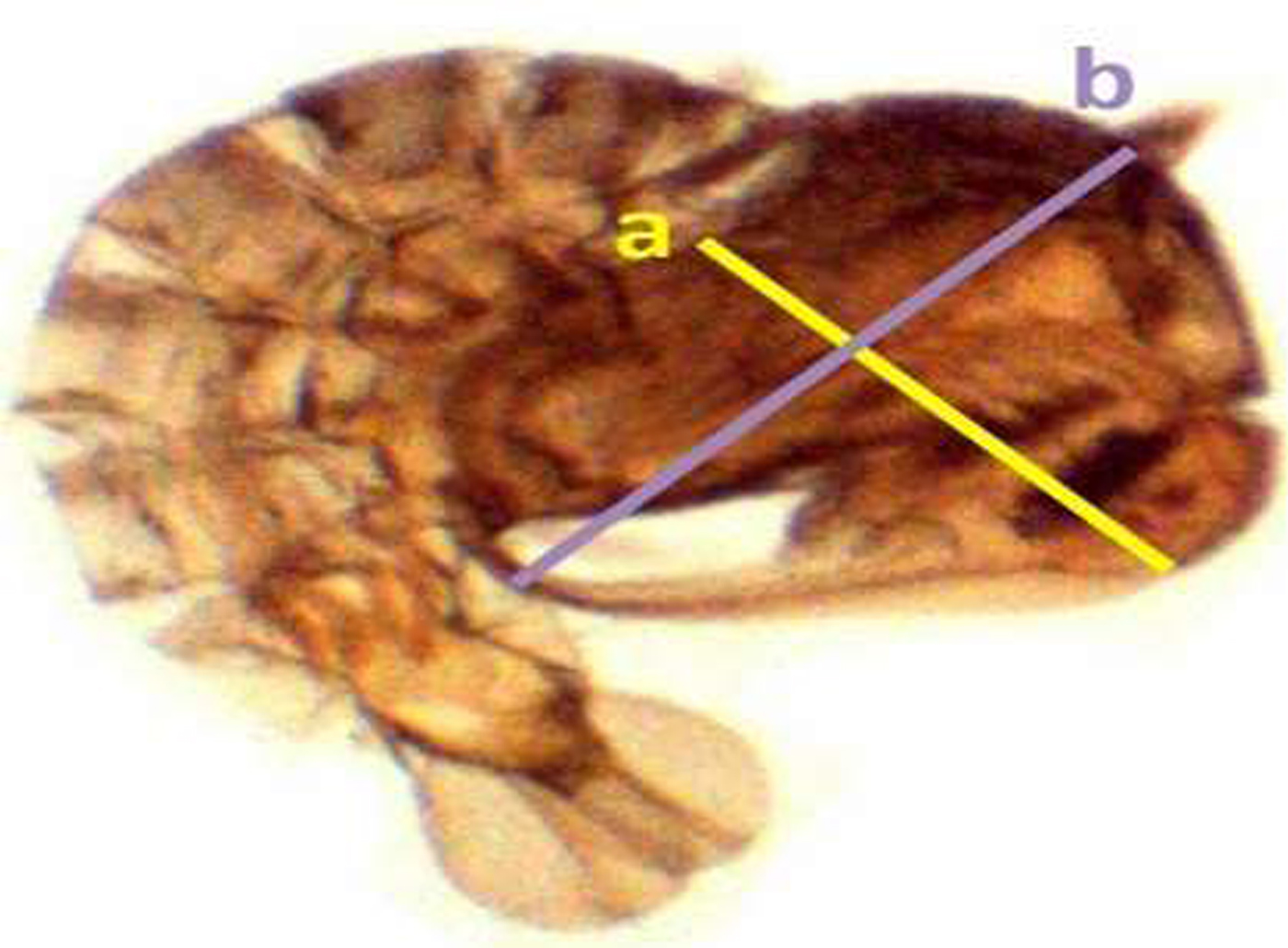
Samples of the fourth instar larvae (n = 30) and pupae (n = 30) were obtained randomly from each trial under different respective concentrations. Collected individual larvae and pupae were placed in a small cup containing 2 ml of cold water (2.5 ± 2 °C), which immobilized the pupae. The larval and pupal body size were detected by measuring different parameters according to the standard methods described by Timmerman & Briegel (Reference Timmermann and Briegel1999) [Larvae: head length, width of head, length of thorax, width of thorax, length of abdomen, width of abdomen, and the total length of larva (fig. 1); Pupae: length and width the cephalothorax (fig. 2)], using a dissecting microscope (BOECO BST-606, Germany) fixed with a microscopy digital USB camera (Optika 4083.B6) under 8× magnification and OPTIKA version 2.12 image processing software. The larvae and pupae, which were taken for measurements, were transferred to respective experimental setups for further investigations.
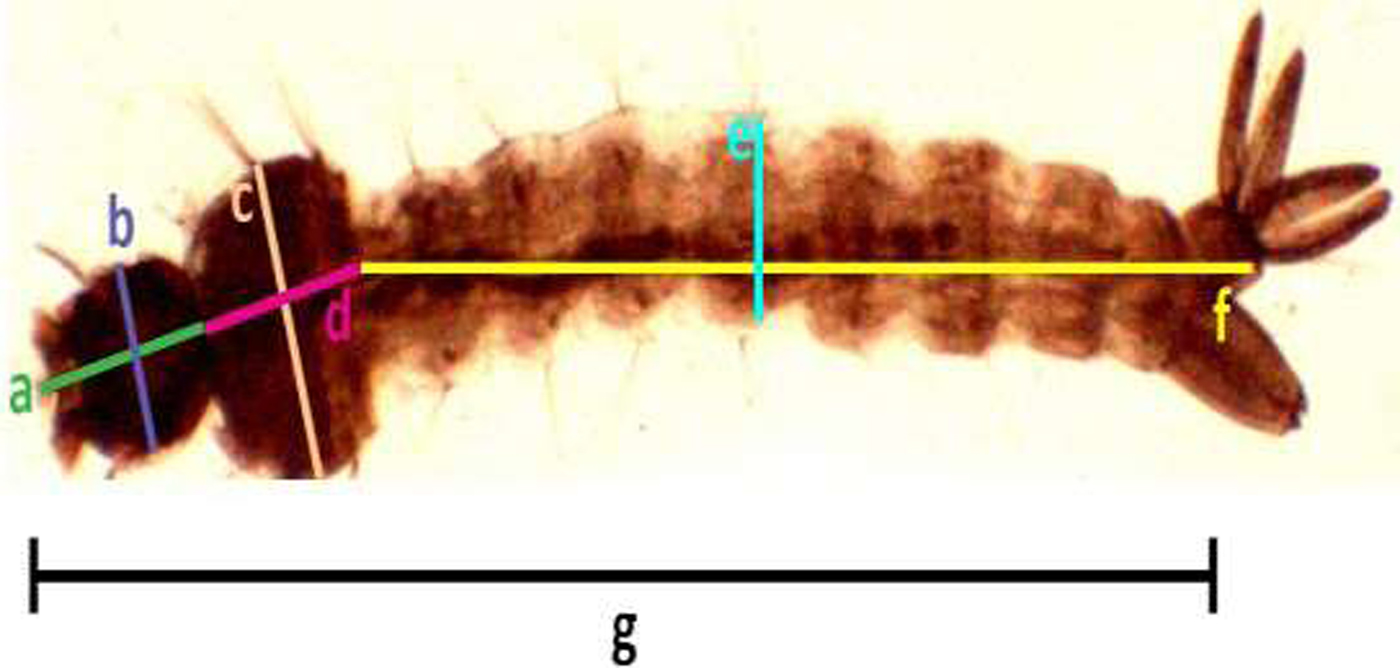
Fig. 1. Measurement of Aedes aegypti 4th instar larvae (head length (a), width of head (b), length of thorax (c), width of thorax (d), length of abdomen (e), width of abdomen, (f) and the total length of larvae (g), respectively).
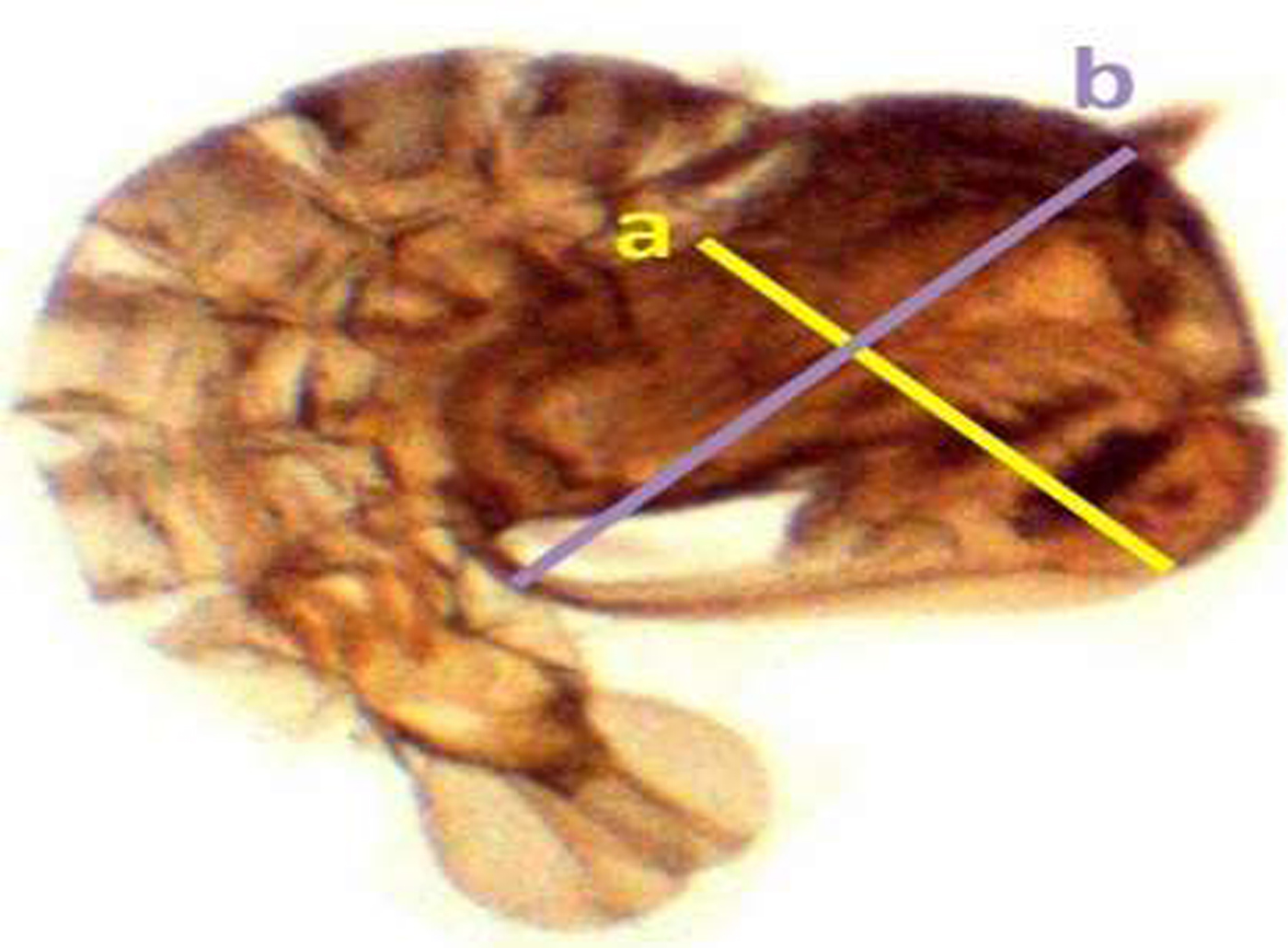
Fig. 2. Measurement of Aedes aegypti pupae for selected parameters (length of the cephalothorax (a), width of the cephalothorax (b), respectively).
