Introduction
Food quality concerns (Leifert et al., Reference Leifert, Rembialkowska, Nielson, Cooper, Butler, Lueck, Niggli, Leifert, Alföldi, Lück and Willer2007) has led to a considerable increase in the amount of organically-managed farmland in the UK and Europe. The inability to use inorganic fertiliser and crop protection sprays in organic systems increases the requirement to optimise sustainable approaches to both crop production and pest control. Maximising beneficial invertebrate predator and parasite activity is a priority in a farming system using no artificial pesticides (Gurr et al., Reference Gurr, Wratten and Luna2003). Carabidae (ground beetles) are a major group of mainly predatory natural enemies, with Shah et al. (Reference Shah, Brooks, Ashby, Perry and Woiwod2003) reporting more activity in organic than in conventional cereals. However, Armstrong (Reference Armstrong1995) and Purtauf et al. (Reference Purtauf, Roschewitz, Dauber, Thies, Tscharntke and Wolters2005) found no difference in activity caused by management differences in cabbage and wheat. Staphylinidae (rove beetles) appear to be more active in conventional fields (Andersen & Eltun, Reference Andersen and Eltun2000), and more spider activity was reported in organic than in conventional wheat (Feber et al., Reference Feber, Bell, Johnson, Firbank and Macdonald1998). Differences between grassland and arable crops had more effect on Carabidae activity than management system (Weibull & Östman, Reference Weibull and Östman2003), and landscape features have been shown to affect the activity of spiders and parasitic Hymenoptera in organic crops (Schmidt et al., Reference Schmidt, Roschewitz, Thies and Tscharntke2005; Thies et al., Reference Thies, Roschewitz and Tscharntke2005). Research has been concentrated on cereal crops, especially wheat, but Berry et al. (Reference Berry, Wratten, McErlich and Frampton1996) found more Hymenoptera, Staphylinidae and Neuroptera in organic than in conventional carrot crops. Bengtsson et al. (Reference Bengtsson, Ahnstrom and Weibull2005) concluded that, in general, predatory insects react positively to organic farming; but the effects of change from conventional to organic management on beneficial invertebrates has proved to be inconclusive and has been, in some cases, contradictory (Hole et al., Reference Hole, Perkins, Wilson, Alexander, Grice and Evans2005).
The conversion of half of Nafferton Farm in northern England to organic farming provided an opportunity to compare beneficial invertebrate activity on two halves of the same farm with contrasting management and crops. Previous work with invertebrates, comparing the effects of management systems, has tended to be limited to a few groups in one crop, but organic systems have more and different crops (Norton et al., Reference Norton, Johnson, Joys, Stuart, Chamberlain, Feber, Firbank, Manley, Wolfe, Hart, Mathews, Macdonald and Fuller2008). Activity of 12 groups of invertebrate predators and parasites, sampled by standardised trapping procedures in four conventional and five organic crops and in four types of field boundary, was monitored on Nafferton Farm in 2005 and 2006. A number of questions were addressed:
(i) What were the effects of management system and crops on the activity of a broad range of beneficial invertebrates, not just on a limited number of epigeal groups?
(ii) Were there differences in beneficial invertebrate assemblages in the crops on the organic and conventional halves of the farm, and what effect did weeds have?
(iii) What influenced beneficial invertebrate activity in field boundaries?
(iv) What was the relationship between invertebrate activity in crops to that in field boundaries, and vice-versa, and to crop management and type?
Methods
Study area
Nafferton Farm, in south Northumberland, managed by the University of Newcastle upon Tyne, was a typical mixed commercial conventional farm in northern England with a dairy herd and mixed cropping of arable and grassland until 2001, when conversion to organic farming of 160 ha of the 320 ha farm commenced. Half of the farm was fully organic in late 2004. It is still a commercial farm. The field pattern of the farm, and an inset showing the location in northern England, is shown as a map (supplementary fig. 1). Field numbers are cross referenced to supplementary table 1, which gives the crop type, the fertiliser, herbicide and fungicide applications on the two halves of the farm in both years. Chemicals used on the conventional crops were not thought to have major detrimental effects on beneficial invertebrates. The crop rotation on the conventional half of the farm was limited to wheat, barley, oilseed rape and grass, with more variation on the organic half. Beans were used to improve soil fertility, and cabbage and potatoes were also grown. Spring barley on the organic half was undersown with grass/clover, and all grass and grass/clover fields were used for silage production. All arable crops on the conventional half were sown in the autumn of the previous year, with those on the organic half sown in spring of the harvest year.

