Introduction
The structure of termite colonies is based on a reproductive division of labor between reproductive and sterile individuals, in which the former monopolizes the reproduction inside the nests (Korb, Reference Korb2007). According to Wilson (Reference Wilson1985), this division of labor leads to a task specialization that induces a greater efficiency of the colony and therefore benefits the whole society. Termite colonies undergo three distinct growth stages: juvenile, mature, and senile, and only the mature ones produce alate reproductives, a sexual caste responsible for the dispersal of the species through the establishment of new colonies (Noirot, Reference Noirot, Krishna and Weesner1969). Society survival and its success during the growth stages depend on the performance of collective tasks, as well as self-regulatory mechanisms, such as caste ratio and development (Thorne, Reference Thorne1997; Chouvenc and Su, Reference Chouvenc and Su2014).
The subterranean termite Coptotermes gestroi is an important pest species, due to its ability to cause structural damage to infrastructure (Costa-Leonardo, Reference Costa-Leonardo2002). It is native to Southeast Asia and has been introduced into many countries due to anthropogenic activities (Jenkins et al., Reference Jenkins, Jones, Lee, Forschler, Chen, Lopez-Martinez, Gallagher, Brown, Neal, Thistleton and Kleinschmidt2007). Thus, C. gestroi has been recorded in tropical and subtropical regions, and at least 24 countries (Li et al., Reference Li, Fujisaki and Su2013). Moreover, human maritime activity has facilitated the arrival of this termite in Europe (Ghesini et al., Reference Ghesini, Puglia and Marini2011). Global warming and reproductive strategies of this termite species may also contribute to its expansion and success in invasive areas (Costa-Leonardo and Arab, Reference Costa-Leonardo and Arab2004; Robinet and Roques, Reference Robinet and Roques2010). However, since the introduction of C. gestroi into these regions, little progress has been made regarding its reproductive biology (Costa-Leonardo and Arab, Reference Costa-Leonardo and Arab2004).
C. gestroi exhibits a bifurcated caste differentiation, comprising an early divergence between the apterous and sexual lines. Second-instar larvae originate the sexual line, composed of six wing-budded nymphal instars (N1–N6), which ultimately differentiate into alate reproductives (Barsotti and Costa-Leonardo, Reference Barsotti and Costa-Leonardo2005). Social organization in termites is strongly influenced by caste differentiation, including the ability to develop neotenic reproductives. Within Rhinotermitidae, the occurrence of replacement reproductives seems to be a pattern, although their origin varies according to the genus (Myles, Reference Myles1999).
Reproductive sampling is quite difficult in field colonies of C. gestroi, since they display a cryptic habit and may have polycalic nests. Therefore, most sampled nests are satellites and do not contain reproductive castes (Lelis, Reference Lelis1999). A mature colony of C. gestroi may possess an imaginal physogastric queen with variable mass and egg-laying rate. Nevertheless, the development of neotenic reproductives has also been reported for this species, although their fertility ranges from non-functional individuals to physogastric queens (Lelis, Reference Lelis1999; Costa-Leonardo et al., Reference Costa-Leonardo, Arab and Casarin2004; Costa-Leonardo and Arab, Reference Costa-Leonardo and Arab2004; Chouvenc et al., Reference Chouvenc, Mullins and Su2015a). A suitable way to access the knowledge about termite reproductive biology is through the rearing and monitoring of laboratory colonies. Such a strategy has been used for sampling different-age reproductives and monitoring colony development in subterranean termites (Costa-Leonardo and Barsotti, Reference Costa-Leonardo and Barsotti1998, Reference Costa-Leonardo and Barsotti2001; Costa-Leonardo et al., Reference Costa-Leonardo, Arab and Casarin2004; Chouvenc et al., Reference Chouvenc, Mullins and Su2015a, Reference Chouvenc, Basille and Su2015b; Chouvenc, Reference Chouvenc2019).