All measurements were made from digital images of wings, thoraces, and abdomens using a microscope mounted camera (BOECO BST 606 made in Germany) at 8× magnification
Body size of adult mosquitoes
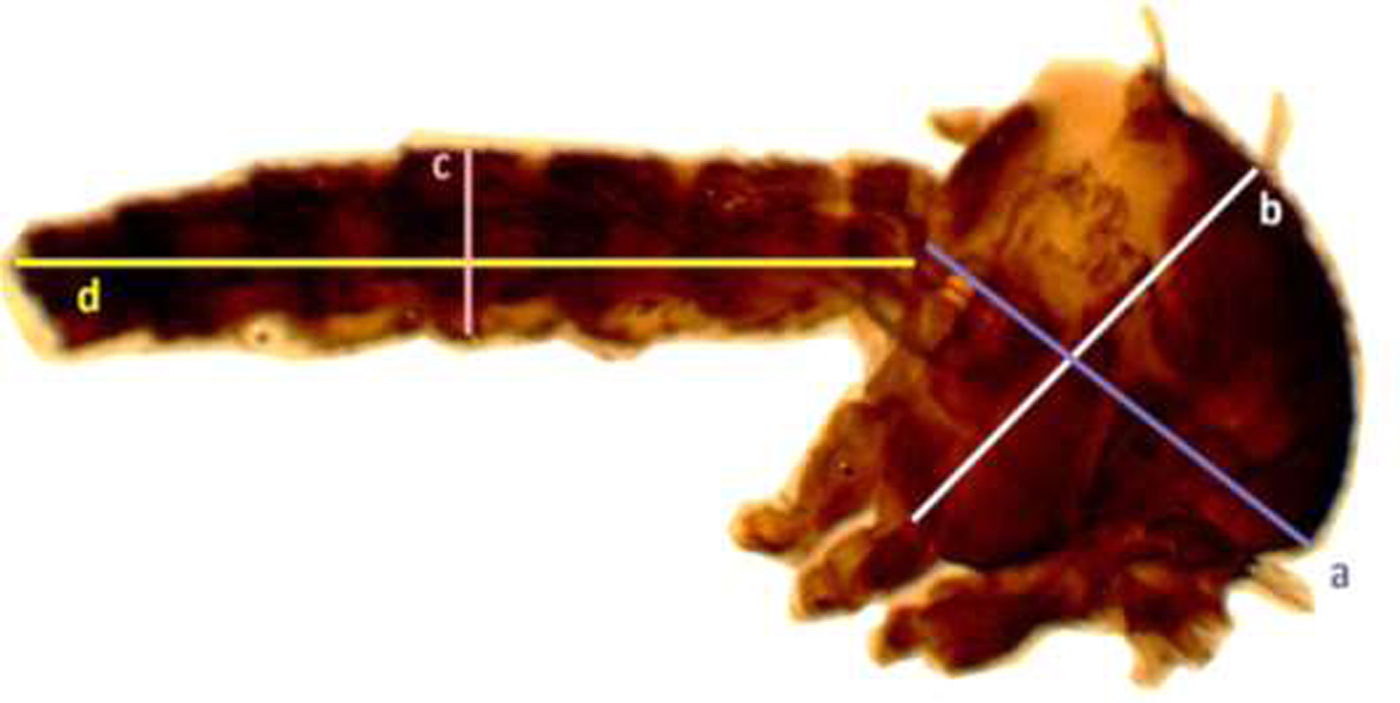
Adult males (n = 30) and females (n = 30) that emerged from each diet treatment were collected randomly into separate collection tubes using mouth aspirators, and samples were frozen immediately in −20 °C. A wing was removed and thorax with the abdomen of each adult was dissected under a dissecting microscope. Adult wing and the thorax with abdomen were mounted separately using Canada balsam in Xylene on glass slides (fig. 3).
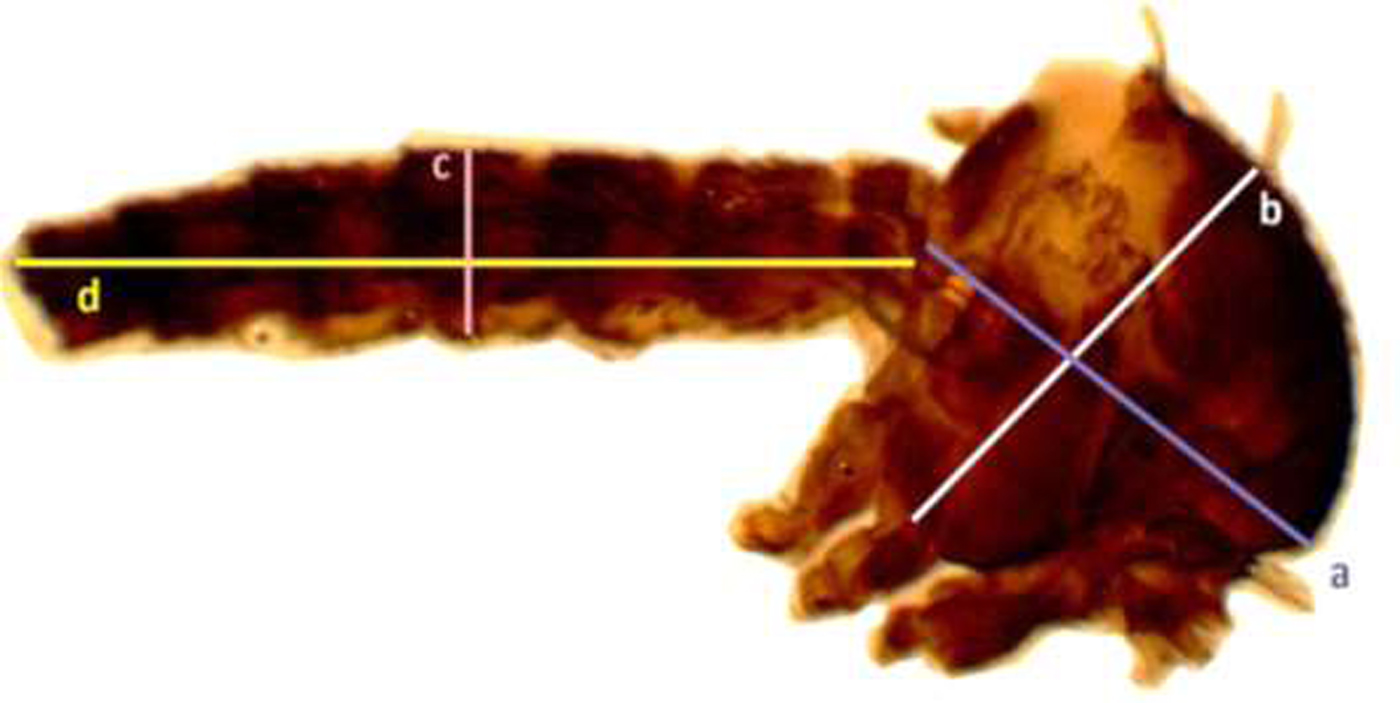
Fig. 3. Measurements of the thorax and abdomen of Aedes aegypti adult mosquito (thorax length (a), width of the thorax (b), length of the abdomen, (c) and width of the abdomen (d) respectively).
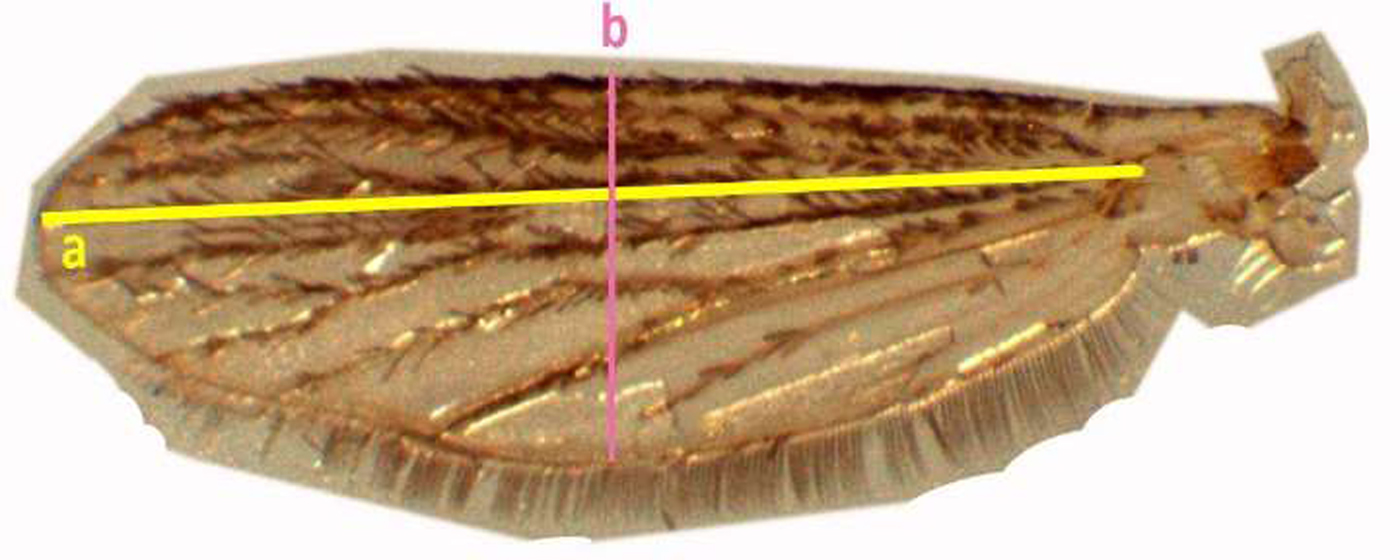
Standardized wing length was measured from the distal end of alula to the end of radius vein excluding fringe scale (Puggioli et al., Reference Puggioli, Balestrino, Damiens, Lees, Soliban, Madakacherry, Dindo, Bellini and Gilles2013) and the width of the wing was measured at the greatest breadth excluding fringing scales (fig. 4).