Fig. 1. Biplot derived from the redundancy analysis relating 2005 crop invertebrate group activity to crop (organic grass/clover and arable, conventional grass and arable), weed and boundary (short herbaceous, tall herbaceous, hedges, woods) variables.
Table 1. The mean number (±standard error) of each invertebrate group in the three groups derived from the classification of the crop sites in 2005 and 2006. Invertebrate group order is as in axis 1 of the principal components analysis.

Weather parameters on the farm were monitored using a Delta-T Weather Station (type WS01) situated in field 9 (supplementary fig. 1). The mean daily air temperatures in May, June, July, August, September and October 2005 were 9.6, 13.7, 14.6, 14.5, 13.5 and 11.2°C, respectively, with the means for the same months in 2006 being 9.7, 14.0, 17.4, 14.4, 14.8 and 11.2°C. The total precipitation for the six months in 2005 was 28.2, 55.0, 69.8, 25.6, 54.2 and 92.8 mm, and in 2006 it was 74.4, 27.8, 13.2, 67.7, 71.4 and 85.4 mm.
Sampling
Pairs of sampling points were used in the crops, one at 5 m from the boundary into the field, and one in the centre of the field. At each sample point, lines of ten pitfall traps (8.5 cm diameter, 10 cm deep), 0.5 m apart and part-filled with saturated salt (NaCl) solution containing a small amount of strong detergent as a preservative, were used. Next to each line of pitfall traps used to sample epigeal invertebrates, a yellow pan trap (a box 22×31 cm, 20 cm deep) containing 1 cm of preservative, was employed to sample aerial invertebrates, in a similar approach to that of Duelli et al. (Reference Duelli, Obrist and Schmatz1999). There was a total of 20 sampling points in the conventional crops in 2005 (arable: four in winter barley, four in winter wheat, four in oilseed rape; non-arable: eight in grass) and 24 in the organic crops (arable: four in spring barley, six in spring wheat, two in beans, two in cabbage; non-arable: ten in grass/clover). The corresponding totals in 2006 were 28 sampling points in conventional crops (arable: 12 in winter wheat, four in winter barley, four in oilseed rape; non-arable: eight in grass) and 32 in organic crops (arable: four in spring barley, eight in spring wheat, eight in beans, four in potatoes; non-arable: eight in grass/clover). Opposite each pair of sample points in the fields, a line of pitfall traps and a pan trap was set in the field boundary. The field boundary covers were short herbaceous vegetation (usually grassy with forbs, such as dandelion Taraxacum, to a height of 0.5 m), tall herbaceous vegetation (generally 1–1.5 m high with tall grasses and forbs, mainly nettles Urtica and thistles Cirsium), hedges (mainly hawthorn Crataegus) and woodland (mainly coniferous). A number of field boundaries were shared between fields so that 20 boundaries were sampled in 2005 and 26 in 2006. The traps were set in the first week of May of both years and five monthly samples were generated. The pitfall and pan trap samples were sorted in the laboratory, and invertebrates were kept in 70% industrial methylated spirit.
The total numbers of Carabidae (ground beetles), Staphylinidae (rove beetles), Linyphiidae (money spiders) and Lycosidae (wolf spiders) were counted from the pitfall samples. Total numbers of Cantharidae (soldier beetles), Coccinellidae (ladybirds), Syrphidae (hoverflies), Neuroptera (lacewings), Hemiptera (predatory bugs; Anthocoridae, Nabidae) and parasitic wasps (Hymenoptera: Ichneumonidae, Proctotrupoidea, Braconidae) were counted from both pitfall and pan trap samples.
