Introduction
Entomopathogenic nematodes (EPN) (Steinernema and Heterorhabditis) are parasites of insects that are mutually associated with insect-pathogenic bacteria (Xenorhabdus and Photorhabdus, respectively). EPN were first applied to control Japanese beetles in the 1930s, and since the 1980s they have been widely used against several important insect pests (Georgis et al., Reference Georgis, Koppenhofer, Lacey, Belair, Duncan, Grewal, Samish, Tan, Torr and van Tol2006). EPN are generally used in inundative biological control programs, where large numbers of nematodes are released with a view to achieving rapid reduction in the pest population. The infective juveniles (IJs) actively seek out insects in the soil and enter insects via their natural openings or penetrate through the cuticle. The nematodes find their way to the insect's haemocoel where they release the bacteria, resulting in death of the insect by septicaemia within days (Kaya & Gaugler, Reference Kaya and Gaugler1993).
There is a risk that entomopathogenic nematodes applied at the high densities required for inundative control might negatively impact wild or released parasitoids. Natural enemies may interfere with each other either through competition or intraguild predation (IGP). Intraguild predation occurs when two species that share a host also engage in a trophic interaction (predation or parasitism) with each other (Rosenheim et al., Reference Rosenheim, Kaya, Ehler, Marois and Jaffee1995). Entomopathogenic nematodes are known to have an adverse effect on the development of some parasitoids (Kaya, Reference Kaya1978a,Reference Kayab; Kaya & Hotchkin, Reference Kaya and Hotchkin1981; Kaya et al., Reference Kaya, Joos, Fallon and Berlowitz1984; Zaki et al., Reference Zaki, Awadallah and Gersraha1997; Shannag & Capinera, Reference Shannag and Capinera2000; Sher et al., Reference Sher, Parrella and Kaya2000; Head et al., Reference Head, Palmer and Walters2003; Lacey et al., Reference Lacey, Unruh and Headrick2003). If female parasitoids avoid ovipositing on nematode-infected insects, the negative impact of nematodes on parasitoid populations might be reduced. Heterospecific host discrimination (the rejection of hosts previously parasitised by another parasitoid species) is possible when parasitoids are able to detect changes associated with prior parasitism (Turlings, Reference Turlings1985). Alternatively, females may be unable to detect prior parasitism or choose to oviposit in previously parasitized hosts (multi-parasitism). In general, previous parasitism reduces the quality of a host for a subsequent parasitoid female (Godfray, Reference Godfray1994), though the reverse has also been reported with authors proposing that previous parasitism may reduce the hosts' defences (Zaviezo & Mills, Reference Zaviezo and Mills2001). The decision to multi-parasitize should reflect larval survival probability (McBrien & Mackauer, Reference McBrien and Mackauer1991); therefore, where there is a risk of parasitoid mortality, either through competition or intraguild predation, multi-parasitism should be avoided. There is little information on the response of parasitoids to nematode-infected hosts, and to date all studies have involved Steinernema species. Neither Diglyphus begini nor Diglyphus isaea (Hymenoptera: Eulophidae) discriminated significantly between healthy leafminers and those parasitized by Steinernema spp., but this may reflect low overall numbers, as over 90% of eggs were laid alongside healthy larvae in each case (Sher et al., Reference Sher, Parrella and Kaya2000; Head et al., Reference Head, Palmer and Walters2003). The ichneumonids, Mastrus ridibundus and Liotryphon caudatus, avoided codling moth hosts that had been killed by nematodes; and significant avoidance was observed as early as 12 h after the hosts had been exposed to Steinernema carpocapsae, a time when the majority of the insects were still alive (Lacey et al., Reference Lacey, Unruh and Headrick2003).
In this study, we evaluated the interaction of the entomopathogenic nematode Heterorhabditis downesi Stock, Griffin and Burnell (Nematode: Heterorhabditidae) with the parasitoid Bracon hylobii Ratzeburg (Hymenoptera: Braconidae), both important biocontrol agents of the large pine weevil, Hylobius abietis L. (Coleoptera: Curculionidae). The large pine weevil is the most important pest of coniferous reforestation in much of central and northern Europe, where adult feeding causes deformation, reduced growth and mortality of young conifer transplants (Eidmann & Lindelöw, Reference Eidmann and Lindelöw1997). EPN are currently applied inundatively on a semi-operational scale against this pest in the UK and Ireland, where B. hylobii is the principal native natural enemy of this weevil.
Hylobius abietis larvae develop in the stumps of recently felled coniferous trees, where development from egg to adult takes 12–36 months (Leather et al., Reference Leather, Day and Salisbury1999; Moore et al., Reference Moore, Brixey and Milner2004). Application of nematodes around the stumps of felled conifers to kill developing weevils is a promising strategy to reduce the number of adult H. abietis emerging onto a site (Brixey et al., Reference Brixey, Moore and Milner2006; Dillon et al., Reference Dillon, Ward, Downes and Griffin2006, Reference Dillon, Downes, Ward and Griffin2007, Reference Dillon, Moore, Downes and Griffin2008a), and this technique is currently being applied on an area wide basis (Forestry Commission, 2006; COFORD, 2007). Although S. carpocapsae is the nematode species currently used, H. downesi is consistently more effective at suppressing adult weevil populations in small-scale trials (Dillon et al., Reference Dillon, Ward, Downes and Griffin2006, Reference Dillon, Downes, Ward and Griffin2007, Reference Dillon, Moore, Downes and Griffin2008a).