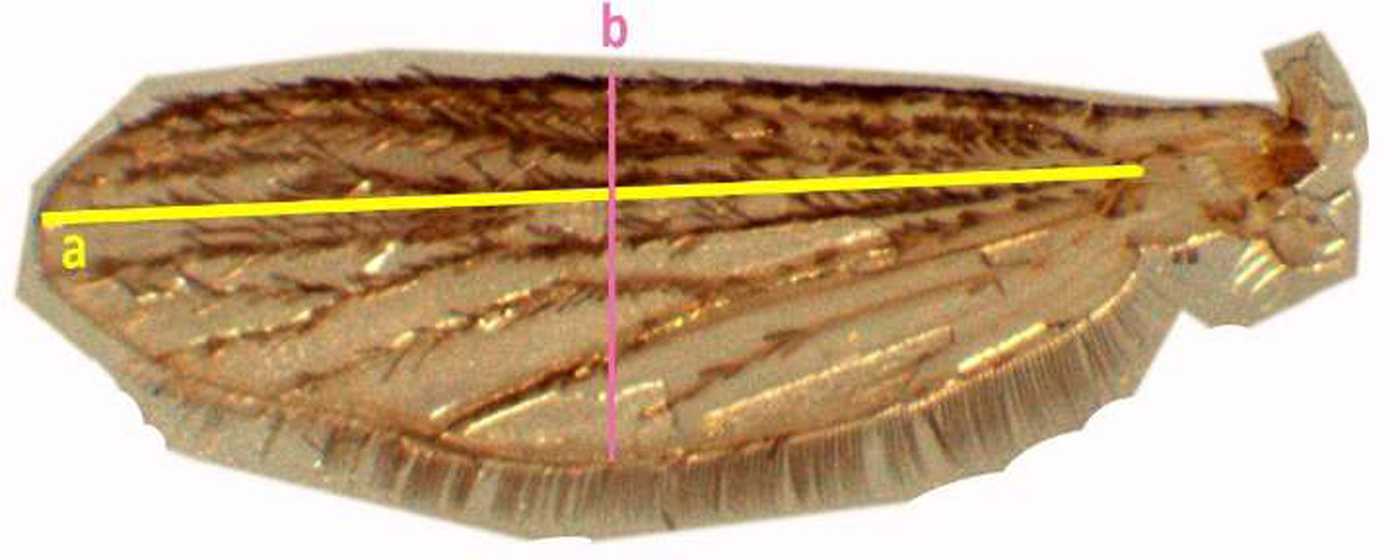
Fig. 4. Measurement of the adult mosquito wing of Aedes aegypti (wing length (a), width of the wing (b), respectively).
In addition, measurements were made of thorax width from dorsum to coxae and from the base of neck to base of the abdomen (fig. 3). Abdomen width was measured at the widest point and the length of the abdomen was measured from base to the tip.
Egg production and hatching rate
Females of the mosquito colonies emerged from different larval diet concentrations were fed with cattle blood meal artificially by metal plate method as described by Gunathilaka et al. (Reference Gunathilaka, Ranathunge, Udayanga and Abeyewickreme2017).
Egg laying cups for females were placed in cages in order to facilitate oviposition. The number of eggs in the filter paper lined in each egg-laying cup was enumerated after 2 days using a stereomicroscope (MEIJI EMZ5) at ×10 magnification. The fecundity was calculated by the average number of eggs laid per female. Egg papers from each treatment were allowed to embryonate for at least 7 days in the laboratory maintained conditions. Eggs were hatched according to the method described by Balestrino et al. (Reference Balestrino, Medici, Candini, Carrieri, Maccagnani, Calvitti, Maini and Bellini2010). Percentage of hatchability was calculated as the ratio of the number of larvae hatched to total number of eggs.
Statistical analysis
The significance of the effect of larval diet concentration on different larval and pupal measurements was analyzed by using General Linear Modeling (GLM) followed by Tukey's pair-wise comparisons. The effect of diet, gender, and interaction of diet concentration and gender on adult measurements was analyzed using GLM in IBM SPSS Statistics (version 23). Further, the significance of the effect of larval diet concentration on pupation success, adult success, the time required for pupation and emergence of adults, sex ratio, fecundity and hatching rate were analyzed using GLM. Pearson's correlation analysis was used to investigate the significance and nature of the relationship of different development parameter with varying diet concentration. All data analysis operations were performed using SPSS Statistics (version 23).
Results
Larval development parameters
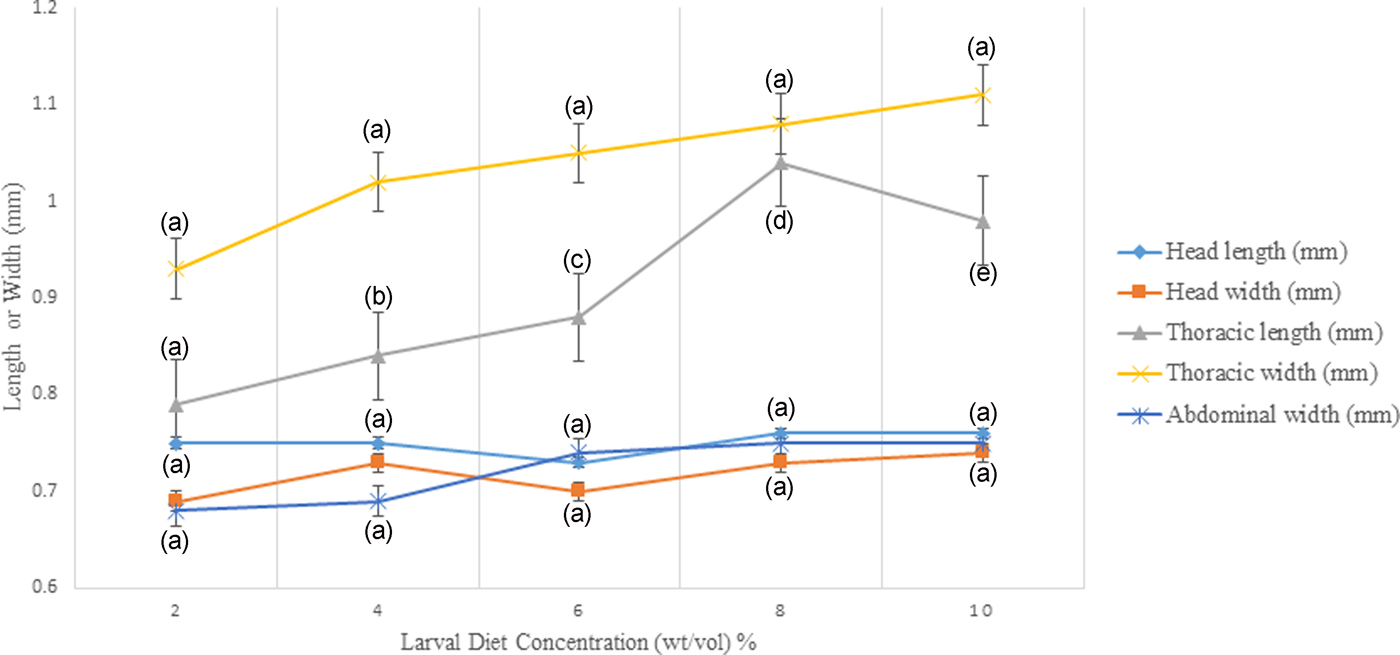
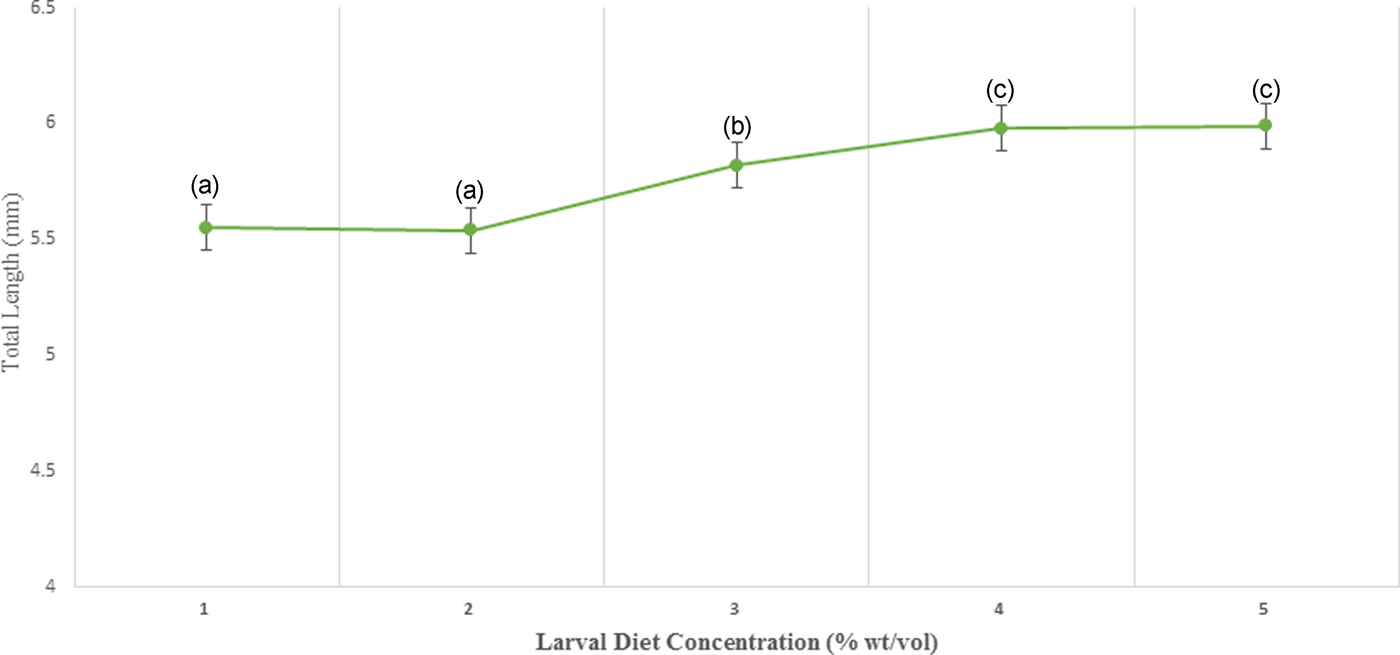
According to the results of GLM, significant differences (P < 0.05) were identified in the total body length (F 4,24 = 3.783) and the thoracic length (F 4,24 = 6.539) of 4th instar larvae with varying diet concentrations at 95% level of confidence. There was a gradual enhancement of body length, head length, and thoracic width of larvae with increasing food availability in the medium. The maximum values of all these parameters were observed at 10% larval diet concentration, which was taken as the highest diet concentration (figs 5 and 6, Annexure 1). The larvae with the smallest body size (5.50–5.60 mm) were found in 2% treatment. However, it was interesting to note that the head length, head width, thoracic width, and abdominal width did not show any significant variances (P > 0.05) at different diet concentrations (Annexure 1).
