Introduction
The poultry red mite, Dermanyssus gallinae, is a haematophagous mite from the order Mesostigmata. Whilst the poultry red mite has a broad host range and has been found associated with gerbils and horses, its main association is with birds (Lucky et al., Reference Lucky, Sayers, Argus and Lucky2001; Mignon & Losson, Reference Mignon and Losson2008). D. gallinae is considered the most significant ectoparasite of egg-laying poultry in Europe (Chauve, Reference Chauve1998) although it has a worldwide distribution. Deleterious effects on the avian host can include mild or fatal anaemia, loss in egg production and elevated stress levels (Kowalski et al., Reference Kowalski, Sokół and Jedlińska-Krakowska2006). Substantial D. gallinae infestations have been reported to impact upon poultry workers by causing dermatitis (Rosen et al., Reference Rosen, Yeruham and Braverman2002). D. gallinae is currently controlled using a regime of hygiene and application of synthetic chemicals, but reports of increasing acaricide resistance suggest alternative approaches are needed. New approaches under investigation by our group include vaccines (Arkle et al., Reference Arkle, Harrington, de Luna, George, Guy and Sparagano2008) and plant-derived products (George et al., Reference George, Callaghan, Guy and Sparagano2008), approaches which often require the maintenance of populations of D. gallinae under laboratory conditions.
Populations of D. gallinae can be maintained in the laboratory by allowing mites to feed periodically on live hens (Tucci, Reference Tucci1997), a method which has inherent welfare disadvantages. Depending upon the number of mites feeding, the bird can experience stress and general discomfort during the procedure. Kilpinen et al. (Reference Kilpinen, Roepstorff, Permin, Norgaard-Nielsen, Lawson and Simonsen2005) infested groups of laying hens with D. gallinae and observed both their behavior and health in comparison to non-infested controls. The study found that following introduction of D. gallinae into the floor pen on two occasions, the mite populations ranged from approximately 3000 to 12,000 mites per 100 ml litter at the peak of infestation, equating to approximately 200–800 mites per bird. Significantly increased self-grooming behaviour was noted in infested birds, as well as increased feather pecking, reduced packed cell volume (PCV) and mortality due to anaemia. Instead of using a live host, a number of authors have shown that D. gallinae can also be fed using chicken skin under artificial conditions (Kirkwood, Reference Kirkwood1968; Zeman, Reference Zeman1988; Bruneau et al., Reference Bruneau, Dernburg, Chauve and Zenner2001; McDevitt et al., Reference McDevitt, Nisbet and Huntley2006). However, the conditions to feed D. gallinae in the laboratory are quite specific. Despite being cosmopolitan in its choice of host in vivo, D. gallinae typically will only feed under in vitro conditions when the feeding model consists of day-old chick skin. The use of day-old chick skin clearly has an ethical and welfare disadvantage in that it involves the euthanasia of young animals. A reduction in the number of animals used for either live feeding or provision of skin can contribute to the overall aim of national guidelines on reduction and refinement of procedures used on experimental animals. Artificial feeding models are attractive for a second reason, namely they can reduce intra-assay variability, especially where variability due to individual host-parasite relationships can have an effect (Kröber & Guerin, Reference Kröber and Guerin2007a). Such lower-cost models can also allow for an increased number of replicates for a given treatment, thereby increasing the power of statistical analyses. Artificial feeding models have a wide range of applications, including the investigation of arthropod transmitted pathogens, identification of biologically active arthropod molecules and testing of antibodies against arthropod antigens and insecticide/acaricide testing (Kröber & Guerin, Reference Kröber and Guerin2007a). A range of other methods, such as wool soaked with serum, capillary tubes and silicone membranes, have been successfully used in in vitro feeding systems of other arthropod ectoparasites (Kocan et al., Reference Kocan, Yoshioka, Sonenshine, de la Fuente, Ceraul, Blouin and Almazán2005; Thind & Ford, Reference Thind and Ford2007) whilst synthetic membranes have been used in tick feeding models to test acaricide efficacy against Ixodes ricinus (Kröber & Guerin, Reference Kröber and Guerin2007b).
