Introduction
Lucilia sericata is an insect belonging to the order Diptera, family Calliphoridae. The larvae of these flies have been used in modern medicine – maggot therapy. They consume dead tissue, cleaning and healing wounds in the process. In Chinese medicine, this method has been used since the beginnings of that civilization, L. sericata larvae have been given the name ‘WuGuChong’. The larvae were presumably used for the superficial treatment of purulent diseases, such as boils or carbuncles. In the 1930s, the surgeon William Baer made observations on the healing and successful treatment of children with severe osteomyelitis by colonizing the wounds with L. sericata. It was a breakthrough in European medicine, but, shortly after this event, penicillin was discovered, and maggot therapy was forgotten about. However, in the 20th century, interest in this therapy was renewed as an increasing number of bacteria species gained resistance to antibiotics (Cazander et al., Reference Cazander, van Veen, Bernards and Jukema2009). Maggot therapy turned out to be effective in the treatment of venous ulcers, diabetic foot and leg ulcers (Zhang et al., Reference Zhang, Wang, Diao, Zhang and Lv2010). This method of treatment is now used in more than 30 countries. Over the past 20 years, some 60,000 patients have undergone this therapy (Mumcuoglu & Özkan, 2009).
Extracts of insects contain saturated and unsaturated hydrocarbons, mainly n-alkanes, carboxylic acids, aldehydes and esters. Fatty acid extracts from dried L. sericata larvae promote wound healing in that the healing time is considerably shortened, probably because of their associated powerful angiogenic properties. The extracts caused no irritation or pain. It is known that fatty acid extracts respond to the vascular endothelial growth factor (VEGF) involved in the formation of a network blood vessels in the embryo and angiogenesis, which are necessary in the inflammatory phase, as well as shrinkage of wounds, scarring, granulation during remoulding and the proliferation of capillary cells (Zhang et al., Reference Zhang, Wang, Diao, Zhang and Lv2010). The investigated substances found in excretions/secretions (E/S) of L. sericata suppressed methicyllin-resistant Staphylococcus aureus (MRSA) and eliminated other bacteria in infected wounds. E/S contain a lot of alkaline compounds like ammonium carbonate, allantoin and urea, which inhibit the growth of bacteria. Infection elicits the synthesis of antimicrobial peptides that kill not only gram-negative and gram-positive bacteria but also fungi. Lucifensin, which can be found in the salivary glands, adipose tissue and haemolymph, is one of the antiseptic agents in E/S of L. sericata (Cerovský et al., Reference Cerovský, Zdárek, Fucík, Monincová, Voburka and Bém2010).
Hydrocarbons play a variety of functions: they are specific to species, sex and, in the case of social insects, colony and caste (Singer, Reference Singer1998). In insect colonies, tri-, tetra-, penta- and hexacosanes occur in large quantities in the reproductive queen, and these are noticeable among the non-productive workers. This variation accents their place in the swarm (Smith et al., Reference Smith, Holldober and Liebig2009). The primary role of cuticular hydrocarbons is to protect insects from desiccation. Methyl branching lowers their melting or transition temperature. The arrangement of the methyl groups, as well as their number, is relevant in protection against excessive water loss. It appears that 2-methylpentacosane reduces their melting point by 10°C and 11-methylpentacosane by 30°C (Gibbs & Pomonis, Reference Gibbs and Pomonis1995). The more methyl branches, the more significant the lowering of the melting point (Gibbs & Pomonis, Reference Gibbs and Pomonis1995; Nelson & Lee, Reference Nelson and Lee2004).
A correlation was noted between the temperature and the length of the n-alkane chains. n-alkanes with longer chains have higher boiling points, which is related to lower volatility and therefore the better adaptation of insects to warmer climates (Gibbs & Pomonis, Reference Gibbs and Pomonis1995). In certain species, resistance to water loss can increase sixfold during the winter, while at the same time the amount of hydrocarbons increases up to 40 times (mostly branched methylated alkanes), the higher the temperature the greater the permeability. It was noticed by Davies (Reference Davies1948) that the permeability of the eggs of L. sericata increased when the temperature was raised to 38°C.