Bracon hylobii is a gregarious ectoparasitoid of H. abietis larvae. It often causes natural mortality of up to 30% of larvae (Munro, Reference Munro1914; Crooke & Kirkland, Reference Crooke and Kirkland1956; Dillon et al., Reference Dillon, Moore, Downes and Griffin2008a), and instances of higher mortality have been recorded in Britain in pine (90%) and spruce (68%) (Hanson, Reference Hanson1943; Henry, Reference Henry1995). The parasitoid has a wide distribution within the range of H. abietis (Heqvist, Reference Heqvist1958; von Waldenfels, Reference von Waldenfels1975; Gerdin, Reference Gerdin1977). Female B. hylobii respond to the stimuli associated with H. abietis larvae actively feeding on bark (Faccoli & Henry, Reference Faccoli and Henry2003). The female's ovipositor is inserted through the bark of the stump, to inject the larva with paralysing venom (Wharton, Reference Wharton1993) prior to depositing a clutch of eggs on or near the body of the host. Average clutch size is approximately 6–7 eggs per host but is influenced by host size and temperature (Hanson, Reference Hanson1943; Henry & Day, Reference Henry and Day2001a). All but 1st instar H. abietis (<100 mg) are susceptible to parasitism by B. hylobii, and larger hosts are preferred (Henry & Day, Reference Henry and Day2001a). Generation time is approximately 20 days at 20°C (Henry & Day, Reference Henry and Day2001a). Augmentation of B. hylobii populations by mass release of laboratory-reared parasitoids has been attempted in the past (Henry & Day, Reference Henry, Day, Alfaro, Day, Salom, Nair, Evans, Liebhold, Lieutier, Wagner, Futai and Suzuki2001b) and is still being actively investigated as a means of suppressing pine weevil populations (COFORD, 2007).
Our objectives were (i) to ascertain the susceptibility of various life stages of B. hylobii to H. downesi, (ii) to test whether ovipositing B. hylobii females will discriminate between healthy and nematode-infected pine weevil larvae and, if so, at what time after infection are larvae rejected and (iii) to test whether B. hylobii respond to nematode-infected hosts by adjusting clutch size or whether the response is simply to accept or reject. Our study is the first to investigate whether a gregarious parasitoid actively adjusts clutch size in response to nematode infection of the host.
Lack (Reference Lack1947) proposed the concept of optimal clutch size, suggesting that females should lay the number of eggs that maximizes fitness. Host size is a key factor influencing parasitoid fitness (Godfray, Reference Godfray1994), and many gregarious parasitoids, including B. hylobii, can assess the quality of a host and alter clutch size accordingly (Henry & Day, Reference Henry and Day2001a). While the resources available to each offspring are greater in larger hosts, host quality is determined by the amount of nutrients available to the parasitoid and not necessarily by host weight alone (Hackermann et al., Reference Hackermann, Rott and Dorn2007). One would expect diseased or dying hosts to be nutritionally suboptimal, and the ability of a parasitoid to avoid wasting eggs on dying hosts would be advantageous. The oviposition response of gregarious parasitoids to pathogen-infected hosts is highly variable (Brooks, Reference Brooks, Beckage, Thompson and Federici1993), though a reduction in clutch size where parasitoids laid on infected hosts has been reported (Kyei-Poku & Kunimi, Reference Kyei-Poku and Kunimi1997; Nguyen et al., Reference Nguyen, Kakai, Takatsuka, Okuno, Ishii and Kunimi2005). Lacey et al. (Reference Lacey, Unruh and Headrick2003) reported that two ichneumonid parasitioids (M. ridibundus and L. caudatus) laid fewer eggs in the presence of nematode-infected hosts; however, results were due to a reduction in the percentage of parasitized codling moths, as opposed to clutch size.
Materials and methods
Nematode and insect cultures
Fourth and fifth instar pine weevil larvae were collected from stumps of felled conifers. Collected weevils were stored individually at 9°C with moist tissue paper for up to two weeks prior to use. Bracon hylobii were collected as cocoons from the wild and cultured in the laboratory using the choice test arena described in the next section. Nematodes (H. downesi K122 strain) were cultured in last instar larvae of Galleria mellonella L. (Woodring & Kaya, Reference Woodring and Kaya1988). After harvest, nematode infective juveniles (IJs) were washed three times by sedimentation in tap water and stored at 9°C. Nematodes were 2–6 weeks old at the time of application.
Choice test: oviposition by B. hylobii on H. downesi-infected or uninfected pine weevil larvae
Pine weevil larvae for choice tests were infected by short-term exposure to a high concentration of nematodes (8000 IJs for 4 h). Weevils were placed individually in eppendorf tubes (1.5 ml) lined with filter paper wetted with a suspension of H. downesi (8000 IJs in 0.1 ml of tap water). Control (healthy) weevils were treated similarly but with tap water only on the filter paper. A ventilation hole was made in the cap of the tube. Tubes were incubated at 20°C for four hours, after which time weevils were taken out and washed thoroughly to remove adhering IJs. There were two experiments.
In experiment 1, the weevil larvae used in the choice test were incubated at 20°C for one, three, five or seven days prior to exposure to the parasitoid. Incubation was in clean 24-well multiwell plates with moist tissue paper lining the lid. When the delay period (1–7 days) had expired, one infected and one healthy (control) weevil larva were placed in a choice test arena and a mated female B. hylobii was introduced. The choice test arena consisted of a chamber (90 mm dia. and 23 mm high) formed by taping the bases of two Petri dishes together. A 5-mm diameter access port was drilled in the side of the upper dish. Two H. abietis larvae (either one healthy and one that had been exposed to nematodes or two healthy) were placed in each arena. Larvae were confined in 10-mm diameter ‘oviposition cells’ drilled in a 3.5-mm high rectangle of perspex (30×80 mm). Cells were approximately 25 mm apart. A single strip of Picea sitchensis Carr. bark (2–3-mm thick) (thin enough for B. hylobii to oviposit through) was taped to the perspex so as to cover both oviposition cells. A single mated female B. hylobii was introduced through the access port. Females were less than five days old and had no prior oviposition experience. Each parasitoid was used only once. A 3-mm2 piece of filter paper soaked in a 50% honey and water solution was also introduced to provide a food source for the parasitoid. The port was plugged with tissue paper, which was kept moist throughout the experiment. For the duration of the oviposition choice test, the arena was kept at 20°C in constant light (B. hylobii females are inactive in the dark (Henry & Day, Reference Henry and Day2001a)). The oviposition choice test was run for 24 h, after which time the number of eggs on each weevil was recorded. Taking account of both the delay period and the one day test period, the window for oviposition by B. hylobii on H. abietis was 1–2, 3–4, 5–6 and 7–8 days post infection with nematodes. Mortality of the weevils was monitored for up to six days. Nematode infection was recognized by the characteristic pink colour of the insect cadaver (denoting the presence of Photorhabdus). Where the nematode-exposed weevil did not die, the results for those replications were discarded from the choice test. The experiment was repeated three times with 10–20 replications (parasitoids) in each.