Weed cover around each sampling point on the organic half of the farm was estimated in 10% bands (i.e. 1=1–10%, 2=11–20% etc., to a maximum of ten) at each sampling time and the maximum weed cover recorded was used in analyses. Weed cover in the conventional fields was always less than 5%. To generate a representative idea of the field boundary structure nearest to sampling points, the area of the four cover types was used. The area of each cover 25 m from the middle of the boundary sampling point, to either side and behind, was calculated.
Data analysis
The data generated could not be analysed factorially and, therefore, multivariate techniques have been used. The invertebrate assemblage data, from the crop and boundary sites separately, were classified using fuzzy set clustering (Bezdek, Reference Bezdek1981), based on principal components analyses (PCA), in a similar procedure used by Eyre et al. (Reference Eyre, Labanowska-Bury, Avayanos, White and Leifert2009). Site scores on the first two axes of the ordination were used for the classification. Constrained ordination, using redundancy analyses (RDA), was used to investigate the relationship between the invertebrate assemblages and crop and boundary variables. Nine variables were used in the RDAs. These were the areas of the four non-crop covers (short herbaceous vegetation, tall herbaceous vegetation, hedges and woodland) together with the percentage weed cover and with categorical variables for the basic type of crop (organic grass/clover, conventional grass and organic and conventional arable). In RDA analyses with the field boundary invertebrate data, eight of the variables were used with weed cover omitted. Automatic forward selection of variables within RDAs was employed and the significance calculated using Monte Carlo permutation tests. The PCAs and RDAs were carried out using the CANOCO package, using invertebrate numbers transformed by log10n+1 (Ter Braak & Šmilauer, Reference Ter Braak and Šmilauer1998). In addition, Spearman rank correlations, relating invertebrate numbers to weed cover, were carried out in the R statistical environment (R Development Core Team, 2009).
Results
2005 and 2006 crops
The classification of the 2005 and 2006 crop invertebrate data both produced three assemblage groups. The mean numbers of each of the 12 invertebrate groups in the three groups in both years are shown in table 1. Group 1 in 2005 consisted of 16 invertebrate assemblages, only two of which were from organic fields, both in grass/clover. Of the 14 from conventional assemblages, there were four each from winter barley, winter wheat and oilseed rape and two from grass. This was a group in which conventional arable invertebrate assemblages dominated, with the highest numbers of Carabidae and Staphylinidae, high numbers of Ichneumonidae and Proctotrupoidea but the fewest of both spider families, Linyphiidae and Lycosidae. Group 2 had 15 assemblages, nine from organic fields and six from conventional. Six of the organic assemblages were from grass/clover, and three were from spring barley, undersown with grass/clover. All six conventional assemblages were in grass. This group had the most Linyphiidae but the fewest Carabidae and relatively few of the three parasitic wasp groups. The 13 assemblages in group 3 were all organic. Two were from grass/clover, six from spring wheat, two from beans, two from cabbage and one from spring barley. The invertebrate assemblages from these organic, mainly arable fields, had the most Syrphidae and Lycosidae, high numbers of Carabidae and parasitic wasps but fewest Staphylinidae. There was no discernable pattern of edge and centre sampling point distribution in groups. In 2005, there were nine, six and six edge assemblages and seven, nine and six centre assemblages in groups 1, 2 and 3, respectively, with similar assemblage distributions in 2006.