This paper describes the cuticular and internal lipid composition of L. sericata. The lipids of flies were separated into classes of compounds using high performance liquid chromatography. Qualitative and quantitative analyses were done by gas chromatography combined with mass spectrometry (GC/MS) in selected ion monitoring (SIM) and total ion current (TIC) modes.
Methods and material
Insects
Lucilia sericata raised from eggs laid on fresh beef by adult flies were reared at 25°C with 50% relative humidity and a 12:12 h photoperiod. Maternal generation was maintained in the same conditions. The insects were fed on beef, and it took them approximately seven days from hatching to puparium formation and another seven days to adults appearance. For the experiments, third-instar (15–20 mm long), not feeding larvae before wandering; pupae, 10–24 h after pupation, as well as male and female adults, 1–3 days old, were used. Adult flies narcotized with CO2 were sexed under a stereo-microscope. All insects were quickly frozen and kept at −20°C until used.
Extraction of lipids
The cuticular and internal lipids were extracted by methods described previously (Gołębiowski et al., Reference Gołębiowski, Maliński, Boguś, Kumirska and Stepnowski2008a). All samples were extracted by immersing larvae, pupae, and male and female imagines separately in petroleum ether for 10 s (extracts I). The insects were then transferred to dichloromethane for 5 min (extracts II). Finally, the insects were extracted with dichloromethane for ten days (extracts III). Extracts I and II contained cuticular lipids, and extracts III contained internal lipids of the insects.
High performance liquid chromatography with laser light scattering detector (HPLC- LLSD)
The lipids were separated by methods described previously (Gołębiowski et al., Reference Gołębiowski, Boguś, Paszkiewicz and Stepnowski2010). All lipid extracts (larvae, pupae, male and female imagines) were separated using high performance liquid chromatography (HPLC) with a laser light scattering detector (LLSD). Separation was performed on a silica gel column (Econosil Silica 5 Micron, Alltech, 25 cm×4.6 mm id). Binary gradient elution with eluent A (hexane) and eluent B (15% of acetone in dichloromethane) was applied with a linear gradient from A to B within 30 min. Total flow was maintained at 0.8 ml min−1. The hydrocarbon fractions obtained were analysed by GC/MS.
Gas chromatography-mass spectrometry
The cuticular and internal hydrocarbons were analysed by GC/MS (SSQ 710 - Finnigan Mat). The mass spectrometer was used in EI mode (70 eV) and set to scan the 40–700 amu mass range at a rate of one scan per second. The samples were introduced through a Hewlett-Packard 5890 gas chromatograph equipped with a 30 m×0.25 mm id, Rtx-5 silica capillary column and a 0.25 μm thick film. The column temperature was programmed at 4°C min−1 from 80°C (held eight minutes) to 320°C (held ten minutes). The transfer line and injector temperatures were maintained at 320°C. Helium was the carrier gas at a constant flow of 2 ml min−1. The ion source was kept at 200°C.
The analysis of n-alkanes was carried out by GC/MS in selected ion monitoring (SIM) and total ion current (TIC) modes.
Statistical analysis
In order to quantitatively determine each of the n-alkanes analyzed, GC analysis was performed with an internal standard (n-decane). The content of the compounds in the analyzed samples were calculated from the chromatogram peak areas. Results were expressed as means±standard deviation of three GC analyses.
Results
Total lipids and n-alkanes
The lipid extracts were separated by HPLC-LLSD. The n-alkane fraction was obtained after four minutes, then analysed by GC/MS. N-alkanes in the cuticular and internal lipids of L. sericata were identified on the basis of the characteristic ions: 43, 57, 71, 85, 99 and M+. (molecular ion). Additionally, for qualitative purposes, the instrument was operated in SIM mode, monitoring the fragment ion m/z=85 and monitoring the molecular ions.