In Southeast Brazil, buildings infested by C. gestroi were first reported in 1923; since then, this species has been an important structural and economic pest (Martins et al., Reference Martins, Fontes, Bueno and Martins2010). However, its reproductive biology has been poorly investigated (Laranjo et al., Reference Laranjo, Haifig and Costa-Leonardo2019), especially regarding nymph, alate, and neotenic differentiation, and ovarian development during the lifecycle of the females. Termite females possess ovaries constituted of panoistic ovarioles, which are found in primitive insect groups, and are characterized by the absence of specialized nurse cells (Büning, Reference Büning and Büning1994). Each ovariole is composed of an anterior terminal filament followed by a germarium, containing germinal cells, and a vitellarium, in which yolk deposition takes place (Grassé, Reference Grassé1982; Grandi, Reference Grandi1991; Morini and Costa-Leonardo, Reference Morini and Costa-Leonardo1992).
According to Raina et al. (Reference Raina, Park and Florane2003), queens of the congeneric Coptotermes formosanus went through a first oviposition cycle during the first 60–70 days. Such an oviposition is followed by a 4-month period of inactivity, in which the ovaries decrease in size with the absence of developing oocytes. In the congeneric species C. gestroi, however, information concerning ovary development in different-aged females is not well understood. Thus, the current study deals with a comparative analysis on the ovaries of C. gestroi females, as we hypothesize that their development and maturation are influenced by female age, reproductive status, and external factors such as colony population and presence of sexual partners. As a complementary information to the reproductive biology of C. gestroi, we discuss the likely origin and functionality of neotenics in this species.
Material and methods
Establishment of incipient colonies
Alate reproductives of C. gestroi (Wasmann, 1896) were collected during the swarming season in the city of Rio Claro, SP, Brazil (22° 23′ S, 47° 31′ W) and sexed under a Zeiss Stemi V6 stereomicroscope. Alate females and males were paired and placed in 6 cm Petri dishes containing decayed and moistened Pinus sp. sawdust. All incipient colonies were kept in the dark at 25 ± 3 °C.
Termites
We used only female individuals: fourth-instar nymphs, non-functional nymphoid neotenics, alates, and different-aged queens of C. gestroi collected in the city of Rio Claro, SP, Brazil. Nymphs were obtained from foraging sites using corrugated paper, while non-functional neotenic females were sampled from a field nest. Neotenics were identified according to their yellowish-pigmented body and sclerotized wing buds. Their non-functional classification was made due to the absence of spermatozoa in the spermatheca and mature terminal oocytes. Alate females were collected from swarming flights, whereas functional queens (80-d, 2- and 4-year-old) were obtained from incipient colonies (described above) kept under laboratory conditions at the São Paulo State University (UNESP), Rio Claro, SP, Brazil. According to their development, colonies were transferred to larger plastic chambers before sampling. A likely 10+-year-old queen, collected from a field nest, was also included in the experiment. The age of the latter queen was estimated based on its conspicuous physogastry.
Analysis of the ovaries in different-aged females of C. gestroi
Total mounting
Abdomens of nymphs, alate females, and 2-year-old queens were dissected under a Zeiss Stemi V6 stereomicroscope, and their ovaries were isolated. Then, they were individually placed on histological slides, stained with acetic orcein or methylene blue solutions (with exception of ovaries from 80-d-old queens), and photographed under Leica ICC50 photomicroscope and Zeiss Stemi V6 stereomicroscope. Three females of each age were selected, except for 2-year-old queens, for which four samples were dissected.
Histology
Abdomens of nymphs, alate females and queens were fixed in FAA (absolute alcohol, glacial acetic acid, 40% formaldehyde, in the ratio 3:1:1) or 4% paraformaldehyde for 24 h. At least three fourth-instar nymphs, three non-functional neotenics, six alates, and four 4-year-old queens were used. Posteriorly, the samples were dehydrated in a series of ethanol (70, 80, 90, and 95%), transferred to a Leica® historesin solution, and kept in the refrigerator for seven days. The abdomens were then embedded in special molds, which were filled with historesin (JB-4 Polaron Instruments/Bio Rad) plus catalyzer for polymerization. Sections of 2–4 μm thick were performed using a Leica RM 2145 microtome and stained with hematoxylin-eosin and toluidine blue. At least three non-functional neotenic females were fixed in FAA, included in paraffin and sectioned with 6–7 μm thickness and their sections stained according to the described above. All sections containing oocytes and the spermatheca were analyzed and photographed under Leica ICC50 photomicroscope, using the software LAS v4.0. Developmental stages of the oocytes were classified according to Grandi et al. (Reference Grandi, Barbieri and Colombo1988).