The biplot (fig. 1), derived from the RDA, shows the relationship between the 2005 crop invertebrates and the crop and non-crop variables. Organic arable was opposite to conventional grass on the positive axis 1 (eigenvalue 0.280) but grass/cover and conventional arable were also along the negative axis 1. There was a close relationship between organic arable and weed cover along axis 1, whilst axis 2 (eigenvalue 0.120) showed variation between grass/clover, short herbaceous boundaries and hedges opposite conventional arable and tall herbaceous boundaries. Coccinellidae, Lycosidae and Braconidae were strongly associated with short herbaceous boundaries, organic arable and weed cover with Syrphidae, Hemiptera and Cantharidae also related to weed cover. The positions of Ichneumonidae, Proctotrupoidea and Neuroptera, along the negative axis 2, indicated that neither arable variable was dominant. Carabidae were most strongly related to a mixture of conventional arable and long herbaceous boundaries. There was a strong association of Staphylinidae with the grass crop, opposite to conventional arable, and Linyphiidae were associated with grass and grass/clover. The results of the Monte Carlo permutations showed that organic arable explained most additional variance (F=10.04, P=0.002), with hedges (F=7.17, P=0.002), conventional arable (F=4.64, P=0.002), weed cover (F=2.48, P=0.016) and grass/clover (F=2.32, P=0.024) also significant in explaining the distribution of invertebrate groups.
Invertebrate activity in 2006 was considerably different from that in 2005 with far more individuals trapped (table 1). The group 1 of 2006 had 21 sampling points, of which 20 were organic. Eight of the assemblages in this group were from beans, six were from spring wheat, four from spring barley, two from potatoes and one from conventional winter wheat. This was a group dominated by organic arable fields with the most of the three parasitic wasp taxa, Syrphidae, Coccinellidae and Linyphiidae and relatively few Hemiptera. The 27 assemblages in group 2 were split into ten organic and 17 conventional. Of the organic, eight assemblages were from grass/clover and two from spring wheat, and ten of the conventional were from winter wheat, three from winter barley and four from oilseed rape. This group had the most conventional arable assemblages, and the most Carabidae, Staphylinidae and Lycosidae, with high numbers of Linyphiidae but relatively few Coccinellidae. Eight of the 12 assemblages in group 3 were from conventional grass, one was from winter wheat, one from winter barley and two from organic potatoes. There were most Hemiptera recorded from these fields, but this group had low overall numbers, with high numbers of Staphylinidae and Linyphiidae but the fewest of other invertebrate taxa except Coccinellidae.
The RDA biplot using the 2006 data (fig. 2) had an axis 1 (eigenvalue 0.351) showing the main variation to be between the organic arable and weed cover variables and conventional grass. The short herbaceous boundaries were associated with organic arable and weed cover and opposite to tall herbaceous boundaries, hedges and woods, which had relatively little influence. Organic arable and conventional grass were also the furthest along the negative axis 2 (eigenvalue 0.100), opposite to the organic grass/cover and conventional arable. Coccinellidae, Neuroptera and Braconidae were strongly associated with organic arable, weed cover and short herbaceous boundaries, with Syrphidae, Ichneumonidae and Proctotrupoidea also along the positive axis 1. Carabidae and Cantharidae were split between the organic arable and grass/clover, and the two spider families, Linyphiidae and Lycosidae, were associated with grass/clover. Staphylinidae showed a strong preference for conventional arable and tall herbaceous boundaries, with Hemiptera mainly associated with conventional grass. The variable explaining most of the additional variance was weed cover (F=18.95, P=0.002), with other significant variables affecting invertebrate assemblage distribution conventional grass (F=15.24, P=0.002), organic arable (F=5.43, P=0.002), organic grass/cover (F=5.38, P=0.002), woodland (F=3.91, P=0.002) and short herbaceous boundaries (F=2.93, P=0.006).

Fig. 2. Biplot derived from the redundancy analysis relating 2006 crop invertebrate group activity to crop (organic grass/clover and arable, conventional grass and arable), weed and boundary (short herbaceous, tall herbaceous, hedges, woods) variables.