However, the use of synthetic membranes to feed D. gallinae has met with limited success. Zeman (Reference Zeman1988) and Bruneau et al. (Reference Bruneau, Dernburg, Chauve and Zenner2001) both reported that D. gallinae would not feed on Parafilm ‘M’ or a synthetic skin substitute. Zeman (Reference Zeman1988) identified the role of kairomones (a sub-class of pheromones that benefit the receiver) in skin and feathers of poultry as an attractant to D. gallinae and, consequently, produced the only report of successfully feeding D. gallinae via synthetic membranes.
Therefore, the aim of this experiment was to investigate the use of alternative synthetic membranes and capillary tubes to feed D. gallinae in vitro by combining the principles of skin and feather compound extraction of Zeman (Reference Zeman1988) with the production of silicon membranes described by Kröber & Guerin (Reference Kröber and Guerin2007b).
Material and methods
Experimental design
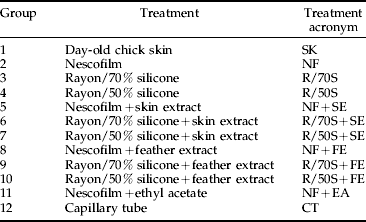
The experiment comprised 12 experimental treatment groups as shown in table 1 and was undertaken in two blocks. Treatments consisted of a feeding chamber with one of 12 different membranes: day-old chick skin (SK); capillary tube (CT); or combinations of Nescofilm (NF) or silicone and rayon membranes of differing silicone concentrations (R/S) and ethyl acetate either alone or in combination with skin or feather extract (EA, SE and FE, respectively). Five replicates per block were used in the first block and four replicates were used in the second block (due to a reduced number of available unfed D. gallinae) with the exception of treatments 11 and 12 where five replicates were used in block two only.
Table 1. Experimental treatment groups
Manufacture of synthetic membranes and preparation of skin
Synthetic membranes were constructed from Nescofilm® (Nippon Shoji Kaisha Ltd, Osaka, Japan), a sealing film for use in laboratories, or a combination of rayon and silicone sealant. Membranes using Nescofilm (NF) were made by stretching the film as thin as possible without tearing it. Silicone membranes (R/S) were made following an adaption of the method of Kröber & Guerin (Reference Kröber and Guerin2007b). Silicone sealant Ceresit 10B (Henkel Loctite Adhesives Ltd, Winsford, UK) with a shore A hardness of 25° (a measure of the relative hardness of an elastic material; the lower the value, the softer the material) was mixed with 30% DC 200 silicone oil (Sigma-Aldrich Ltd, Poole, UK) in order to achieve either a 50:50 silicone:silicone oil mix or 70:30 silicone:silicone oil mix. Hexane was then added at a rate of 1.5 g per 1 g silicone mixture to render the mixture temporarily more fluid to assist application. Pieces of rayon (regenerated cellulose; Lens Saver® lens cloth, Hear Saver Ltd, Stoney Creek, Canada), approximately 70×60 mm, were laid on a layer of plastic film, and the silicone mixture was applied using a silicone spatula. The membranes were allowed to air dry for a minimum of seven days before use. All membranes were examined under 10× magnification to ensure there were no holes in them prior to use. Day-old skin was harvested from euthanased day-old layer chicks, which was plucked, scraped of fat and stored frozen at −20°C. Skin was thawed prior to use.
Skin and feather extracts
Feather and skin extracts for use with the membranes were prepared following the method of Zeman (Reference Zeman1988). Skin and feathers were collected from 18 week-old laying hens euthanased by cervical dislocation. Skin (5.5 g) was finely chopped and added to 11 ml 99.5% ethyl acetate. The mixture was placed at 2–8°C for five days, and then the aqueous phase was removed and stored at −20°C and the skin was discarded. Approximately 2 g of feathers were finely chopped and added to 10 ml 99.5% ethyl acetate. The sample was allowed to sit at room temperature for 30 min and then spun at 13,000×g for 15 min. The supernatant was removed and stored, and the feathers were spun again for 15 min at 13,000×g. The supernatant was removed and added to the first supernatant sample collected. The supernatant was stored at −20°C and the feathers were discarded. Where appropriate, 10 μl of feather or skin extracts were added to membranes of groups 5–10. Group 11 received 10 μl ethyl acetate only. Following the addition of ethyl acetate or extract, membranes were air dried for 45 min before use in the chambers.