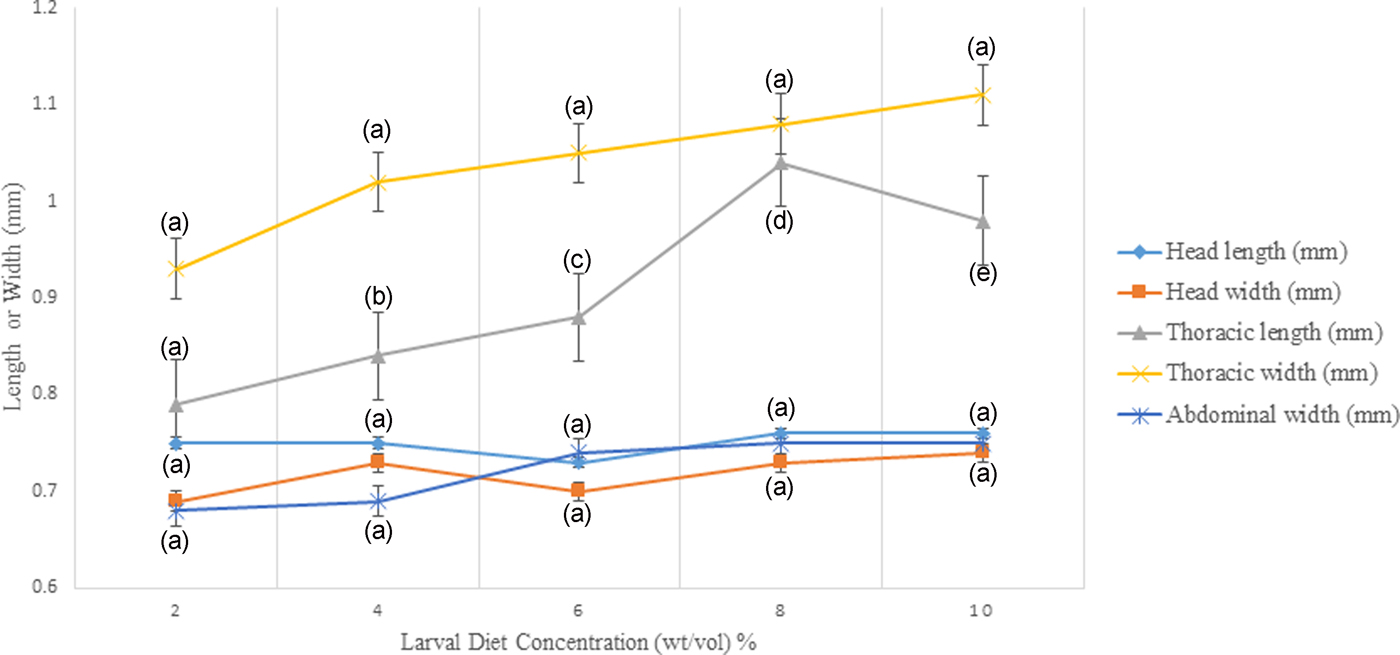
Fig. 5. Variation of head length, head width, thoracic length, thoracic width, and abdominal width of the 4th instar Aedes aegypti larvae fed with different diet concentrations.
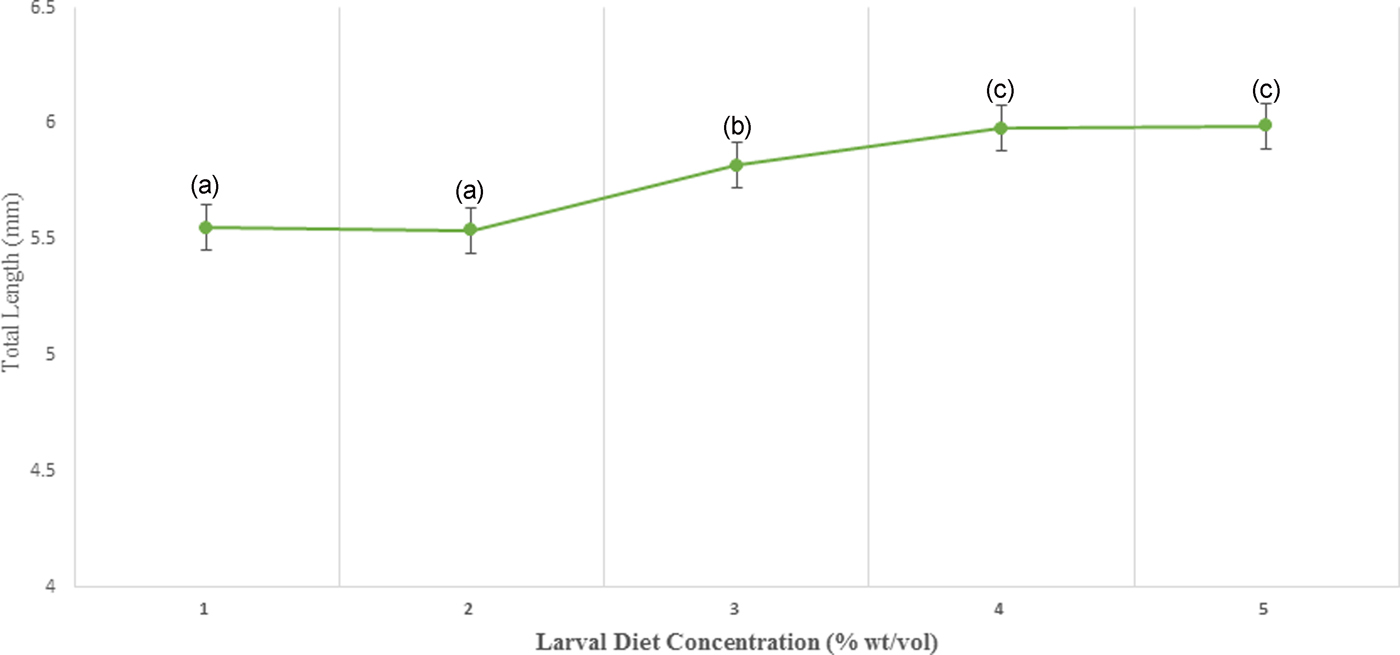
Fig. 6. Variation of the total length of 4th instar Aedes aegypti fed with different diet concentrations.
The highest width of the head, thoracic length, and abdominal width of larvae were detected at 8% concentration. However, there was a sudden collapse of all these parameters of the larvae treated with 10% larval diet. There was a fluctuation in the head width and head length of 4th instar larvae reared at the concentration series of larval diet (fig. 5).