Weed coverage on the organic half of the farm varied according to crop. In 2005, grass/clover had few weeds (<10%), both spring wheat and barley a little more (10–20%), with the most in beans (50–60%) and cabbage (60–70%). In 2006, weed cover in grass/clover and potatoes was low (<10%), somewhat higher in spring wheat (10–20%) and considerably higher in spring barley (50–60%) and beans (70–80%). Only one group, Lycosidae, gave a significantly negative response to weed cover (table 2), in 2006, with fewer in weedier crops. Hemiptera, Syrphidae and the three parasitic wasp groups were significantly related to increasing weed cover in 2005. Hemiptera activity was not significantly related to weeds in 2006 but Coccinellidae, Syrphidae, Ichneumonidae, Braconidae and Proctotrupoidea all had significantly more activity in the weedier crops.
Table 2. The correlation coefficients (r) and probabilities (P) from Spearman rank correlations of weed cover in the organic crops with invertebrate group numbers in 2005 and 2006.
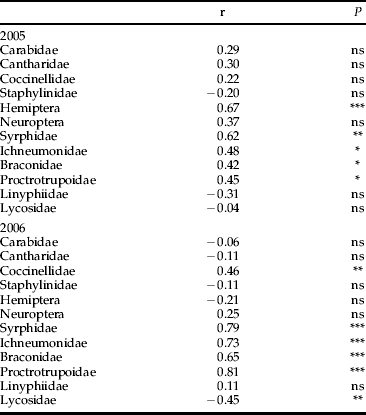
ns, not significant; * P<0.05; **, P<0.01; *** P<0.001.
2005 and 2006 field boundaries
Two invertebrate assemblage groups were generated in both years using the data from the field boundaries. The mean numbers of the invertebrate groups are given in table 3. There were nine assemblages in group 1 of the 2005 classification, of which six were from boundaries of conventional arable fields, two were next to organic arable and one next to grass/clover. All these boundaries had tall herbaceous vegetation and four backed on to woods and three on to hedges. Of the 11 assemblages in group 2, seven were from boundaries with tall herbaceous vegetation but six also had short vegetation, with four backing on to woodland and one on to a hedge. Four of these group 2 boundaries were by organic arable, five by grass/clover and four by conventional grass. Group 1 assemblages had more of most invertebrate groups with only more Braconidae, Coccinellidae and Linyphiidae in group 2.
Table 3. The mean number (±standard error) of each invertebrate group in the two groups derived from the classification of the field boundary sites in 2005 and 2006. Invertebrate group order is as in axis 1 of the principal components analysis.

The biplot from the RDA of the 2005 boundary data (fig. 3) showed a split along axis 1 (eigenvalue 0.317) between grass and grass/clover fields and short boundaries opposite to conventional arable fields, hedges and tall boundary vegetation. Organic arable fields and boundary woods were along the positive axis 2 (eigenvalue 0.119), but there were no variables directly opposite in the negative half of the axis. There were strong associations between the number of boundary Coccinellidae and Syrphidae with adjacent organic arable fields, which also had an influence on Ichneumonidae and Braconidae. Neuroptera and Cantharidae were between the two arable field types with Hemiptera, Proctotrupoidea, Lycosidae and, to a lesser extent, Staphylinidae, associated with conventional arable fields, tall herbaceous boundaries and hedges. Boundary Linyphiidae were mostly associated with grass fields with Carabidae not strongly influenced by any variable, although there was an indication that more were found by organic arable fields and woodland boundaries. The Monte Carlo permutation showed that conventional arable fields (F=3.96, P=0.004), organic arable fields (F=3.24, P=0.006), short boundary vegetation (F=2.25, P=0.028) and woodland (F=2.23, P=0.044) were significant in explaining the distribution of the 2005 boundary assemblages.

Fig. 3. Biplot derived from the redundancy analysis relating 2005 field boundary invertebrate group activity to crop (organic grass/clover and arable, conventional grass and arable) and boundary (short herbaceous, tall herbaceous, hedges, woods) variables.