Capillary tube
Blood (see subsequent section) was drawn up into heparinised capillary tubes (Hawksley & Sons Ltd, Lancing, England), and the tube was sealed at one end using Cristaseal clay (Hawksley & Sons Ltd, Lancing, England).
Source of D. gallinae
D. gallinae were obtained from a commercial poultry farm and kept at 22°C in the dark in flat-bottomed tissue flasks sealed with a fine gauze for 7–10 days to allow digestion of the blood meal. Mites were then kept at 4°C in the dark for a minimum of four weeks to ensure that they were starved.
Source of blood
Nine laying hens (42 days old) with no known exposure to D. gallinae were euthanased by cervical dislocation, and blood was collected by cardiac puncture into glass vacutainers containing 200 units sodium heparin (Sigma-Aldrich Ltd, Poole, UK). Blood was stored at 2–8°C and used within two days of collection. Prior to use in the feeding model, blood was allowed to warm to room temperature and the blood samples pooled to provide the required volume of blood for testing synthetic membranes on that occasion.
Design and assembly of feeding chamber
The feeding apparatus used has been described previously by Harrington et al. (Reference Harrington, Mohi El Din, Guy, Robinson and Sparagano2009). Briefly, it comprised a clear glass vial with a modified silicon/poly(tetrafluoroethene) PTFE ring (a disc, with the centre cross cut removed) placed inside a snap ring plastic cap with a hole in the centre. The membrane to be tested was fitted to the ring, cut to size and placed between the ring and cap. A 0.5-ml tube with the end removed was secured to the top of the lid using modeling clay to act as a blood reservoir. For day-old chick skin, the skin was stretched across the ring such that the outer surface of the skin was facing into the vial. Capillary tubes were used in the feeding chamber by using unmodified silicon/PTFE discs; the tube was pushed through the cross cut and held in place using modeling clay. As described earlier, synthetic membranes were cut to size and placed in the chamber.
Unfed mites (20 females) were collected into the vial and the vial sealed with the ring/membrane/cap. The reservoir was filled with 200 μl fresh heparinised blood. Mite chambers were kept in an incubator (WTB Binder, Tuttlingen, Germany) in the dark at 37°C and 75–85% RH. Mites were examined 17 h after being allowed to feed, and the number of mites which had fed was observed. Mites were defined to have fed if they were engorged and/or had changed colour from pale grey/brown to bright red (McDevitt et al., Reference McDevitt, Nisbet and Huntley2006).
Statistical analysis
To evaluate feeding rates on the synthetic membranes, data were transformed to conform to a normal distribution and analysed by analysis of variance (ANOVA) using the general linear model (GLM) command fitting treatment and treatment as factors. Tukey's t-test was used for pairwise comparisons between treatments. Treatment 12 (CT) was excluded from statistical analysis since no mites fed in this group and inclusion of the group created a highly skewed data distribution. Statistical significance was declared at P⩽0.05. Data are presented as arithmetic mean±standard error (SE) and the F statistic is shown. The total number of mites in each chamber was the denominator in all calculations of the mean. All statistical analyses were performed using Minitab 15.1.2 for Windows (Minitab Inc., College, USA).
Results
Assessment of feeding rate
No mites fed on blood presented by CT. In the first block, feeding rates in all treatments ranged from 0.0 to 33.8%, whilst in the second block feeding rates ranged from 0.0 to 45.1% (table 2). The highest feeding rates were recorded for SK.
Table 2. Total number of mites alive and fed (F), alive and unfed (UF), dead and fed (DF) and dead unfed (DUF) for each experiment and mean percentage (±SE) of fed mites in each treatment for both blocks and the mean of blocks 1 and 2 (except treatment 11).

Within a column, values with different superscripts are significantly different at P⩽0.05.
* Mean percentage of total fed mites and total number of fed mites differ due to rounding errors from means of replicates. ** Except treatment 11, n=100.
Overall, proportion of fed mites was highest in the treatment using SK, where 38.8% mites fed, whilst the use of NF+SE resulted in the numerically highest percentage of mites feeding compared to all other groups using synthetic membranes, 32.3% (table 2). The membranes R/70S resulted in the lowest numerical proportion of fed mites on any of the synthetic membranes with just 5.3% of mites feeding. There was a highly significant effect of treatment upon the mean proportion of fed mites (F10,79=4.38, P<0.001). A significantly higher proportion (P<0.01) of mites fed on SK than R/70S, R/50S, R/50S+SE and R/70+FE. The proportion of fed mites was significantly higher (P<0.05) on NF+SE than R/70S, R/50S and R/50S+SE.