Qualitative analysis revealed the presence of n-alkanes with 12 to 31 carbon atoms in the chain, depending on sex, developmental stage and type of extraction. The largest amounts of lipids were present in larvae, 7% more than hydrocarbons in adult males. The smallest amounts were found in adult females (30% less than in larvae). In all developmental stages, the largest amounts of lipids were obtained during the longest extraction. The first extraction yielded large amounts of hydrocarbons in male and female imagines. Larvae and pupae had ca. 96–97% fewer surface lipids than adult males. Irrespective of stage, the second fraction derived from the shorter extraction with dichloromethane was the least effective. Figure 1 shows the masses of the cuticular and internal lipids.

Fig. 1. The masses of cuticular and internal lipids. The total lipid contents ranged from 21.7 (female) to 31.0 (larvae) mg g−1 of the insect body.
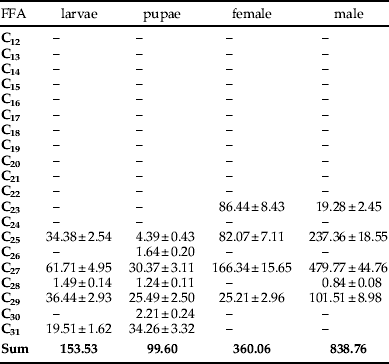
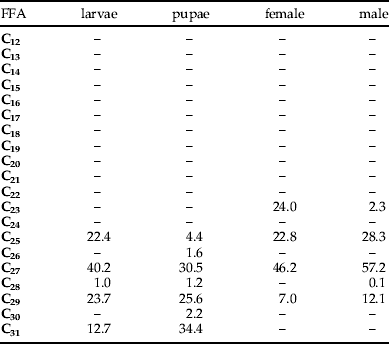
The amount of cuticular n-alkanes isolated from adult males was 330.84 μg g−1. This last quantity is about twice the average in adult females (158.93 μg g−1); whereas, in larvae, quantities were significantly lower: 31.46 μg g−1 were extracted from larvae and 42.08 μg g−1 from pupae. The total mass of lipids is the greatest in larvae, but the content of n-alkanes in the total mass of larvae lipids is about ten times smaller than in males. The respective internal n-alkane contents of pupae, larvae, adult females and males were significantly higher than the cuticular n-alkanes: 99.60, 153.53, 360.06 and 838.76 μg g−1 of the insect body.
Cuticular n-alkanes in pupae and larvae
Larvae contained n-alkanes with 23–31 carbon atoms except for chains with 24 and 30 carbons (table 1). Pupae contained significantly more compounds with 14–31 carbons than larvae (table 2). C23 and C26 in larvae were present in trace amounts, whereas C24 and C30 were not detected in any extracts. There was a greater diversity of n-alkanes in the pupae, but many of them (C15, C17, C18, C20, C21, C22, C23, C24) were present only in trace quantities. The most distinctive n-alkane turned out to be C29 (59.5%): in larvae this made up more than 41% regardless of the type of extraction, whereas in pupae its content was >51%. Another n-alkane occurring in large amounts was C27: from larvae, 12.8% in the first fraction and 24.5% in the second. The situation was similar in pupae: 11.9% in the first extraction and 26.3% in the second. C25 (8.5%) and C31 (30.4%) were the n-alkanes that occurred in larval cuticular lipids in large amounts. These compounds constituted 2.1% and 15.4%, respectively, in the pupal cuticular lipids.
Table 1. The composition of cuticular n-alkanes found in larvae of Lucilia sericata.

Table 2. The composition of cuticular n-alkanes found in pupae of Lucilia sericata.