Group 1 of the 2006 classification had 14 boundary assemblages, three by organic arable, two by grass/clover, eight by conventional arable and one by grass. All these assemblages came from boundaries with tall herbaceous vegetation with 11 by hedges and one by a wood. Five of the 12 assemblages in group 2 were by organic arable, two were next to grass/clover, three next to grass and two next to conventional arable. Six assemblages were from boundaries with both short and long vegetation, with five having boundary woodland and one a hedge. Both groups had similar numbers of Hemiptera, Neuroptera and Cantharidae (table 2), but there were more Staphylinidae, Lycosidae and Syrphidae in group 1 assemblages. There were about twice as many Proctotrupoidea and Linyphiidae in group 2 assemblages and many more Carabidae, Coccinellidae, Ichneumonidae and Braconidae.
The biplot (fig. 4) showed that the main variation along axis 1 (eigenvalue 0.278) was between short vegetation boundaries, grass/clover and organic arable fields in the positive half opposite to conventional arable and grass fields and long vegetation boundaries along the negative half. Axis 2 (eigenvalue 0.110) was mainly related to differences between wood and hedge boundaries. Coccinellidae, Braconidae, Cantharidae and Ichneumonidae were strongly associated with short vegetation boundaries and grass/clover fields, with Carabidae and Proctotrupoidea related to organic arable fields. Linyphiidae were also influenced by short vegetation boundaries but also by hedges. Neuroptera were found mainly in boundaries with woods with Staphylinidae and Hemiptera also associated with woods and with grass fields. Boundary Syrphidae were the only invertebrate group related to conventional arable fields, and also to hedges, with Lycosidae not strongly associated with any of the variables. Only two variables were significant in explaining the distribution of the 2006 boundary assemblages, the grass/clover fields (F=2.26, P=0.046) and the short herbaceous boundaries vegetation (F=2.14, P=0.048).

Fig. 4. Biplot derived from the redundancy analysis relating 2006 field boundary invertebrate group activity to crop (organic grass/clover and arable, conventional grass and arable) and boundary (short herbaceous, tall herbaceous, hedges, woods) variables.
Discussion
There were very obvious differences in the numbers of invertebrates trapped in the two years of the survey, possibly related to the warmer weather in 2006, but the numbers recorded could also have been influenced by crop and boundary type differences. The average daily temperature in July 2006 was 2.8°C higher than in July 2005, and weather patterns are known to affect invertebrate surveys, especially with spiders (Schmidt et al., Reference Schmidt, Roschewitz, Thies and Tscharntke2005). The 2005 classification produced more well-defined groups than that of 2006, with distinct groups dominated by organic and conventional arable by grass and grass/cover assemblages. In 2006, one group was again composed of organic arable assemblages; but there was a mix of conventional arable and grass/clover assemblages in another, separated from the third group with mainly conventional grass assemblages. However, the vegetation cover of autumn-sown conventional wheat and barley fields is not dissimilar to that of grass/clover silage fields, especially in spring. Vegetation cover is known to affect Carabidae activity (Eyre, Reference Eyre2006), and sowing time has been shown to affect ground beetle species assemblage composition (Purvis et al., Reference Purvis, Fadl and Bolger2001). The most obvious difference between the spring-sown organic arable and vegetable fields and all the others is the lack of vegetation cover until late spring or early summer. Booij & Noorlander (Reference Booij and Noorlander1992) found differences in Carabidae activity in a number of arable crops, and Hummel et al. (Reference Hummel, Walgenbach, Hoyt and Kennedy2002) found that activity of a number of invertebrate groups differed in several different vegetable crops. Weibull et al. (Reference Weibull, Östman and Granqvist2003) also reported differences in Carabidae activity in a comparison of cereal and grass crops, but the major difference in the distribution of the 12 invertebrate groups in this study was a basic difference in sowing time and management of organic arable and vegetable crops. Management intensity is known to affect the distribution and composition of Carabidae and spider assemblages (Rushton et al., Reference Rushton, Luff and Eyre1989; Cole et al., Reference Cole, McCracken, Downie, Dennis, Foster, Waterhouse, Murphy, Griffin and Kennedy2005), whilst sowing and harvesting differences affected Carabidae assemblages (Kromp, Reference Kromp1999). Carabidae activity was less in the grassy fields than in arable in both years and was greater in conventional arable fields than in organic in 2005, but with differences fewer in 2006. Few differences were observed in Carabidae activity in wheat grown under the two management systems by Döring & Kromp (Reference Döring and Kromp2003) and Fuller et al. (Reference Fuller, Norton, Feber, Johnson, Chamberlain, Joys, Mathews, Stuart, Townsend, Manley, Wolfe, Macdonald and Firbank2005). There was a strong relationship between Carabidae activity in 2005 and tall herbaceous field boundaries; but, in 2006, there was no obvious relationship with any boundary type.