There was no significant effect of block or extract type when all proportion of fed mites on all synthetic membranes were compared. Mean feeding rate of both experiments for all synthetic membranes without extract, all synthetic membranes with SE and all synthetic membranes with FE was 8.8, 19.2 and 11.8%, respectively. Similarly, when data for silicone-based membranes only (R/70S, R/50S) were analysed for effect of extract, there was no significant effect of block or extract. However, analysis of the effect of extract on the proportion of fed mites on NF membranes, showed that significantly more mites fed on NF+SE than NF alone or NF+FE (F2,23=7.39, P<0.05).
Discussion
This current study was designed to investigate the use of synthetic membranes for in vitro feeding of D. gallinae in comparison to using day-old chick skin. There is only one report in the literature of synthetic membranes being used successfully for in vitro D. gallinae feeding, and the use of silicone membranes has not been previously reported for D. gallinae. In the current study, the proportion of mites feeding on a synthetic membrane was not significantly different from that observed with skin.
Zeman (Reference Zeman1988) has previously demonstrated the importance of kairomones as a stimulus for D. gallinae to feed artificially although, when feeding on a live host, other factors, such as temperature and carbon dioxide, have been shown to play a role (Kilpinen Reference Kilpinen2001, Reference Kilpinen2005). Using fresh blood stabilized in 2% EDTA, Zeman (Reference Zeman1988) found that mites did not feed on Parafilm membranes unless they had been impregnated with a feather or skin extract. Bruneau et al. (Reference Bruneau, Dernburg, Chauve and Zenner2001) reported similar findings, where no mites fed on either of the synthetic membranes used in their study (Parafilm ‘M’ and a synthetic skin substitute, pansement hydrocolloïde 0.3 mm). In contrast, in the current study, D. gallinae fed on synthetic membranes that had not been impregnated with either skin or feather extract, although the feeding rates were relatively low, 5.3 to 12.4%. The use of ethyl acetate without skin or feather extract also appeared to have some attractant effect for mite feeding and led to increased feeding rate compared to Nescofilm alone. Mean feeding rates were higher for Nescofilm membranes+ethyl acetate than in all other synthetic membranes with the exception of Nescofilm+skin extract although, in all cases, the difference was not statistically significant. Zeman (Reference Zeman1988) did not report data on mite feeding rates on Parafilm+organic solvent only, and our data would suggest that whilst kairomones are important, a contributory factor to their effectiveness in vitro is the solvent itself.
Following the impregnation of membranes with feather or skin extract, feeding rates increased in some but not all treatments. Nescofilm impregnated with skin extract resulted in the highest proportion of fed mites of all synthetic membranes (32.3%), which was not significantly different from that seen with skin (38.8%). This is in contrast to the observation reported by Zeman (Reference Zeman1988), where the highest proportion of mites was observed to feed on Parafilm ‘M’ impregnated with feather extract. In that study, 30.1% mites fed, which was 9.4% lower than that observed with two-week-old chick skin. In the current study, Nescofilm impregnated with feather extract achieved only a 9.9% mean feeding rate, although this was not significantly different from any other treatment. Similarly, when the effect of extract was examined irrespective of synthetic membrane type, membranes impregnated with skin extract had the highest proportion of mite feeding, 19.2%, compared to 8.8% without extract or 11.8% with feather extract. The difference in the effectiveness of feather and skin extract observed between the current study and that of Zeman (Reference Zeman1988) could be related to the extraction method used. In the current study, whilst the method for skin extraction was the same as used by Zeman (Reference Zeman1988), the method for feather extraction differed. Whilst Zeman (Reference Zeman1988) used a combination of extraction in ethyl acetate followed by evaporation of ethyl acetate under vacuum followed by solubilizing the residue in benzene, in the current study only ethyl acetate was used. However, effectiveness of extract is also likely to be related to properties of the membrane, including thickness. Kröber & Guerin (Reference Kröber and Guerin2007a) describe the importance of the thickness of the silicone membrane in feeding assays to account for both species and developmental stage differences in hypostome length, such as a softer and thinner (60–150 μm) silicone membrane for Ixodes ricinus, which have a shorter hypostome than Amblyomma hebraeum in ticks. Membrane thickness was not measured in the current study, but given that adult female D. gallinae are considerably smaller in size than adult female I. ricinus (1 mm and 2–3 mm, respectively), membrane thickness could obviously be a significant factor in mite feeding with smaller hypostomes than ticks. Similarly, the composition of the membrane is important to ensure it is soft enough for the mite's mouthparts to penetrate. The higher feeding rates observed with Nescofilm are most probably a result of the lower thickness of the membrane. Carroll et al. (Reference Carroll, Young and Bruce1992) successfully used sacs made of stretched Parafilm to feed the northern fowl mite, Ornithonyssus sylviarum, a mite similar in size to D. gallinae.