Experiment 2 focused on the first 24 h after exposure to nematodes. The methodology was as above, except the delay period and oviposition choice test period were reduced. The delay period between infection of the larvae with nematodes and their use in the oviposition choice test was 0, 6, 12 or 24 h. The oviposition choice test lasted for only eight hours. Thus, the window for oviposition was 0–8, 6–14, 12–20 and 24–32 h post infection. The experiment was repeated until at least 25 parasitoids had been tested for each treatment.
Susceptibility of developing B. hylobii to H. downesi
Female B. hylobii were allowed to oviposit on late instar weevil larvae for 24 h (using the oviposition chambers described above), and the number of eggs on each weevil was recorded. The parasitized weevils then were transferred to wells of a 24-well plate lined with filter paper (one weevil per well). Bracon hylobii eggs hatched two days after oviposition, and the number of parasitoid larvae was counted. Nematodes were applied to samples of weevil larvae one, three, five or seven days after the parasitoids hatched. Nematode suspension (250 IJs in 100 μl tap water) or 100 μl tap water (control) was applied to the filter paper lining the well. The number of B. hylobii larvae infected by the nematode-bacterial complex was counted 3–8 days after nematode application. The number of parasitoid cocoons formed on each weevil was counted subsequently. Bracon hylobii that formed cocoons were retained and scored for emergence of adult parasitoids. The experiment was run three times with three, seven and seven replicates (weevil larvae), respectively.
Susceptibility of B. hylobii cocoons and adults to infection by H. downesi
An intact clutch of B. hylobii cocoons was placed in a 50-ml universal vial and covered with peat moss to a depth of 3 cm. Each vial received either 100 μl of nematode suspension containing 10,000 H. downesi IJs or 100 μl of tap water. The vial was placed inside an inverted glass jar. Newly emerged B. hylobii adults were able to escape contact with the nematode-infected substrate by exiting the vial and dispersing inside the jar. Jars contained a strip of filter paper (1×5 cm) soaked with a 50% honey and water solution to maintain the escaped B. hylobii. Jars were incubated at 20°C for one week. The number of dead and alive adults was counted. Adults that died were dissected in a drop of water to look for nematodes. Cocoons from which adults failed to emerge were opened, and the status of the occupant (dead or alive) was noted. A sample of non-eclosed B. hylobii was dissected. The experiment was run three times, with 5–7 clutches of parasitoid cocoons per treatment per experiment.
Statistical analysis
Routine statistics were performed using MINITAB Release 14 for Windows (Minitab Inc., 2003). Significance levels were taken at P<0.05, unless otherwise stated. Data were tested for normality using the Anderson Darling test and, where non-normal, data were normalized by transformation if possible. More than two treatments were compared using ANOVA or Kruskal Wallis. Two treatments were compared using a two-sample t-test. In the first oviposition choice test (1–7 days), analysis was on arcsine square root transformed data for percent of parasitoids laying eggs and on log transformed data for number of eggs laid. In the second choice test experiment (0–24 h), the proportion of parasitoids laying eggs on healthy versus infected weevils was compared by χ2 using totals rather than means of each experiment, due to the small number of replicates in some experiments. Death rate of weevils used and not used for oviposition was compared using χ2. In the second oviposition choice test, analysis of the number of eggs per B. hylobii was performed on untransformed data. The proportion of weevils selected for oviposition in the choice arena (healthy versus infected) and in the presence of two healthy weevils was compared using χ2. The number of eggs laid per B. hylobii and clutch size in the choice arena was compared with values obtained in the presence of two healthy weevils using a t-test. Analysis of the susceptibility of developing B. hylobii to H. downesi was performed on means of three experiments. The number of larvae parasitized by H. downesi and the number of adults emerging from H. downesi-treated weevils were compared using Kruskal Wallis; the number of cocoons formed in the control and the nematode treatment were compared using ANOVA. In the cocoon experiment, emergence and mortality in nematode treated arena was compared with controls using a t-test.
Results
Oviposition by B. hylobii on H. downesi-infected weevils with a long (1–7 day) delay between nematode exposure and oviposition
In oviposition choice experiment 1, all of the weevils that had been exposed to nematodes were infected and died, mostly within two days of infection. Two thirds of the weevils (68%, 34/50) offered to B. hylobii one day after infection were still alive at the start of the oviposition choice test, while only 10% were still alive at the end of the test (two days after infection). All of the nematode-infected weevils offered to B. hylobii three, five or seven days after infection were dead at the start of the oviposition test. Where B. hylobii females were each presented with a healthy weevil and a weevil infected by nematodes 1–7 days previously, 74% of the B. hylobii laid on the healthy weevil, with no nematode-infected weevils selected for oviposition. There was no difference between delay periods (1, 3, 5 or 7 days after infection) either in the proportion of healthy weevils on which eggs were laid (F=2.69, df=3, 8, P>0.05) or in the number of eggs laid by ovipositing parasitoids (clutch size) (F=0.84, df=3, 8, P>0.05). The average B. hylobii clutch size was 16.6±0.6 eggs, n=147.