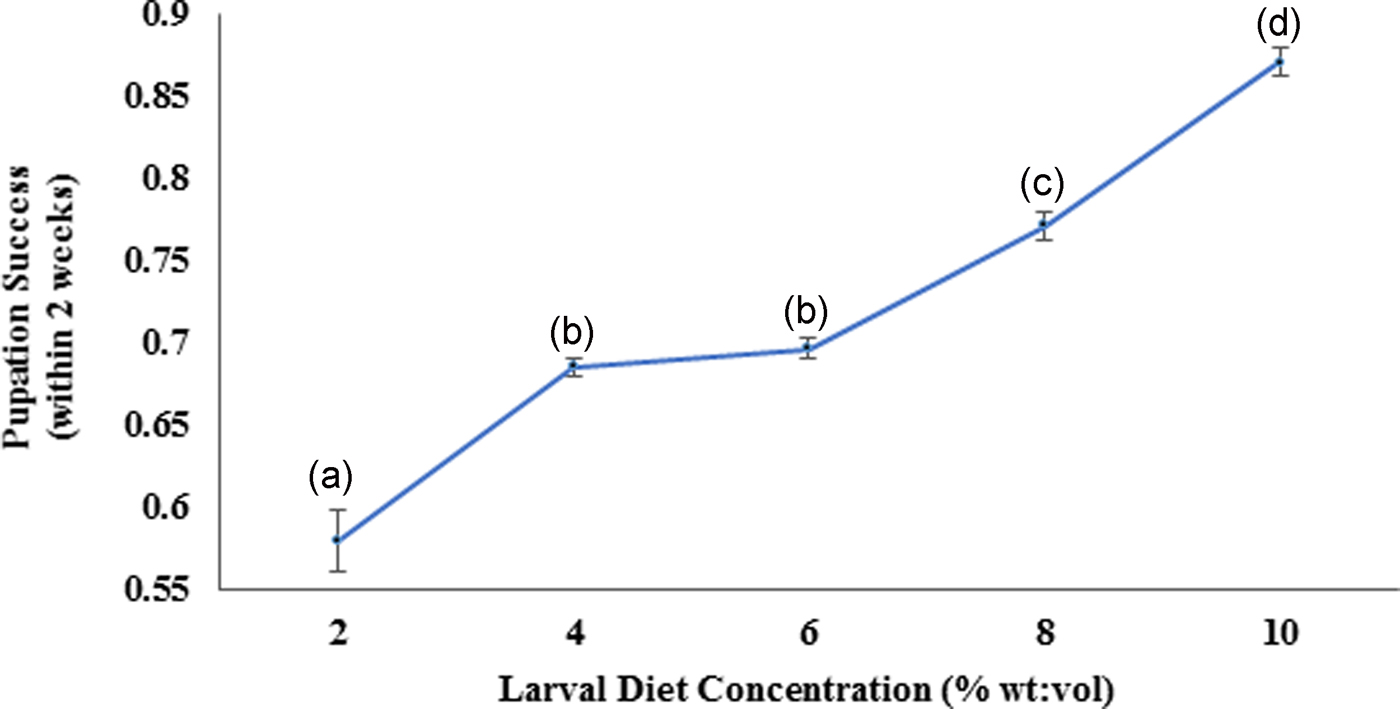
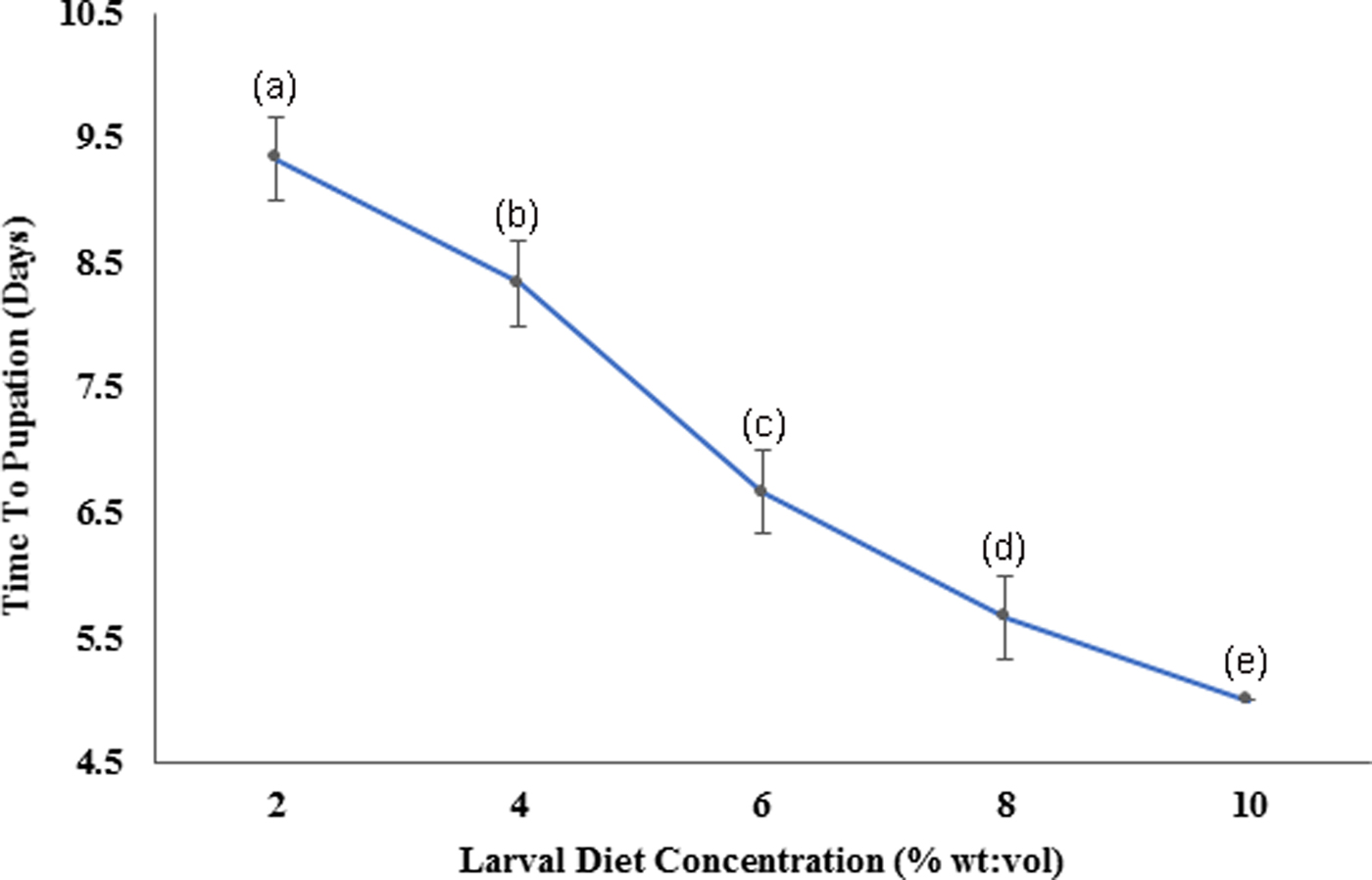
According to the Pearson correlation, strong significant positive correlations (P < 0.05; Pearson correlation coefficient [r] > 0.75) were observed among total body length, thoracic length, and abdominal width of larvae with the diet concentrations. Pupation success of larvae was observed to be characterized by a gradual increment with increasing diet concentration. The highest pupation rate (85–89%) within minimum duration (4.91–5.09 days) was observed at 10% larval diet concentration (fig. 8). The pupation rate of Ae. aegypti larvae was differed significantly (F4,24 = 103.539, P > 0.05 at 5% level of significance) with changing diet concentration, thereby the pupation rate decreased with elevating larval diet concentration (fig. 7, Annexure 1). Furthermore, a strong significant positive correlation (P < 0.05; r = 0.965) was observed among pupation rate with larval diet concentration. This was also confirmed by the GLM (F 4,24 = 36.875, P < 0.05) (Appendix 1).
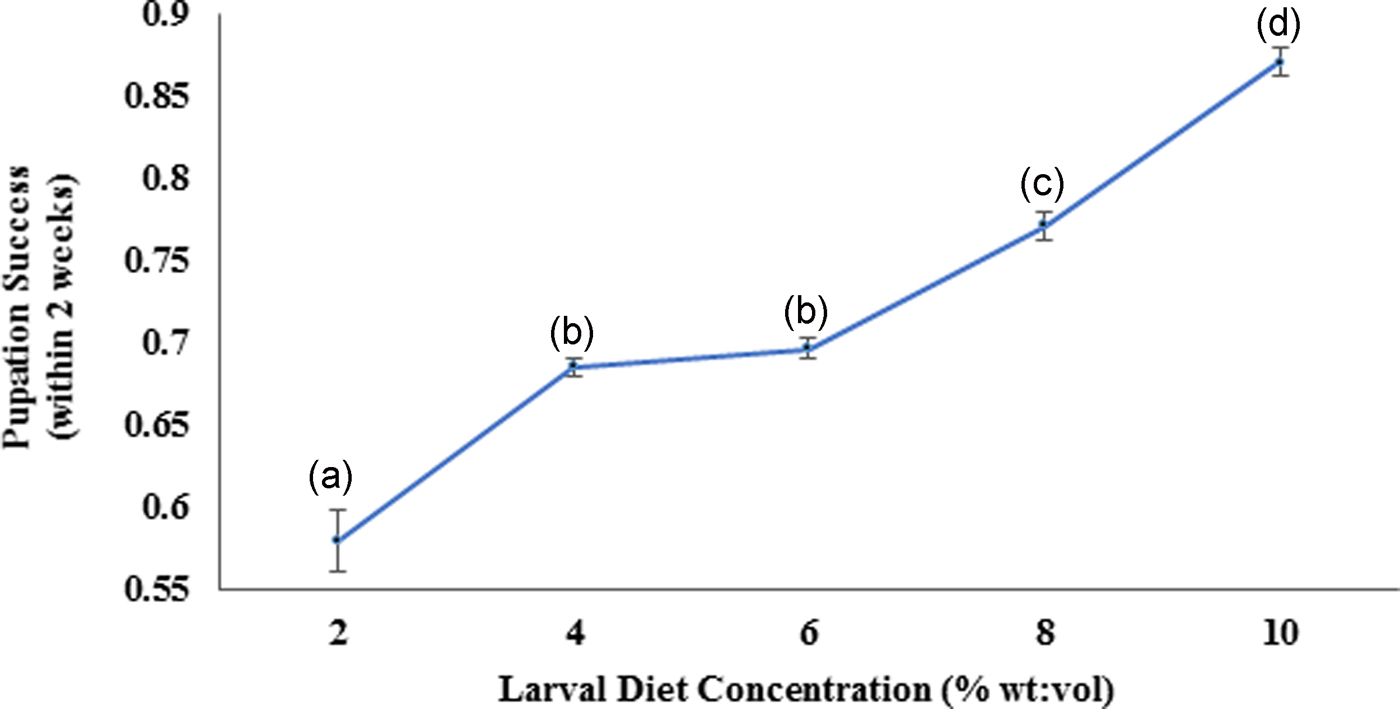
Fig. 7. Pupation success of the Aedes aegypti larvae fed with different diet concentrations.