As mentioned previously, both biological and synthetic membranes have been used to present the blood meal to D. gallinae. In the current study, D. gallinae did not feed on blood-filled capillary tubes without membranes. The role of ticks in the transmission of Anaplasma marginale has been studied by adhering capillary tubes to the head of the tick, Dermacentor variabilis (Kocan et al., Reference Kocan, Yoshioka, Sonenshine, de la Fuente, Ceraul, Blouin and Almazán2005). This is impractical for D. gallinae since it is a short-term feeder, and the bore of a capillary tube is larger than the mite itself. However, it is possible that the provision of blood to mites using very fine needles or blunt cannula, could overcome the need for membranes, although the lack of the kairomone stimulus would still need to be addressed in an area of further research.
McDevitt et al. (Reference McDevitt, Nisbet and Huntley2006) suggested the importance of ‘conditioning’ D. gallinae to promote high feeding rates. They indicated that starving mites for seven days at 20°C following by a period of cooling at 1°C for 30 days resulted in 68–72% of mites feeding on blood (treated with the anticoagulant sodium heparin) through day-old chick skin, compared to a feeding rate of 24% in mites kept at 20±3°C (‘not conditioned’). In contrast, feeding rate on skin in the current study was 38.8%, despite using similar conditions for mite conditioning as described by McDevitt et al. (Reference McDevitt, Nisbet and Huntley2006). This suggests that there are additional factors which determine optimal feeding rates of mites in individual laboratories, perhaps due to factors other than just temperature, such as humidity or the nature of the storage vessels for the mites themselves.
Further studies are required to optimize synthetic membranes as successful substitutes to day-old chick skin in D. gallinae feeding models to encourage higher mite feeding rates. Statistically, there was no single treatment that resulted in significantly higher D. gallinae feeding rates than all others, despite large numerical differences. This is a problem of the artificial assay and an area of further development necessary to reduce the intra-assay variation in feeding rates observed in the current study. Bruneau et al. (Reference Bruneau, Dernburg, Chauve and Zenner2001) suggested that mites adapt to the in vitro feeding system following high mortality in the first generation. Whilst completion of the mite life cycle was not a parameter studied in the current experiment, maintenance of mite colonies adapted to synthetic membranes could reduce the intra-assay variation observed, although testing to ensure the laboratory mite population was still representative of the ‘wild’ population would be necessary.
Optimization of the assay should focus on improving liveability of mites in chambers, as well as refining membranes. However, this study does demonstrate that alternative membranes to Nescofilm or Parafilm are possible (with further development). Synthetic membranes or alternative methods of blood meal presentation should be an active area of D. gallinae research to address the ethical consideration of reducing animal use in research. Samples for use in skin and feather extracts can be sourced from commercial poultry slaughterhouses, thereby reducing the need to maintain and euthanase birds specifically for this purpose.
Conclusion
D. gallinae were observed to feed through synthetic membranes comprising either Nescoflim or a silicone sealant/silicone oil mixture and rayon. Feeding rates were highest in a membrane consisting of Nescofilm impregnated with skin extract, which gave a statistically similar feeding rate to that observed with day-old chick skin. However, optimization of the membrane properties and testing conditions is still required.
Acknowledgements
This work was supported by the Biological and Biotechnology Research Council. We would like to thank Dr Robert Shiel for help with data analysis.