Staphylinidae activity was greatest in grassy and conventional arable fields, especially in 2006; and, in both years, there was an association with hedges and woods. Kroos & Schaefer (Reference Kroos and Schaefer1998) found more Staphylinidae activity in conventional wheat than in organic, although no differences were reported by Clough et al. (Reference Clough, Holzschuh, Gabriel, Purtauf, Kleijn, Kreuss, Steffan-Dewenter and Tscharntke2007). Linyphiid spider activity was related to both grass crops on both halves of the farm in 2005, but there was more activity in organic grass/cover in 2006 and no consistent relationship between activity and field boundary type. An inconsistent pattern was also found with lycosid spiders, most active in organic arable fields in 2005 and in grass/clover in 2006, with again no obvious relationship to any boundary type. Greater spider activity was reported in organic wheat (Feber et al., Reference Feber, Bell, Johnson, Firbank and Macdonald1998; Fuller et al., Reference Fuller, Norton, Feber, Johnson, Chamberlain, Joys, Mathews, Stuart, Townsend, Manley, Wolfe, Macdonald and Firbank2005), and the amount and type of surrounding non-crop habitat also affected spider activity in wheat (Schmidt et al., Reference Schmidt, Roschewitz, Thies and Tscharntke2005).
There has been far less work on the comparison of management and crop differences on other invertebrate group activity than Carabidae and spiders and on comparisons in crops other than cereals. In both years, Coccinellidae, Syrphidae and Braconidae activity was strongly associated with organic arable, weeds and the short herbaceous boundaries and activity of Ichneumonidae and Proctotrupoidea was also related to organic arable and weeds. Enhanced activity of Syrphidae and of the three parasitic wasp groups has been achieved by using planted weed strips and field margins (Hausammann, Reference Hausammann1996; MacLeod, Reference MacLeod1999), whilst increased weed cover has been advocated in order to increase parasitic wasp activity in particular (Stephens et al., Reference Stephens, Schellhorn, Wood and Austin2006). Increased activity of these groups was related to increased pollen and nectar food supply from weed flowers, and it was apparent that there was greater activity in crops on the organic half with flowering weeds. Landscape complexity has also been implicated in influencing activity of these groups (Thies et al., Reference Thies, Roschewitz and Tscharntke2005; Meyer et al., Reference Meyer, Jauker and Steffan-Dewenter2009). Pfiffner & Luka (Reference Pfiffner and Luka2003) reported more spider activity in weedier cereal fields, but it was interesting that weed cover on the organic half of the studied farm had relatively little effect on the activity of the epigeal invertebrates sampled with pitfall traps. Low numbers of Cantharidae, Neuroptera and Hemiptera were recorded, with, in general, more of the first two groups active in organic arable fields but Hemiptera gave inconsistent results.