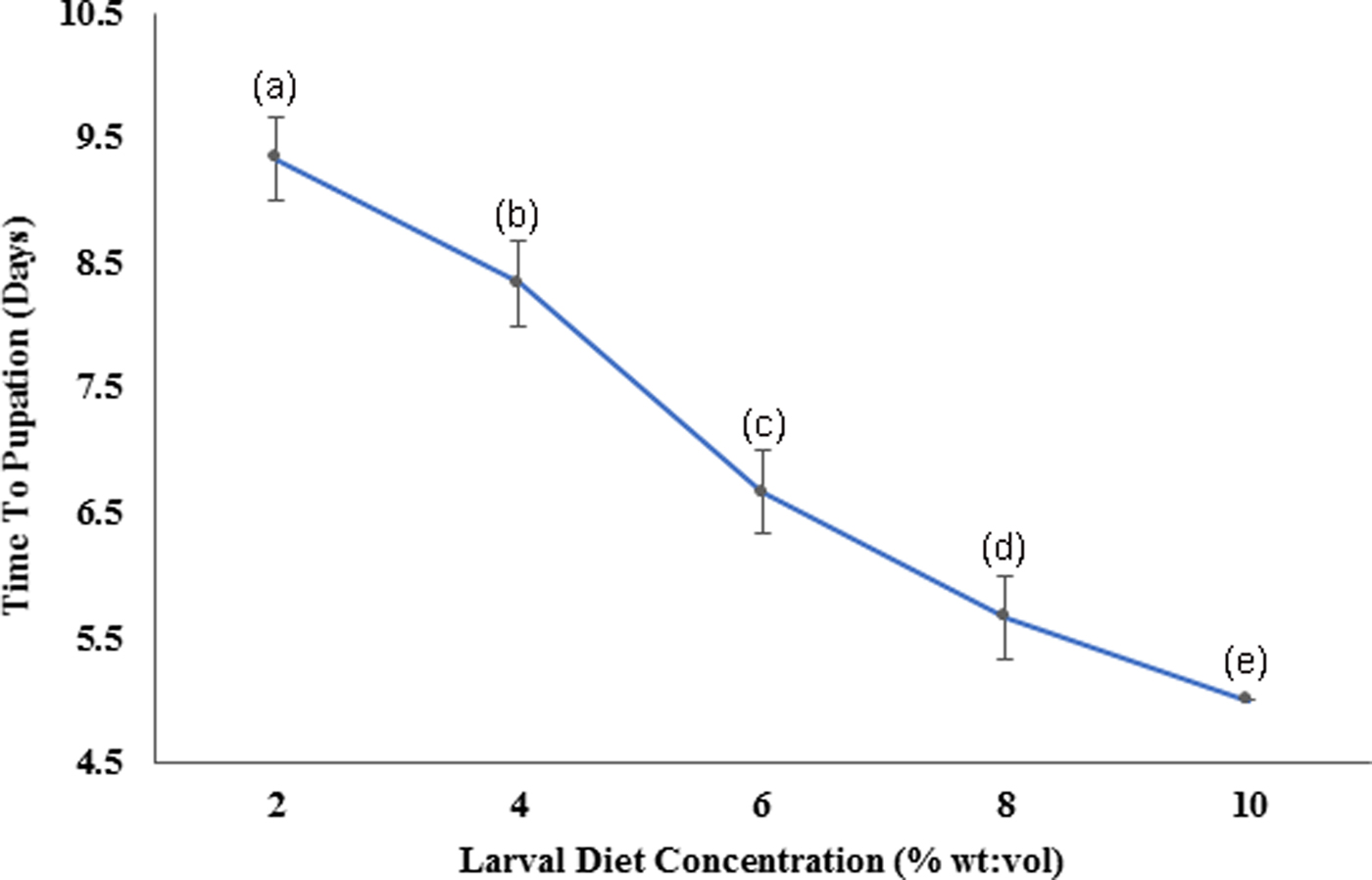
Fig. 8. Variation of the time required for pupation of Aedes aegypti larvae fed with different diet concentrations.
Pupal development parameters
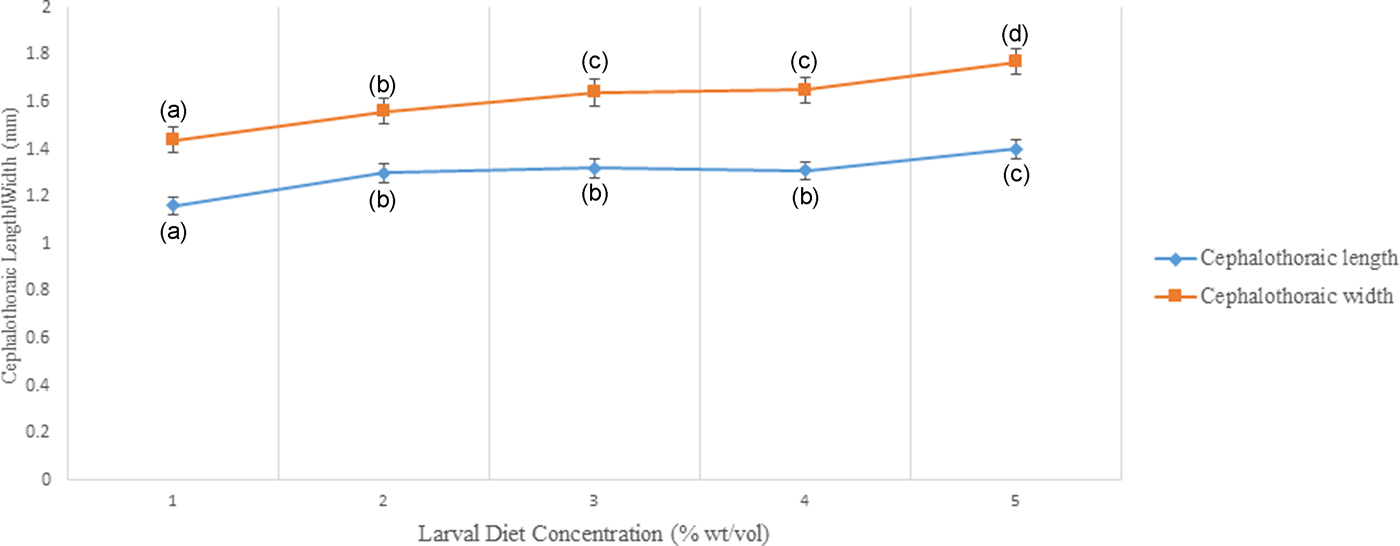
Cephalothoracic length (F 4,24 = 5.415) and width of pupae (F 4,24 = 6.390) differed significantly (P < 0.05) indicating an increasing trend with elevating larval diet concentrations (fig, 9, Annexure 2). Significant positive correlations were observed from the cephalothoracic length (P < 0.05; r = 0.750) and width (P < 0.05; r = 0.830) of pupae with diet concentration. The highest length and width of cephalothoraces were identified from the pupae emerging from the 10% larval diet treatment.
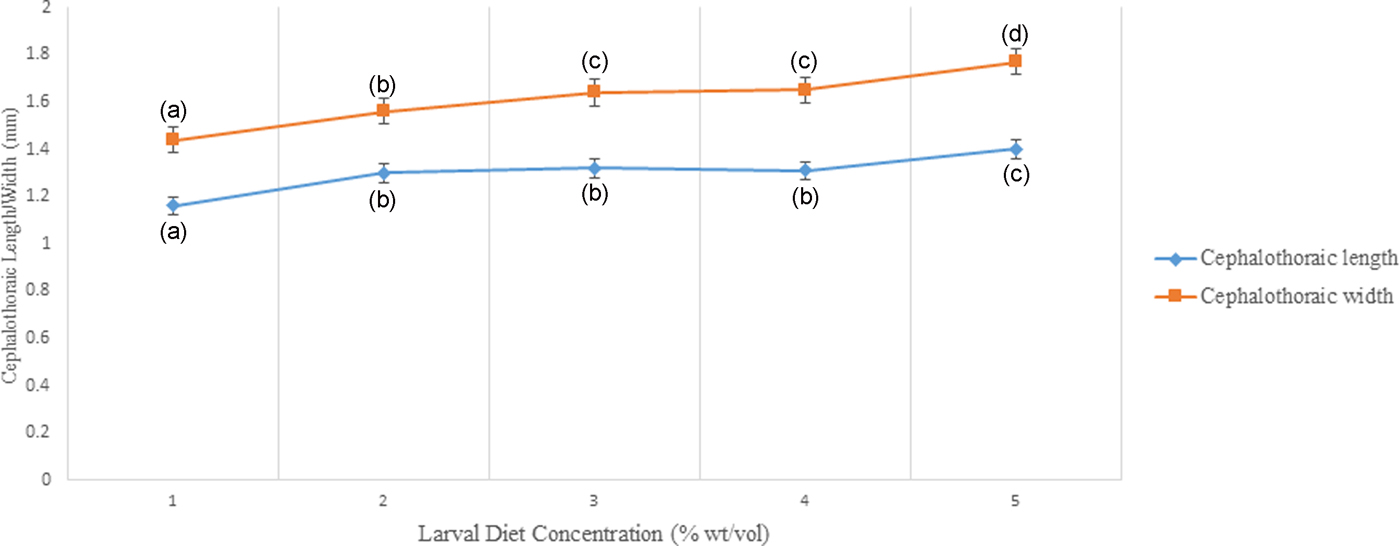
Fig. 9. Variation of Cephalothoraic length and width of Aedes aegypti treated at different diet concentrations.