Oviposition by B. hylobii on H. downesi-infected weevils with a short (0–24 h) delay between nematode exposure and oviposition
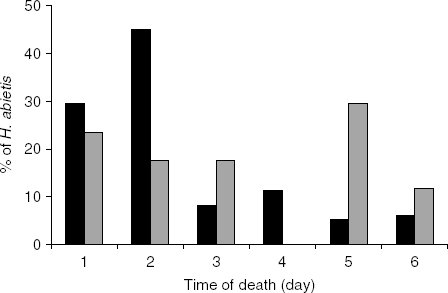
In oviposition choice experiment 2, 73.5% (166/226) of the weevils exposed to nematodes were infected by the nematodes. Most of the nematode-infected weevils died within two days of infection, but mortality continued for up to six days (fig. 1). Bracon hylobii used some nematode-infected weevils for oviposition in all treatments. With no delay and a six hour delay between nematode infection and the start of the test period, there was no difference in the percentage of healthy and infected weevils parasitized by B. hylobii (fig. 2a). With a longer delay, fewer nematode-infected than healthy weevils were parasitized, but differences were only significant at the 12 h delay (P<0.01 and P=0.08 at 12 and 24 h, respectively). Altogether, twice as many healthy (46/166) as infected weevils (23/166) were selected for oviposition (χ2=9.678, df=1, P<0.01). Five B. hylobii laid on both weevils in the choice arena. Nematode-infected weevils that were used for oviposition by B. hylobii tended to die later than those that were not (fig. 1). The day of death was recorded for a subset of 120 nematode-infected weevils. Of those weevils that were selected for oviposition, only 41% (7/17) died of nematode infection within two days compared to 74% (73/103) of those that were not used for oviposition. This difference in time of death between infected weevils used and not used for oviposition was significant (χ2=5.791, df=1, P<0.05).
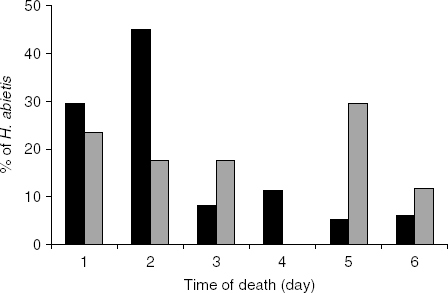
Fig. 1. Time to death of Hylobius abietis exposed to Heterorhabditis downesi and then exposed to Bracon hylobii after a 0–24 h delay; n=17 for weevils that were oviposited on by B. hylobii and 103 for those that were not (■, Not oviposited, ![]() , Oviposited).
, Oviposited).

Fig. 2. Oviposition by Bracon hylobii in a choice test (8 h) with one healthy Hylobius abietis larva and one larva that had been exposed to Heterorhabditis downesi 0–24 h previously. (a) Total percentage of healthy and nematode-infected H. abietis larvae with eggs. (b) Clutch size (mean±SE) of ovipositing B. hylobii on healthy and nematode-infected weevil larvae in the choice test. The number on a column or pair of columns indicates (a) the number of parasitoids tested or (b) the number of H. abietis on which the parasitoid oviposited. ** denotes significance at P<0.01 (Chi square test) (■, Healthy;
![]() , Infected).
, Infected).
Bracon hylobii either rejected the infected weevils or laid a full clutch of eggs on them (fig. 2b). There was no effect of either delay period (0, 6, 12 or 24 h) or treatment (healthy versus infected) on clutch size (F=0.28, df=3, 61, P>0.05 and F=1.05, df=1, 61, P>0.05, respectively). There was no interaction between these two factors (F=0.60, df=3, 61, P>0.05).
Oviposition of B. hylobii on two healthy weevils
Partitioning of load across available hosts
In experiment 1, when offered two healthy weevil larvae, 88% (44/50) of the B. hylobii oviposited on either one or both of them (23/50 and 21/50 parasitoids, respectively). We investigated whether B. hylobii altered the number of eggs she laid or her clutch size when ovipositing on multiple hosts. The number of eggs per ovipositing B. hylobii was higher where parasitoids used two hosts, than when only a single healthy host was selected (24.7±1.6 and 16.5±1.4, respectively) (t=3.75, df=41, P=0.001); but, where two hosts were used, the parasitoid reduced her clutch size (no. eggs host per host) compared to when one host was used (12.3±0.9 and 16.5±1.4, respectively) (t=2.40, df=39, P<0.05). In experiment 2, as only one B. hylobii in the control laid on two hosts, it was not possible to determine whether clutch size was reduced in the presence of multiple healthy hosts.
Effect of ‘patch quality’ on clutch size/oviposition decision
To test whether the presence of a nematode-infected weevil in the arena affects the oviposition behaviour of B. hylobii choice test results were compared with arenas containing two healthy weevils.
Experiment 1: The proportion of B. hylobii that oviposited on either one or both healthy weevils (88%; 44/50) was higher than the 74% (147/198) that oviposited when presented with one healthy and one infected weevil (χ2=4.27, df=1, P<0.05) (table 1). The number of eggs per parasitoid was lower when they were given one healthy and one nematode-infected weevil (16.6±0.6, n=147) than when presented with two healthy weevils (20.4±1.2, n=44) (t=2.74, df=65, P<0.01) (table 1), but clutch size was higher (16.6±0.6 and 13.8±0.8, respectively) (t=2.74, df=65, P<0.01). As the presence of multiple hosts may alter B. hylobii behaviour (see above), only those parasitoids ovipositing on a single host were compared. Parasitoids that used only one of the healthy hosts laid 16.5±1.4 eggs per B. hylobii (n=23), which is similar to the number laid in the choice test where all of the hosts used were also healthy (16.6±0.6, n=147) (t=0.48, df=30, P>0.05) (table 1).