The majority of n-alkanes present turned out to have odd numbers of carbon atoms in their chains. All the n-alkanes in the first larval extractions were odd-numbered, even those present in trace amounts. In the second extractions, however, 98% of the n-alkanes were odd-numbered and 1.6% even-numbered. Larger amounts of n-alkanes were recorded in pupae: 6.6% of even- and 93.2% of odd-numbered n-alkanes in the first extraction, and 3.7% of even- and 96.2% of odd numbered n-alkanes in the second.
Cuticular n-alkanes in L. sericata imagines
Qualitative and quantitative analysis of the cuticular lipids from females showed that n-alkanes contained from 12 to 30 carbon atoms in the chain (but no C18 and C15 were present (table 3). The situation with regard to males was similar but, in addition, C30 and C12 were not detected. C31 was identified only in males in the second extract. The second extract isolated from females consisted of n-alkanes with 12 to 29 carbon atoms, but not C13, C15 or C18. Coincidentally, the results obtained from males were very similar, as C14, C17, C19–C29 and C31 were isolated (table 4). The most abundant n-alkanes detected in the cuticular extracts of males were C21 (2.0%), C23 (16.2%), C25 (26.2%), C27 (47.9%) and C29 (3.4%). The same applied to females; the most abundant n-alkanes in females were C23 (15.7%), C25 (27.7%) and C27 (47.5%). Other cuticular n-alkanes in females were present in much smaller quantities (<2% or traces). C23, C25 and C27 together made up 90.3% of the n-alkanes present in males and 90.9% of those in females. The results showed that imagines of L. sericata, like its other developmental stages, contained significantly larger quantities of odd-numbered n-alkanes. Figure 2 shows the most abundant n-alkanes of adult males and females.

Fig. 2. The composition of male and female n-alkanes occurring in large quantities. The lipid extracts included the major n-alkanes from C22 to C29 (μg g−1 of the insect body). The standard deviation is indicated on the bar. ▪ I extraction female ![]() I extraction male
I extraction male ![]() II extraction female
II extraction female ![]() II extraction male.
II extraction male.
Table 3. The composition of cuticular n-alkanes found in female of Lucilia sericata.

Table 4. The composition of cuticular n-alkanes found in male of Lucilia sericata.

Internal n-alkanes in L. sericata
Qualitative analysis of the internal compounds present in L. sericata showed little diversity of n-alkanes. Only odd-numbered compounds were found in females. In adult males and larvae, C28 was also present in very small quantities (0.84 and 1.49 μg g−1 of the insect body, respectively) (table 5). Three even-numbered n-alkanes were present in the internal lipids of pupae. C26, C28 and C30 together made up 5.0% of the n-alkanes present in pupae. Even-numbered n-alkanes were present in amounts from 0.84 to 2.21 μg g−1 of the insect body.
Table 5. The composition of internal n-alkanes found in Lucilia sericata.
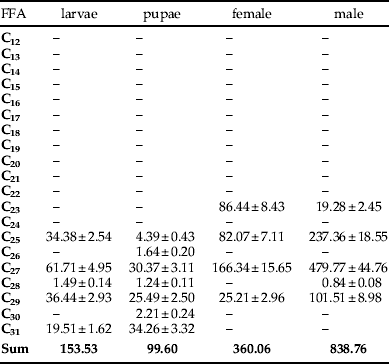
The internal lipids of larvae, pupae, and adult female and male L. sericata contained four odd-numbered n-alkanes (table 6). Heptacosane (C27) was the compound occurring in the largest quantities in all developmental stages. The respective contents of C27 in the internal lipids of the larvae, pupae, adult females and males were 61.71, 30.37, 166.34 and 479.77 μg g−1 of the insect body. Other n-alkanes occurring in high concentrations were C29 in larvae and pupae (23.7% and 26.6%, respectively), C23 in females (24.0%) and C29 in males (12.1%). The n-alkane C23 occurred only in lipids of females and males (24.0% and 2.3%, respectively). However, C31 n-alkane occurred only in the lipids of larvae and pupae (12.7% and 34.4%). The n-alkanes C30 and C26 occurred only in the pupal lipids (1.2%).