The classification of boundary invertebrate assemblages produced, in both years, one group with assemblages from boundaries with tall herbaceous vegetation next to conventional arable fields and another with short herbaceous vegetation next to organic arable, with assemblages from next to grass and grass/clover fields in both groups. The activity of some invertebrate groups in boundaries was strongly associated with the adjacent crop with, for instance, more Staphylinidae in boundaries next to conventional arable crops and more parasitic wasps next to organic arable crops. The extra diversity of crops in organic farming (Norton et al., Reference Norton, Johnson, Joys, Stuart, Chamberlain, Feber, Firbank, Manley, Wolfe, Hart, Mathews, Macdonald and Fuller2008) will affect boundary invertebrate activity, especially the growing of such crops as beans, as well as weed cover. The pattern of boundary invertebrate assemblage distribution and group activity is likely to be dependent on location, since landscapes dominated by conventional agriculture tend to be different from those dominated by organic (Schmidt et al., Reference Schmidt, Roschewitz, Thies and Tscharntke2005; Billeter et al., Reference Billeter, Liira, Bailey, Bugter, Arens, Augenstein, Aviron, Baudry, Bukacek, Burel, Cerny, De Blust, De Cock, Diekötter, Dietz, Dirksen, Dormann, Durka, Frenzel, Hamersky, Hendrickx, Herzog, Klotz, Koolstra, Lausch, Le Coeur, Maelfait, Opdam, Roubalova, Schermann, Schermann, Schmidt, Schweiger, Smulders, Speelmans, Simova, Verboom, van Wingerden, Zobel and Edwards2008).
The inability of Hole et al. (Reference Hole, Perkins, Wilson, Alexander, Grice and Evans2005) to come to any hard and fast conclusions about the effects of management system on invertebrate biodiversity and activity stem from the inconclusive work on organic and conventional cereals (Fuller et al., Reference Fuller, Norton, Feber, Johnson, Chamberlain, Joys, Mathews, Stuart, Townsend, Manley, Wolfe, Macdonald and Firbank2005; Purtauf et al., Reference Purtauf, Roschewitz, Dauber, Thies, Tscharntke and Wolters2005; Clough et al., Reference Clough, Holzschuh, Gabriel, Purtauf, Kleijn, Kreuss, Steffan-Dewenter and Tscharntke2007). Although Bengtsson et al. (Reference Bengtsson, Ahnstrom and Weibull2005) concluded that predatory insects react positively to organic farming, the only epigeal group consistently more active on the organic half of the farm was Lycosidae, with Staphylinidae more active on the conventional half. However, there was considerably more activity of aerially active predators and parasites, sampled mainly by pan trap, in the organic arable fields, especially in the weedier fields.
This study differed from others in that there was the ability to compare crop, management and field boundary effects on the activity of a wide range of beneficial invertebrate groups on one farm. Optimising natural enemy activity is a priority in organic farming systems, and the results from the split farm indicate a number of approaches likely to be useful. Autumn sowing, especially for cereals, may provide a better environment for beneficial groups than highly disturbed spring seedbeds, given that mechanical soil disturbance has negative effects on beneficial invertebrates (Thorbek & Bilde, Reference Thorbek and Bilde2004). A balancing act with weeds may be desirable since they appear to increase invertebrate activity but can have a negative effect on crop yield. Mechanical weeding (Van Der Weide et al., Reference Van Der Weide, Bleeker, Achten, Lotz, Fogelberg and Melander2008) may limit weed cover to acceptable levels while retaining some positive input. A number of approaches have been advocated for increasing beneficial invertebrate activity by vegetation manipulation (Landis et al., Reference Landis, Menalled, Costamagna and Wilkinson2005). The close association between invertebrate activity in organic arable crops and short vegetation boundaries indicated that boundary management could be useful. Planting of appropriate flower mixtures, together with mowing to limit competitive vegetation development, may induce increased aerial invertebrate activity. Field margin planting used to sustain biodiversity on arable land (Critchley et al., Reference Critchley, Allen, Fowbert, Mole and Gundrey2004), incorporating plant species known to increase invertebrate activity, may be a potential approach. A wider understanding of invertebrate activity both in and between crops and field boundaries is required to formulate strategies to increase pest control with beneficial invertebrates.
Acknowledgements
This work was supported by the European Union Integrated Project QualityLowInputFood (EU FP6 Contract CT-2003-506358).
Supplementary material
The online fig. and table can be viewed at http://journals.cambridge.org/ber.
The supporting material provides a figure of the field distribution and location of Nafferton Farm and a table of inputs used in the fields and other information.