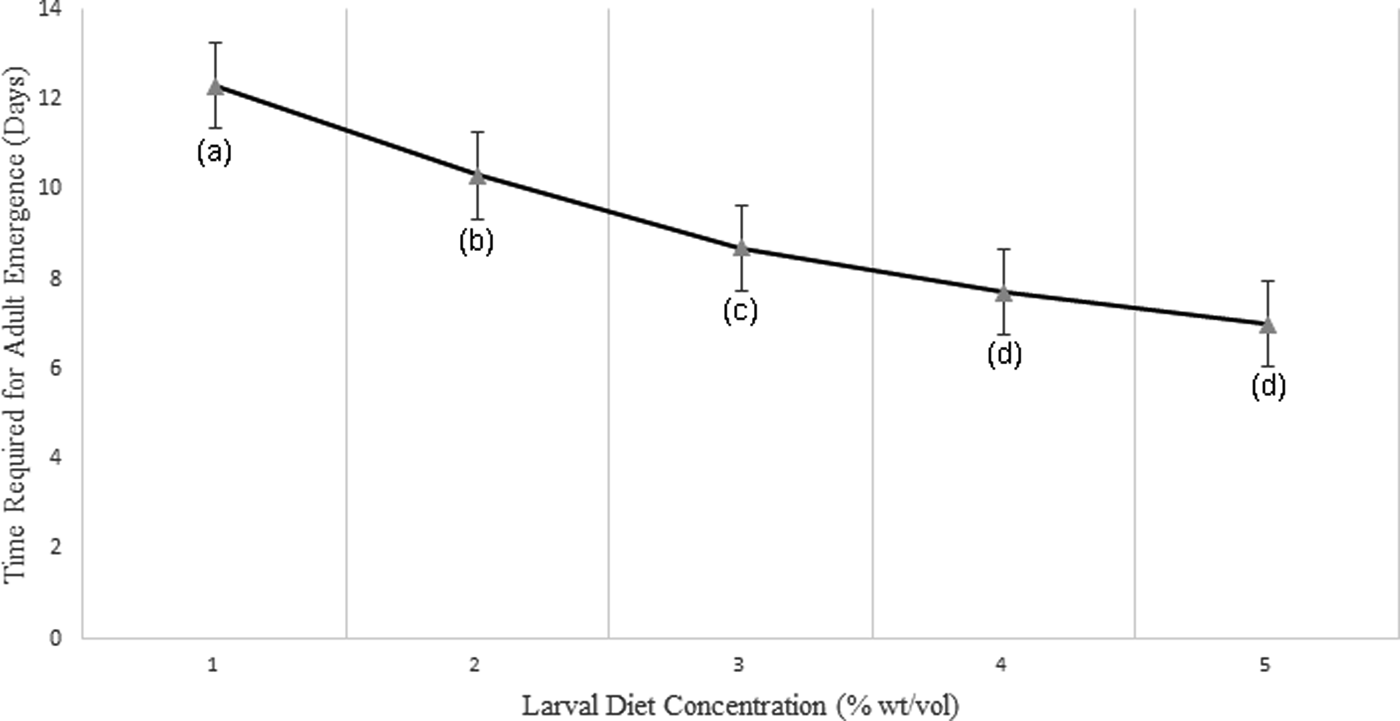
Adult success (the percentage of pupae emerged as adults) also increased significantly with increasing larval diet concentrations (F 4,24 = 52.250, P < 0.05). Larvae reared at the lowest diet treatment of 2% required a considerably prolonged time to emerge as adults when compared with larvae fed with high diet concentrations (8% and 10%). Further, a strong significant negative correlation (P < 0.05; r = −0.956) was observed between the adult success with diet concentration at 95% level of confidence (fig. 10).
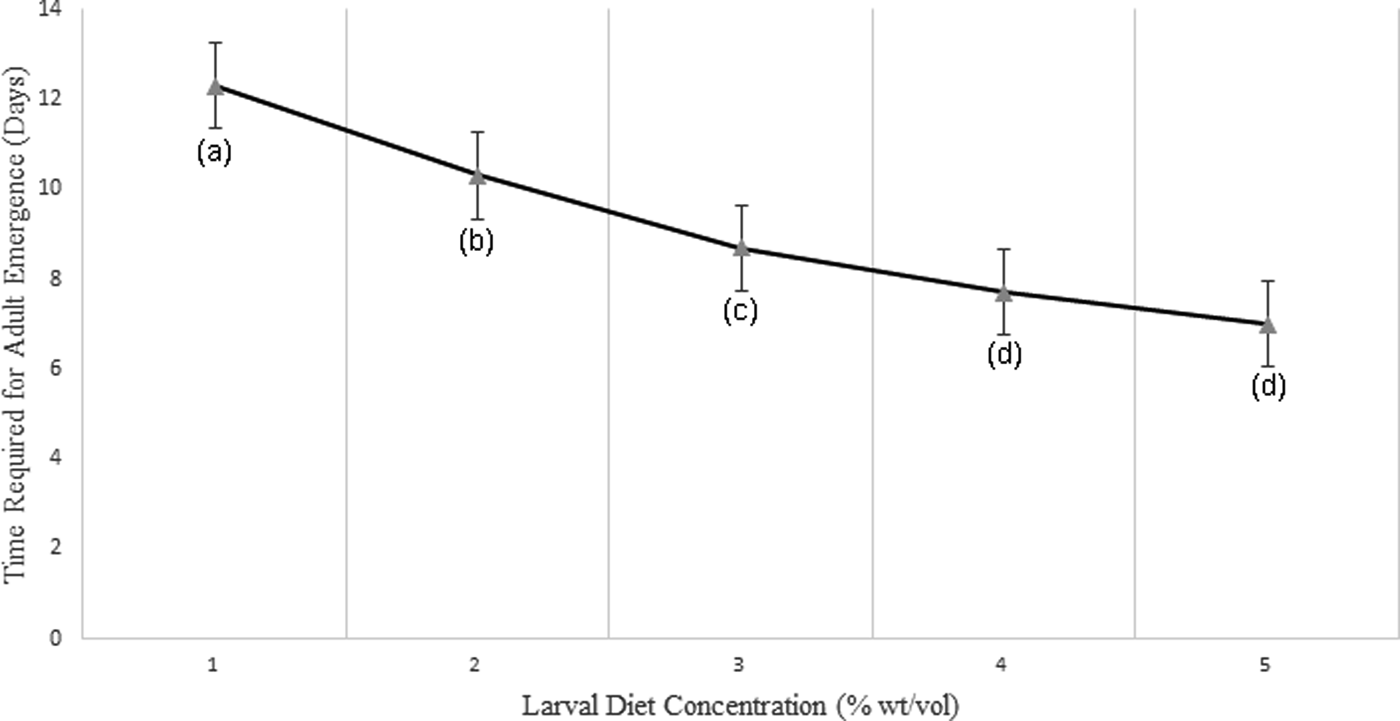
Fig. 10. Variation of time required for adult emergence (in days) of Aedes aegypti larvae treated at different diet concentrations.
Adult development parameters
It was found that the minimum and maximum sex ratios ranged 0.8–2, respectively. The overall sex ratio (male: female) of Ae. aegypti, varied significantly with different dietary concentrations (F 4,24 = 17.289; P < 0.05) at 5% level of significance. Relatively high proportions of males were identified from the larvae reared with low diet concentrations, while the tendency was shifted to females of the larvae treated with high diet concentrations (table 1). A strong significant negative relationship was observed among the sex ratio and increasing diet concentrations (P < 0.05; r = −0.846) at 95% level of confidence.
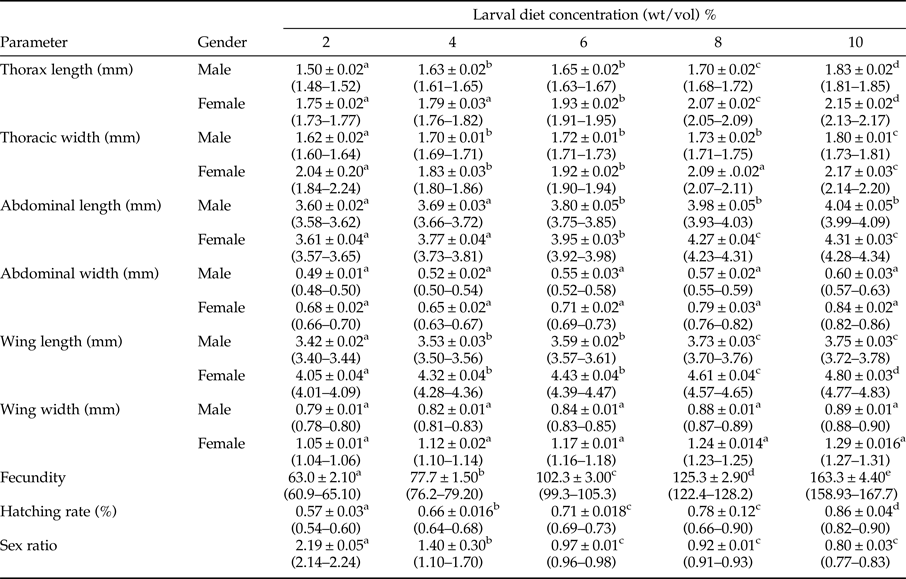
Table 1. Development parameters of adult Aedes aegypti (Mean ± SE) fed with different concentrations of IAEA larval diet.
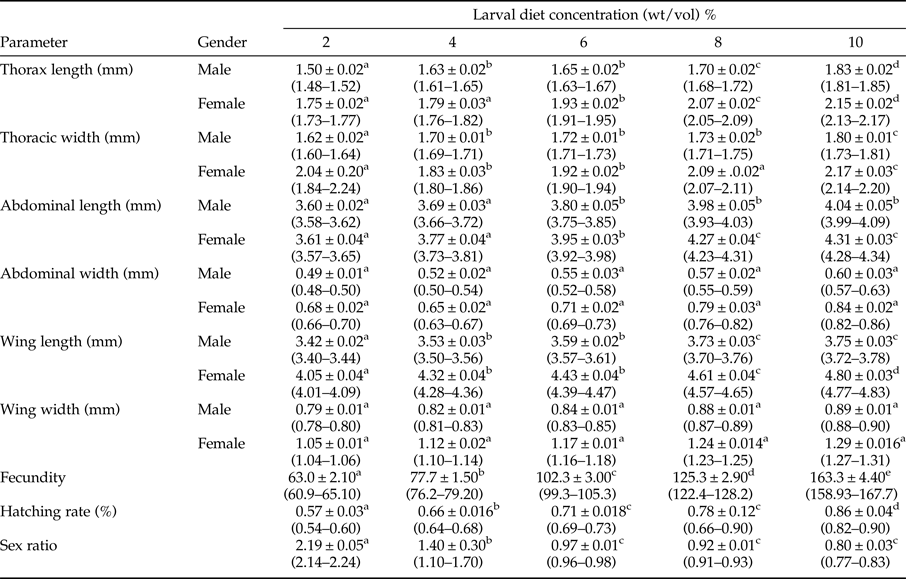
Note: Values are Mean ± Standard Error and range in parenthesis. Different superscript letters in a row denote significant differences (P < 0.05) in accordance with the General Linear Modeling followed by Tukey's pair-wise comparison test.
The mean values of thoracic length, thoracic width, abdominal length, and the wing length of adult Ae. aegypti mosquitoes also increased significantly with different dietary concentrations and gender of the mosquitoes (P < 0.05) at 5% level of significance. Surprisingly, significant increments in mean values of abdominal width and width of the wing were attributed only due to the effect of gender (P < 0.05), as the effect of larval diet concentration, was not significant (P > 0.05), in terms of GLM.