Table 1. Number of B. hylobii ovipositing, and number of eggs laid, when presented with either two healthy (uninfected) weevil larvae or one infected and one healthy larvae for 24 h (experiment 1) or eight hours (experiment 2).

Similar lower (or upper) case within a column for a given experiment indicate overall (or ‘laid on one insect’) values did not differ significantly at P>0.05 (t-test).
Experiment 2: In this experiment, there was no difference in the proportion of B. hylobii that oviposited when given either two healthy (22/60; 36.7%) or one infected and one healthy weevil (64/166; 41.0%) (χ2=0.07, df=1, P>0.05) (table 1). The number of eggs laid per parasitoid when neither weevil was infected (21.7±1.44, n=22) was similar to when one was infected by nematodes (19.9±0.9, n=64) (t=1.06, df=39, P>0.05). Overall clutch size was also similar whether there were two healthy weevils present or one healthy and one infected (20.8±1.6 and 18.5±0.9) (t=1.29, df=36, P>0.05) (table 1). One B. hylobii oviposited on both weevils in the ‘healthy versus healthy’ treatment and five laid on both in the healthy versus nematode-infected treatment. When only those parasitoids ovipositing on a single host were compared, the number of eggs laid (and clutch size) in the presence of an infected host (19.2±0.9 eggs per B. hylobii, n=59) was again similar to that laid in the presence of two healthy hosts (21.2±1.4, n=21) (t=1.20, df=36, P>0.05).
Susceptibility of developing B. hylobii to H. downesi
All of the H. abietis larvae in the nematode treatments were infected by H. downesi, as evident by the characteristic colouration (pink). Hylobius abietis that were not exposed to nematodes remained white, but by day 5–7 had become flaccid due to feeding of the B. hylobii larvae. There were equal numbers of B. hylobii larvae on the weevils that were assigned to the various groups for treatment with nematodes or water 1–7 days after hatching (10.7±0.5, n=135) (F=1.12, df=7, 16, P>0.05).
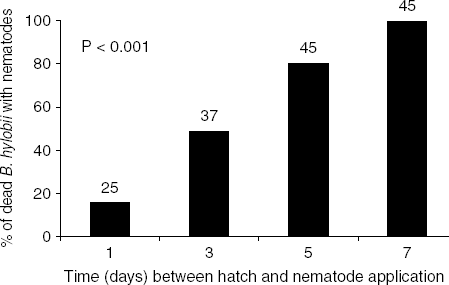
In all of the nematode treatments, some dead B. hylobii larvae were found (average 2–3 per weevil) (fig. 3a). The number of infected B. hylobii larvae per H. abietis was similar irrespective of the time after hatch the nematodes were applied (H=2.45, df=3, P>0.05). All of the dead B. hylobii larvae that were found in the nematode treatments were pink (denoting presence of the nematode's symbiont Photorhabdus), but dissection showed that not all of the pink cadavers harboured nematodes. Bracon hylobii that were older (and hence larger) at the time of nematode application were more likely to harbour nematodes than younger ones (χ2=61.35, df=3, P<0.001; fig. 4).

Fig. 3. Numbers of Bracon hylobii ((a) parasitized larvae, (b) cocoons or (c) emerging adults) on Hylobius abietis larvae to which Heterorhabditis downesi or water was applied 1–7 days after hatch of B. hylobii eggs. Mean (±SE) of three experiments (■, H. downesi; ![]() , Water).
, Water).
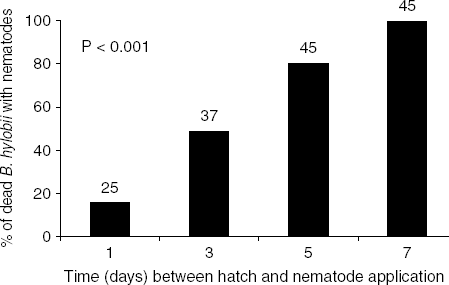
Fig. 4. Percentage of Bracon hylobii larvae killed by entomopathogenic nematode-bacterial complex (as evidenced by pink colour) in which nematodes developed. Heterorhabditis downesi nematodes were applied one, three, five or seven days after hatch of B. hylobii eggs. A number over a column indicates the number of B. hylobii larvae tested.
An average of 3–4 cocoons per H. abietis were formed in the water treatments, representing 20.6% of eggs laid (total eggs=1162) (fig. 3b). The time at which water was applied did not affect either the number of cocoons or the success rate of eggs in maturing to cocoons (F=0.26, df=3, 8, P>0.05; F=0.10, df=3, 8, P>0.05). Bracon hylobii cocoons were formed in the nematode treatments (1–2 cocoons per H. abietis), except when nematodes were applied one day after the B. hylobii hatched. The number of cocoons was reduced relative to controls (F=20.65, df=1, 16, P<0.001) (fig. 3b). Day of application had no effect, and there was no interaction between treatment (nematode or water) and day (F=0.65, df=3, 16, P>0.05 and F=1.28, df=3, 16, P>0.05, respectively). Some adults emerged in all treatments in which cocoons were formed (fig. 3c); overall, adults emerged from 16% of cocoons formed in the nematode treatments (total cocoons=76) and from 54% of cocoons formed in the water controls (total cocoons=239). The number of B. hylobii that emerged per nematode-treated weevil (less than one B. hylobii in each treatment) was significantly reduced relative to water controls (1–3 B. hylobii) (H=16.8, df=1, P<0.001).