Table 6. The composition of internal n-alkanes found in Lucilia sericata.
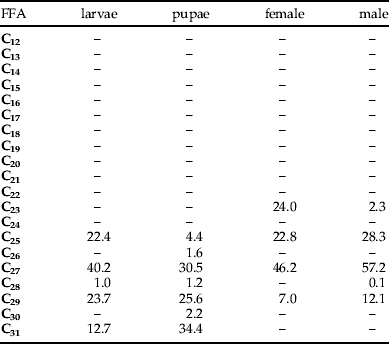
The total n-alkane content in the internal lipids of the larvae, pupae, adult females and males were 153.53, 99.60, 360.06 and 838.76 μg g−1 of the insect body, respectively.
Discussion
The use of short extraction during the study helped in the analysis of the surface hydrocarbons. The first solvent, a non-polar one, was used to isolate non-polar compounds, while more polar lipids were eluted with the second solvent. The third and longest extraction was used for the analysis of internal compounds and yielded the largest amounts of lipids. The majority of n-alkanes were obtained during the third extraction, which is the result of the appropriate organic solvent and the long duration of the process. During the third extraction, all compounds were eluted. The standard methods of analysis for these lipid fractions were GC and GC/MS (Chapman et al., Reference Chapman, Espelie and Peck2000; Gołębiowski et al., Reference Gołębiowski, Boguś, Paszkiewicz and Stepnowski2011). 2D is sometimes used to analyse co-eluted components. This is a method usually used to test for pheromones, as they are produced in tiny amounts (Kalinova et al., Reference Kalinova, Jiros, Zdarek, Wen and Hoskovec2006). In our analysis of n-alkanes, however, this method was not applied, as it was not found necessary. During extraction from the insects, various compounds, such as fatty acids, n-alkanes, esters and sterols, were isolated. The application of HPLC-LLSD enabled n-alkanes to be separated from other compounds (Gołębiowski et al., Reference Gołębiowski, Maliński, Nawrot and Stepnowski2008b). The hydrocarbon fraction was further analysed by GC/MS. Selective ion monitoring (SIM) was applied to achieve high selectivity and relatively good sensitivity.
Comparison of the results of the analysis performed on the different developmental stages of L. sericata showed that males contained the greatest amounts of cuticular n-alkanes, followed by females, pupae and larvae. Similar results were obtained with the Colorado potato beetle (Leptinotarsa decemlineata) (Nelson et al., Reference Nelson, Adams and Fatland2003), but the n-alkanes constituted a much smaller percentage of the isolated compounds. A profile similar to that in L. sericata larvae was also detected in Stenocara gracilipes (Lokey, Reference Lokey1988). The cuticular lipids of S. gracilipes adults contained eight n-alkanes from C23 to C30. Different profiles were identified in Zygogramma exclamationis, where more of the n-alkanes were found in larvae, than in male and female imagines (Nelson & Charlet, Reference Nelson and Charlet2003). The cuticular lipids of Z. exclamationis larvae contained nine n-alkanes, ranging from C25 to C33. Additionally, the males and females contained C24 and C25. The most abundant n-alkane in the female was C27, whereas the major n-alkane of the pupae was C31. The situation was the same in the case of L. sericata females and pupae. In larval and adult Frankliniella occidentalis, similar n-alkanes were identified but in different quantities (Gołębiowski et al., Reference Gołębiowski, Maliński, Nawrot, Szafranek and Stepnowski2007). The cuticular lipids of larval and adult F. occidentalis contained n-alkanes from C25 to C28 and from C25 to C28, respectively. The major cuticular hydrocarbon in both stages was the n-alkane C27. Nevertheless, different compounds were found in Anagasta kuehniella larvae (Hebanowska et al., Reference Hebanowska, Maliński, Dubis, Oksman, Pihlaja, Nawrot and Szafranek1990). In this case, the n-alkanes ranged from C16 to C31. In our studies, the larvae contained n-alkanes from C23 to C31, but the lipids of males, females and pupae contained