It was interesting to note that the effect of the interaction between diet concentration and gender on variations among the investigated growth parameters of adult Ae. aegypti mosquitoes were non-significant (P > 0.05) at 95% level of confidence (table 1). The highest growth parameters were shown by the female mosquitoes developed from the larvae treated with the highest diet concentrations.
Both fecundity (F 4,24 = 183.409) and hatching rate (F 4,24 = 43.507) of female Ae. aegypti mosquitoes elevated significantly (P < 0.05 at 5% degree of confidence) with increasing larval diet concentration, as indicated by the statistics of GLM. The highest fecundity (163.3 ± 4.4) and the hatching rate (0.82–0.90) were observed from the adults emerged from 10% diet concentration, while the opposite was observed at 2% diet level. Increasing diet concentration was significantly and positively correlated with both fecundity (r = 0.981) and hatching rate (r = 0.971) (Pearson, P < 0.95).
Discussion
The present experiment was conducted to determine the effect of different larval diet concentrations on several life-history traits of Ae. aegypti, attempting to define conditions for efficient large-scale rearing, which is a crucial step in the success of Sterile Insect Technology (SIT) applications (Carvalho et al., Reference Carvalho, Nimmo, Naish, McKemey, Gray, Wilke, Marrelli, Virginio, Alphey and Capurro2014; Puggioli et al., Reference Puggioli, Carrieri, Dindo, Medici, Lees, Gilles and Bellini2016). This approach may also facilitate the production of high-quality adults in terms of body size, flight ability, mating success, fecundity, fertility, and separation of sexes in mass rearing (Medici et al., Reference Medici, Carrieri, Scholte, Maccagnani, Dindo and Bellini2011; Puggioli et al., Reference Puggioli, Balestrino, Damiens, Lees, Soliban, Madakacherry, Dindo, Bellini and Gilles2013).
The length of rearing time is an important aspect to be considered for mass rearing, as it affects the production costs. The fastest development (reflected by shortest pupation time) was observed from the larvae treated with 10% diet, which was the highest concentration. Improvement of the survival rate due to the increasing food availability may be the potential reason for such elevated development rates in Ae. aegypti. However, excessive diet can diminish larval survival due to microorganisms involved in proliferating unconsumed food available in the medium (Gilles et al., Reference Gilles, Lees, Soliban and Benedict2011). Therefore, optimization of the larval diet dose is important in mass rearing.
Some studies have shown that, increasing larval density at the lower diet levels prolonged the development time, while at higher food levels the time to pupation was shorter (Gilles et al., Reference Gilles, Lees, Soliban and Benedict2011; Yoshioka et al., Reference Yoshioka, Couret, Kim, McMillan, Burkot, Dotson, Kitron and Vazquez-Prokopec2012). This was not tested in the present study since the larval density in all experiments was maintained constantly, as the main aim was to optimize the larval diet concentration.
In order to produce an optimal protocol for the standardized mass rearing of Ae. aegypti, several parameters were investigated at 24 h intervals after the beginning of pupation, because this is the period when the females best exploit protandry (Puggioli et al., Reference Puggioli, Carrieri, Dindo, Medici, Lees, Gilles and Bellini2016). The highest sex ratio (males: females) was observed at the lowest food treatment as noted in the previous studies. This male-biased sex ratio may suggest an effect of undernourishment but is favorable when selecting the males to be released in a SIT application (Chambers and Klowden, Reference Chambers and Klowden1990; Puggioli et al., Reference Puggioli, Balestrino, Damiens, Lees, Soliban, Madakacherry, Dindo, Bellini and Gilles2013; Balestrino et al., Reference Balestrino, Puggioli, Gilles and Bellini2014). The male pupae production rate at 24 h is a very important parameter, as it determines the number of males that can be selected for release as a proportion of the total males produced. Hence, variations in the survival to adult emergence rate due to lack of nutrients, is detrimental to the development of females, but not to that of males (Chambers and Klowden, Reference Chambers and Klowden1990).
It is convenient that intra-specific competition affects the larval survival and the inadequate quantity of food within a population may negatively affect the larval development as suggested by Gilles et al. (Reference Gilles, Lees, Soliban and Benedict2011). This also could be a reason for the decrease in the proportion of larval survival to pupation, under the minimal larval diet.
With the statistical analysis, time to pupation, and adult emergence varied significantly with larval diet concentration. However, Chambers and Klowden (Reference Chambers and Klowden1990), showed that the ability of larvae to pupate was greatly influenced by the nutritional reserves acquired during feeding. Therefore, larvae reared with higher food supply probably attain their critical weight earlier than those reared under lower diet concentration (Chambers and Klowden, Reference Chambers and Klowden1990), which is an important requirement for the pupation of mosquito larvae. Nevertheless, female larvae should achieve higher critical weight than male larvae (Chambers and Klowden, Reference Chambers and Klowden1990).
Female fecundity and fertility differed significantly with the larval diet concentration. The highest number of eggs laid per female was observed in the larval diet treatment with 10% diet concentration. The lowest number of eggs were laid by the females emerged from 2% treatment with a minimum amount of larval food. Similarly, eggs laid by females fed with elevated levels of food, hatched efficiently compared with the eggs laid by emerged females that were allowed to grow in minimum food levels. Both female fecundity and hatching rate strongly correlated with dietary level.
The fitness and health of colonized adult mosquitoes can be indirectly evaluated by measuring their body size (Petersen et al., Reference Petersen, Marchi, Natal, Marrelli, Barbosa and Suesdek2016). The total length and thoracic length were significantly associated with larval diet level, however, the effects on other size parameters (head length, width of head, thoracic width, abdominal length, and abdominal width) were not significant. Therefore, total length and thoracic length can be used as reliable larval measurements in order to predict the size of larvae.
In addition to larval growth parameters, cephalothoracic length and width of pupae varied significantly with larval diet level. According to Koenraadt (Reference Koenraadt2008), cephalothoracic length is the most reliable pupal parameter to predict the adult male body size (Medici et al., Reference Medici, Carrieri, Scholte, Maccagnani, Dindo and Bellini2011). Therefore, this study also highlights that the length of the cephalothorax can be used as an important parameter in predicting adult male body size.
The disparity of adult body sizes between larval diet levels was indicated from different measurements performed such as thoracic length, thoracic width, abdominal length, and abdominal width, length of wing and width of wing. Furthermore, significant positive correlations were found between these measures and the food quantity provided. Interestingly with the increase of food availability, the adult body size increased relative to length showing an allometric growth pattern. However, total body length is widely used as a proxy measure of size (Petersen et al., Reference Petersen, Marchi, Natal, Marrelli, Barbosa and Suesdek2016). In this case, both head length and thoracic width of larvae, also indicated similar growth patterns, suggesting an allometric growth pattern of Ae. aegypti, with the increased availability of food. Moreover, based on the findings it can be recommended that head length and thoracic width of larvae could be used as proxy measures of adult body size in Ae. aegypti. In case of adults, thoracic length, abdominal length, and wing length provided suitable proxy measures of adult body size.
The current study reveals that largest females produced at the highest food concentrations yielded the highest fecundity and fertility rates. According to the available literature, the body size of female mosquitoes affect the number of eggs laid and mating success (Araújo et al., Reference Araújo and Gil2012). Further, small adult size of male affects the reproduction, reducing spermatogenesis and testis size. Similarly, small females exhibit fewer ovarioles per ovary, which results in reduced fecundity (Medici et al., Reference Medici, Carrieri, Scholte, Maccagnani, Dindo and Bellini2011).
However, for SIT applications, mass rearing is performed to produce male individuals. Selecting optimal rearing conditions to increase the production of high-quality male mosquitoes while reducing the cost of production, is essential for the effectiveness and sustainability of SIT technologies. Therefore, selecting the ideal conditions to optimize the survival rate, a number of males, rearing schedule, the time required for male production in maximum quantity, without affecting the quality while considering economic costs, is important (Puggioli et al., Reference Puggioli, Carrieri, Dindo, Medici, Lees, Gilles and Bellini2016). Hence, the absolute values of the present study are likely to be useful for other research groups undertaking mass rearing for sterile insect technique approaches. Further, the growth metrics of male mosquitoes is also useful, as most of the studies focus on female mosquitoes.
Conclusion
The experiment results emphasize that best quality adults were produced at larval diet concentration of 10%, which provided the highest pupal survival, fastest development of larval instars and adults, highest fecundity, hatching rate and largest body size in adults. However, the 8% larval diet produced the highest male survival rate and male body size measurements that were more or less similar to the males produced at the 10% level. Therefore, we recommend the 8% diet level for the mass rearing of males for SIT applications. Since the quantity of these diets may affect the quality of sterilized Ae. aegypti, it is recommended to optimize the dietary composition at different artificial rearing conditions in order to obtain competitive sterile individuals to contest with the natural population for a productive SIT based vector control approach. Further, it is essential to conduct additional testing, including mating success, before a particular diet is recommended for such applications.
Ethics approval and consent to participate
Ethical clearance for the study was obtained from Ethical Review Committee (ERC) of the Faculty of Medicine, University of Kelaniya, Sri Lanka.
Consent for publication
Written consents were obtained from the study participants prior to the interviews for publication and all data collected was kept confidential.
Availability of data and material
The collected data collected will be kept confidential. Data will not be shared in any of the sources.
Competing interests
The authors have declared that they have no competing interests.
Funding
Laboratorial experiments were funded by the National Research Council Funded Dengue Mega Grant (NRC TO 14-04), Sri Lanka and International Atomic Energy Agency (CRP 170959).
Authors’ contributions
PADHNG- Designing the research, laboratory analysis, supervision of the research work, writing and reviewing the manuscript; UMHUU– Conducting the laboratory analysis; NWBALU- Assisting laboratory analysis, statistical analysis and writing the manuscript; RMTBR – Assisting laboratory analysis and reviewing the manuscript; LDA - Supervision of laboratory analysis and reviewing the manuscript; WA- Overall supervision and reviewing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Financial support by the National Research Council Funded Dengue Mega Grant (NRC TO 14-04), Sri Lanka and International Atomic Energy Agency (CRP 170959). Head and all staff of the Molecular Medicine Unit, Faculty of Medicine, University of Kelaniya, Sri Lanka for facilitating the research work.