Susceptibility of B. hylobii cocoons and adults to infection by nematodes
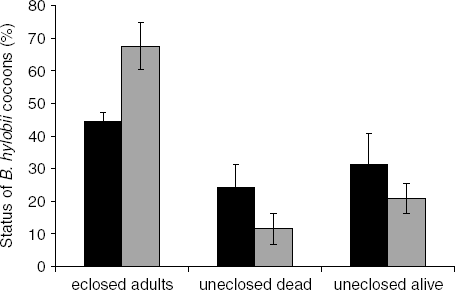
More than half of the cocoons that were treated with water only (controls) eclosed in this experiment. There was a lower hatch rate in the nematode treatment relative to the water control (44.5±2.9 and 67.5±7.2%, respectively) and the difference was significant at the 10% level (t=2.97, df=2, P=0.10; fig. 5). A similar percentage of nematode-treated and control cocoons held dead insects (24±7.1 and 12±4.8%) (t=1.54, df=3, P>0.05); however, 33% (8/24) of the dead B. hylobii in the nematode-treated cocoons harboured nematodes while none of the control cocoons did. A high proportion of the adults that were recovered in the nematode treatment were dead (80%; 39/49), while all of those in the water treatment were still alive (73/73). A sample of dead adults from the nematode treatment was dissected; 32 out of 33 harboured nematodes.
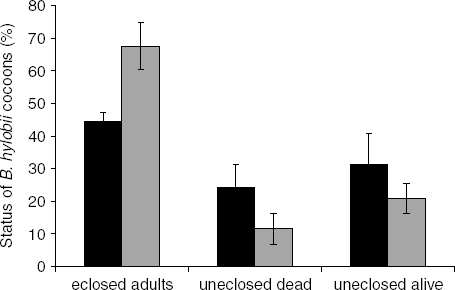
Fig. 5. Fate of Bracon hylobii cocoons exposed to Heterorhabditis downesi nematodes as a percentage of cocoons. Mean (±SE) of three experiments with 5–7 replications (parasitoid cocoon clutches) per experiment (■, H. downesi; ![]() , Control).
, Control).
Discussion
It is clear from the experiments reported here that H. downesi has an impact on almost all of the life stages of B. hylobii, both through competition for host resources and by direct infection of the parasitoid, an example of intraguild predation. Nematodes and their associated bacteria rapidly colonize the host, dramatically altering its quality for other organisms such as B. hylobii. This alteration of the resource would effectively starve those parasitoids that are not directly killed by nematodes. Premature host death is the most common consequence of a host-parasitoid-pathogen interaction, frequently resulting in death of the parasitoid (Brooks, Reference Brooks, Beckage, Thompson and Federici1993) and is the most likely cause of parasitoid failure in the present experiments when nematodes were applied shortly after hatch. When nematodes were applied one day after hatch, no parasitoid larvae survived to cocoon stage. Parasitoid death due to premature host death has been reported in several laboratory studies with nematodes, mostly S. carpocapsae. This is particularly clear in cases where the parasitoid itself is not infected by the nematodes, as for the endoparasitoid braconids Glyptapanteles militaris (Kaya, Reference Kaya1978a), Apanteles ultor (Triggiani, Reference Triggiani1985) and Dacnusa sibirica (Head et al., Reference Head, Palmer and Walters2003), and the tachinid Myxexoristops sp. (Mracek & Spitzer, Reference Mracek and Spitzer1983). Hatched larvae of ectoparasitoids, such as B. hylobii in the present study, are at risk of being killed directly by the nematodes, as well as facing death by starvation. Most of the studies on the effect of nematodes on parasitoids have involved endoparasitoids, which are shown to be susceptible to nematode infection between emerging from the host and completing the cocoon (Kaya, Reference Kaya1978a,Reference Kayab; Kaya & Hotchkin, Reference Kaya and Hotchkin1981; Triggiani, Reference Triggiani1985). Ectoparasitoids, such as B. hylobii, however, are accessible to the nematodes throughout their development.
When nematodes were applied three, five or seven days after parasitoid hatch, a proportion of B. hylobii formed cocoons. As the oviposition period lasted one day and two days elapsed between oviposition and hatch, the actual time that elapsed from deposition of the first eggs to nematode application may have been up to six, eight and ten days in these three treatments, respectively. In B. hylobii, cocoon formation usually starts between six and eight days after oviposition at 20°C and takes some days to complete (Henry, Reference Henry1995). Thus, those cocoons that were formed may have been initiated before the nematodes were applied. Adult parasitoids emerged from some of the cocoons that were formed in the presence of nematodes. In the separate cocoon experiment, it was demonstrated that, once B. hylobii larvae have matured fully and spun cocoons, they are relatively but not totally safe from nematode infection. Similar results were reported for fully-formed cocoons of various braconid and ichneumonid parasitoids of armyworm and codling moth (Kaya, Reference Kaya1978a; Kaya & Hotchkin, Reference Kaya and Hotchkin1981; Lacey et al., Reference Lacey, Unruh and Headrick2003), and Kaya & Hotchkin (Reference Kaya and Hotchkin1981) demonstrated that the resistance of insects to infection in intact cocoons is due to the presence of a pore-free layer of silk within the cocoon that acts as a mechanical barrier to nematodes. Although cocooned parasitoid larvae may be protected to some extent from EPN, the presence of the cocoon does not necessarily confer complete resistance (Lacey et al., Reference Lacey, Unruh and Headrick2003). As the eclosion rate of B. hylobii cocoons formed in the presence of nematodes was significantly lower than that of the controls, it appears that a proportion of these cocooned parasitoids may have been infected by nematodes that entered the cocoon before it was complete, or nematodes penetrated the cocoon after it was fully formed. It is likely that the cocoons are permeable to nematodes until sufficient silk has been laid down to form a complete barrier, which would take at least a day or two. Alternatively, nematodes adhering to a parasitoid larva may have become enveloped with it in the cocoon as it was spun, and the parasitoid could thus become infected after cocoon formation (Kaya, Reference Kaya1978b). Nearly all the adult B. hylobii became infected with nematodes when they were obliged to migrate through heavily nematode-infested substrate after eclosion, even though they had the opportunity to escape from this infested substrate on reaching the surface. Nematodes pose a potential threat to adult B. hylobii either at emergence or when females return to oviposit on a host.