similar n-alkane mixtures: from C13 to C31, C12–C30 and C14–C31, respectively. In A. kuehniella n-alkane mixtures, C23, C25 and C27 predominate. Only three n-alkanes (C27, C28 and C29) were identified in the cuticular lipids of adult Blatella germanica (Augustynowicz et al., Reference Augustynowicz, Maliński, Warnke, Szafranek and Nawrot1987). However, twelve compounds from C21 to C35 were found in adults of Aphthona lacertosa and A. nigriscutis (Nelson et al., Reference Nelson, Olson and Fatland2002). The presence of a large number of these compounds may be explained by the diet of the insect (Nikolova et al., Reference Nikolova, Rezanka, Nikolova-Damyanova and Kalushkov1999). In both insect species (A. lacertosa and A. nigriscutis), just as in our results (larvae and pupae), nonacosane was the main compound. Large amounts of n-nonacosane were also found on the vitelline membrane surface of the eggs of Cochliomyia hominivorax, Cochliomyia macellaria, Musca domestica, Phaenicia sericata, Lucilia cuprina and Anastrepha ludens (Nelson & Leopold, Reference Nelson and Leopold2003). Moreover, in eggs, n-alkanes have up to 31 carbon atoms in the chain. A greater number of carbon atoms is associated with a higher melting point. The eggs are more vulnerable to dessication, so n-alkanes with longer chains provide greater protection (Gibbs, Reference Gibbs2002). Studies conducted on beetles and the wheat plants they inhabit indicate equally large amounts of C27, C29 and C31. In our studies, the dominance of n-alkanes containing 27, 29 and 31 carbon atoms in the chain was well marked.
The composition of cuticular n-alkanes is depended on their biosynthesis. Cuticular n-alkanes may be synthesised de novo from acetate (Blomquist et al., Reference Blomquist, Chu, Nelson and Pomonis1980) and also can be received during feeding (Blomquist & Jackson, Reference Blomquist and Jackson1973), so their presence on the surface of insects depends on the accessibility in food. Insects synthesise n-alkanes by the decarboxylation of long-chain fatty acids (Lokey, Reference Lokey1988). The result of the decarboxylation is n-alkane with one less carbon atom. In the termite, Zootermopsis angusticollis (Chu & Blomquist, Reference Chu and Blomquist1980), for example, tetracosanoic acid is decarboxylated to n-tricosane. Most cuticular fatty acids of L. sericata are even-numbered entities (M. Gołębiowski et al., unpublished data), so after decarboxylation n-alkanes with odd-numbered carbon chains are obtained.
More recently, bioassays have shown that surface hydrocarbons are important tools for recognition systems for insects. Cuticular hydrocarbons serve as contact pheromones when insects encounter each other. They permit insects to identify friends from foes. The existence of such resources could signify an attempt to protect the insect against predators (Nawrot et al., Reference Nawrot, Gawlak, Szafranek, Szafranek, Synak, Warchalewski, Piasecka-Kwiatkowska, Błaszczak, Jeliński and Fornal2010). They constitute a true chemical signature. For example, it was found that both n-alkanes and (Z)-9-alkenes with odd numbers of carbons from 25 to 33 are necessary to discriminate nest mates from foreign conspecifics in Formica japonica (Akino et al., Reference Akino, Yamamura, Wakamura and Yamaoka2004). Moreover, to distinguish Polistes gallicus queens from workers, the hydrocarbon composition of the Van der Vecht organ (mainly linear and monomethyl-branched alkanes with odd-numbered carbon chains) was used (Dapporto et al., Reference Dapporto, Santini, Dani and Turillazi2007).
Acknowledgements
Financial support was provided by the Polish Ministry of Research and Higher Education for 2010–2013 grant: N N303 504238 and by grant: DS 8200-4-0085-1 from the University of Gdansk.