Parasitoids frequently fail to discriminate between healthy hosts and those infected with pathogens, including viruses, protozoa and bacteria (reviewed by Brooks, Reference Brooks, Beckage, Thompson and Federici1993), although avoidance of oviposition on nematode-infected hosts has been reported. The eulophid parasitoids, D. begini and D. isaea, tended to avoid ovipositing on nematode-infected leafminer larvae (Sher et al., Reference Sher, Parrella and Kaya2000; Head et al., Reference Head, Palmer and Walters2003); and two codling moth parasitoids, M. ridibundus and L. caudatus, discriminated between healthy and infected live hosts in choice tests (Lacey et al., Reference Lacey, Unruh and Headrick2003). In our experiments, B. hylobii rejected either all nematode-infected insects (experiment 1) or those that were closer to nematode-induced death (experiment 2), showing that B. hylobii can discriminate and avoid ovipositing on hosts where the risk of offspring survival is low. At 20°C, entomopathogenic nematodes are expected to take two or more days to kill their host, although there is evidence that host allelochemistry is sufficiently altered within eight hours of infection by steinernematids to deter conspecific nematodes (Glazer, Reference Glazer1997). At the time of death, the insect is filled with the nematodes' bacterial symbiont (Photorhabdus spp. or Xenorhabdus spp.). Bacterial activity in nematode-infected hosts has also been implicated in repellence of other organisms (e.g. ants: Zhou et al., Reference Zhou, Kaya, Heungens and Goodrich-Blair2002). In experiment 2, B. hylobii tended to reject weevils that had been infected with nematodes from 12 h post exposure. However, not all infected insects were rejected; those that had a more advanced pathology (death occurred within two days of infection) were more likely to be rejected. Those with a less advanced pathology (perhaps those that had been invaded by fewer nematodes or mounted a more successful immune response) either were unaltered in chemistry or behaviour, or B. hylobii did not recognize or respond to such alterations. The ichneumonid M. ridibundus avoided parasitizing nematode-infected codling moth larvae within the same time period as B. hylobii in experiment 2 (12 h after exposure), although a longer post-infection period was required before L. caudatus exhibited avoidance (Lacey et al., Reference Lacey, Unruh and Headrick2003). As H. abietis are concealed, it is likely that vibrations are more important than chemical cues in host location and/or acceptance (Meyhofer & Casas, Reference Meyhofer and Casas1999). Nematode-infected insects typically become lethargic prior to death; therefore, the parasitoid might reject an infected insect either due to its altered chemistry or its lack of movement.
Adjustment of clutch size in response to the probability of reduced larval survival due to competition with other parasitoids has been addressed by numerous authors (reviewed by Godfray et al., Reference Godfray, Partridge and Harvey1991; Pexton & Mayhew, Reference Pexton and Mayhew2005) and also has been investigated for parasitoids laying on pathogen-infected hosts. In a no-choice experiment, the bethylid Cephalonomia tarsalis laid fewer eggs per host on Beauveria bassiana-infected grain beetles compared to healthy beetles (Lord, Reference Lord2001). Braconids have been shown to reduce clutch size on virus and microsporidium-infected hosts (Kyei-Poku & Kunimi, Reference Kyei-Poku and Kunimi1997; Hoch et al., Reference Hoch, Schopf and Maddox2000; Nguyen et al., Reference Nguyen, Kakai, Takatsuka, Okuno, Ishii and Kunimi2005). In our experiments, B. hylobii females did not alter their clutch size in response to nematode infection. A female parasitoid either did not lay or laid a full clutch. This would seem to indicate that B. hylobii do not respond to incrementally worsening conditions but reject once a stimulus threshold is reached. The percentage of B. hylobii ovipositing was reduced in the presence of nematode-infected H. abietis cadavers, indicating that cues associated with nematode infection may deter parasitoids from ovipositing even on healthy hosts within a patch. This result was seen in choice experiment 1 but not in choice experiment 2 where the weevils had been more recently infected and most were alive at the end of the choice test. This suggests that a cue threshold must be reached in order for parasitoids to exhibit avoidance. In these assays, healthy and infected weevils were confined in a closed container where volatile concentrations would build up. It is unclear whether volatile levels in the soil around an EPN infected insect would be high enough to deter B. hylobii from ovipositing on nearby healthy insects.
In contrast to the behaviour of female parasitoids, infective juveniles of H. downesi accepted weevil hosts that had been parasitized by B. hylobii up to seven days previously and infected both the parasitized weevils and live B. hylobii larvae. Bracon hylobii venom contains numerous insecticidal toxins (Quistad et al., Reference Quistad, Nguyen, Bernasconi and Leisy1994); and, although the venom paralyzes the insect host, these toxins clearly do not make paralyzed/dead insects unattractive to EPN. Similarly, S. carpocapsae was not deterred from leafminer larvae paralyzed or parasitized by D. begini (Sher et al., Reference Sher, Parrella and Kaya2000). Even dead insects are readily invaded by EPN and are capable of supporting nematode reproduction (Pye & Burman, Reference Pye and Burman1978; San-Blas & Gowen, Reference San-Blas and Gowen2008). A proposed benefit derived from invading dead insects is that nematodes do not have to fight the insect's immune response, so the probability of EPN survival might be increased (San-Blas & Gowen, Reference San-Blas and Gowen2008). While the quality and/or quantity of the weevil larvae available to the EPN may deteriorate over time, B. hylobii themselves are more likely to support nematode development and reproduction as they increase in size. Unlike parasitoids which tend to be highly specific, laboratory studies have shown that EPN species can infect a wide range of insect species (>200 species from several orders) (Poinar, Reference Poinar and Franz1986; Peters, Reference Peters1996). Many of the cues used by EPN in host acceptance are non-specific (CO2, vibrations, temperature) (Wright & Perry, Reference Wright, Perry and Gaugler2002), so cues would be emitted by both B. hylobii and weevils. It, therefore, was not unexpected that EPN would invade the easily accessible B. hylobii larvae.
When insect cadavers that appear to have been infected by nematodes (based on characteristic colour changes) are dissected, nematodes are not always found (e.g. Unruh & Lacey, Reference Unruh and Lacey2001). In our experiment, smaller B. hylobii larvae were less likely to contain nematodes than larger parasitoid larvae. The failure to recover nematodes indicates either that B. hylobii death was caused by ingestion of the nematodes bacterial symbiont (Photorhabdus) alone, or invading IJs died and disintegrated after releasing their bacteria. In a related experiment, when B. hylobii larvae were allowed to feed on weevil larvae previously infected by H. downesi, none of the developing B. hylobii showed signs of infection by Photorhabdus (A. Everard, unpublished data). It, therefore, is likely that invading nematodes were killed by the insect's immune response following penetration (Dowds & Peters, Reference Dowds, Peters and Gaugler2002). It is possible that more IJs invaded the larger B. hylobii larvae, thus overcoming the insects' immune response and resulting in survival of invading IJs.
These results demonstrate that both larvae and adult B. hylobii are susceptible to infection by entomopathogenic nematodes. The small number of adult B. hylobii emerging per nematode-treated weevil, coupled with the susceptibility of adult B. hylobii to infection by EPN when forced to migrate through EPN infested soil, indicate that EPN have the potential to reduce B. hylobii populations. The conditions under which the experiments were conducted (insects exposed to high doses of nematodes on artificial substrates) provide ideal conditions for the nematodes and so may overestimate the extent to which insect populations would be affected in the field. We have conducted field trials EPN against H. abietis over seven years (Dillon et al., Reference Dillon, Ward, Downes and Griffin2006, Reference Dillon, Downes, Ward and Griffin2007, Reference Dillon, Moore, Downes and Griffin2008a). Our results suggest that, in the short-term at least, the impact of EPN on B. hylobii is minimal. Assessment of the trials included destruction of stumps, which allowed the proportion of weevils parasitized by applied EPN and by native B. hylobii to be assessed, and also any parasitism of B. hylobii larvae by EPN. The percentage of weevils parasitized by B. hylobii on a site varied from 1 to 22%, but was usually less than 3%. Bracon hylobii infected by EPN were only rarely observed (A. Dillon, unpublished data). There was no evidence of widespread interference between the two agents; percentage parasitism of weevils by B. hylobii was not reduced in EPN-treated stumps, and the percentage of weevils parasitized by either EPN or B. hylobii was almost twice as high in H. downesi-treated stumps as in control (untreated) stumps, where only B. hylobii parasitism was recorded. However, under certain stump conditions, there was evidence of competition between EPN and B. hylobii (Dillon et al., Reference Dillon, Moore, Downes and Griffin2008a). The results obtained by stump destruction represent a snapshot in time four weeks after EPN were applied and can only indicate an effect on the parasitoid life stages present at that time (primarily cocoons, but also some parasitoid larvae). Field trial data did not include an assessment of the viability of the B. hylobii cocoons or mortality of adult B. hylobii. It is also possible that small early instar B. hylobii larvae infected by EPN disintegrated following nematode invasion and, therefore, were not recovered during stump assessment. To ascertain whether B. hylobii populations are impacted in the longer term would require monitoring populations on nematode-treated and untreated sites in years subsequent to treatment.
However, even if intraguild predation of the parasitoid by nematodes is rare, successful application of nematodes to H. abietis larvae in a biological control programme could have detrimental effects on naturally occurring populations of B. hylobii as a result of competition reducing resource availability either by exploitation or interference. Naturally occurring epizootics or extensive field applications of pathogens (bacteria, viruses, fungi) have been shown to reduce population levels of various parasitoids, though rarely to completely eliminate them (Brooks, Reference Brooks, Beckage, Thompson and Federici1993). There are rather few field studies on the impact of EPN on parasitoids. Field application of Steinernema feltiae strongly affected one ichneumonid parasitoid (Xenoschesis fulvipes) of the spruce sawfly but not another (Ctenopelma lucifer) (Battisti, Reference Battisti1994). It was not clear whether this difference was due to differential susceptibility of the parasitoids or to the fact that they parasitized different stages of the host.
Hochberg et al. (Reference Hochberg, Hassell and May1990) developed a model of a host-parasitoid-pathogen interaction and concluded that biological control involving parasites and pathogens may be a sound strategy under certain circumstances. There is empirical evidence for this from systems involving nematodes; Georgis (Reference Georgis1981; cited by Georgis & Hague, Reference Georgis and Hague1982) reported that S. carpocapsae, used jointly with the ichneumonid parasitoid Olesicampe monticola, gave better control of larch sawfly than the parasitoid alone; and, in our studies, parasitism by EPN and B. hylobii was higher in nematode-treated stumps than in untreated stumps, where parasitism was due to only B. hylobii (Dillon et al., Reference Dillon, Moore, Downes and Griffin2008a). Co-existence of competing natural enemies may occur if there is temporal or spatial resource partitioning (MacArthur, Reference MacArthur1972). The majority of B. hylobii parasitized weevils and applied EPN occur close to the bole of the stump (Henry, Reference Henry1995; Dillon et al., Reference Dillon, Rolston, Meade, Downes and Griffin2008b), so there is the potential for severe competition for hosts in this region of the stump. Co-existence may be possible if B. hylobii can develop in nematode-free space. That weevils parasitized by B. hylobii have been recovered out to a distance of 140 cm (Henry, Reference Henry1995) suggests that such a refuge does exist.
Acknowledgements
Research was funded by the National Council for Forest Research and Development (COFORD) through the Irish National Development Plan 2001–2006 and by the European Union INTERREG IIIA administered through the Welsh European Funding Office on behalf of the Welsh Assembly Government.