Oocyte morphometry
Aiming to evaluate the cross-sectional area of the terminal oocytes in alate females and functional queens, histological sections of the ovaries of alates and 4-year-old queens were photographed at a magnification of 400x. Posteriorly, terminal oocytes were outlined using the free-hand tool of the software ImageJ (available at http://imagej.nih.gov/ij/), which automatically provided the enclosed cross-sectional area of each oocyte. Data were compared using a t test with the software RStudio version 1.3.1093.
Results
Analysis of ovaries in different-aged females of C. gestroi
General morphology and location
Similar to other insect females, the reproductive apparatus of C. gestroi females consists of two ovaries and two lateral oviducts that merge in a common oviduct, which ends in a genital chamber. The paired ovaries of the females of this species are disposed dorsolaterally in the abdomen, each one composed of several panoistic ovarioles, long and twisted, which are surrounded by many tracheas. These ovarioles leave the lateral oviducts and extend to the insertion region, located in the dorsal wall of the thorax. This arrangement of ovarioles can be observed in a histological section of a non-functional neotenic female (fig. 1a). The ovarioles connected with the lateral oviduct are arranged in a linear sequence (fig. 1b). In each ovariole, oocytes develop and mature in an anteroposterior gradient, with terminal oocytes located at the base of these ovarioles (fig. 1c). The sampled non-functional neotenics were about 6 mm long, showed a non-physogastric and yellowish body, and originated from nymphs which were ascertained to the presence of wing buds. These nymphoid females had from light brown to brown eyes, many body hairs, and genital plates similar to those present in alate females (fig. 2a–c). Individuals were classified as non-functional due to the absence of stored spermatozoa and mature terminal oocytes.

Figure 1. Histological sections of a non-functional neotenic female of C. gestroi. (a) Longitudinal section of the body indicating the dorso-lateral disposition of the ovary inside the insect body. (b) Note the arrangement of ovarioles (arrows) leaving the lateral oviduct. (c) Detail of the structural organization of an ovariole (arrow) showing three different stages of previtellogenic oocytes (stages I, II and III). Stage III oocyte is ovoid and basal. lateral oviduct = lo, ov = ovary.

Figure 2. External morphology of non-functional neotenic females of C. gestroi. (a) View of a neotenic female with light brown eyes (arrowhead), mandibles with sclerotized edges and wingbuds in the thorax. (b) Another neotenic female with similar morphological features, but exhibiting brown eyes (arrowhead). (c) Detail of the wingbuds (arrows) in a neotenic female.
Total mounting and histology of the ovaries
Fourth-instar nymphs, neotenics, alate females, 80-d, and 2-year-old queens of C. gestroi showed variation in the number of ovarioles and oocyte development (fig. 3a–f, Table 1). Also, ovaries grow asymmetrically and therefore do not have the same number of ovarioles. Based on histological features of the ovaries, these structures show well-differentiated regions from the apex to the base: a terminal filament, devoid of germinal cells, which is followed by a short region occupied by oogonia and prefollicular cells, the germarium. Nevertheless, the most part of the ovariole is composed of cells arranged in a linear disposition, the vitellarium. The results showed remarkable morphological differences among the oocytes located in the different parts of the vitellarium, with three previtellogenic (I, II, and III) and three vitellogenic stages (IV, V, and VI). Such stages are described as follows: Stage I oocytes are characterized by the rounded shape, occurrence of a central germinal vesicle with a distinct nucleolus, and are enfolded by a single layer of flattened follicular cells. Stage II oocytes are larger than stage I, with ovoid shape, and are enfolded by cuboidal follicular cells (fig. 4a–c). Stage III oocytes are remarkably ovoid in shape, with a conspicuous germinal vesicle and central nucleolus. Moreover, the follicular epithelium is composed of columnar cells (fig. 4d).

Figure 3. Total mounting of the ovaries in different-aged females of C. gestroi. (a) Ovary of an alate female. Note the ovarioles and small curved spermatheca. (b) Ovaries of an 80d-old queen, showing developing oocytes. (c–e) Ovary from 2-year-old functional queens. Note the well-developed oocytes of the heaviest sampled queen (10.3 mg) in Fig. (d, f) Detail of a large terminal oocyte and immature oocytes from a 2-year-old functional queen. Arrows indicate terminal oocytes. Stain: acetic orcein. spe = spermatheca.
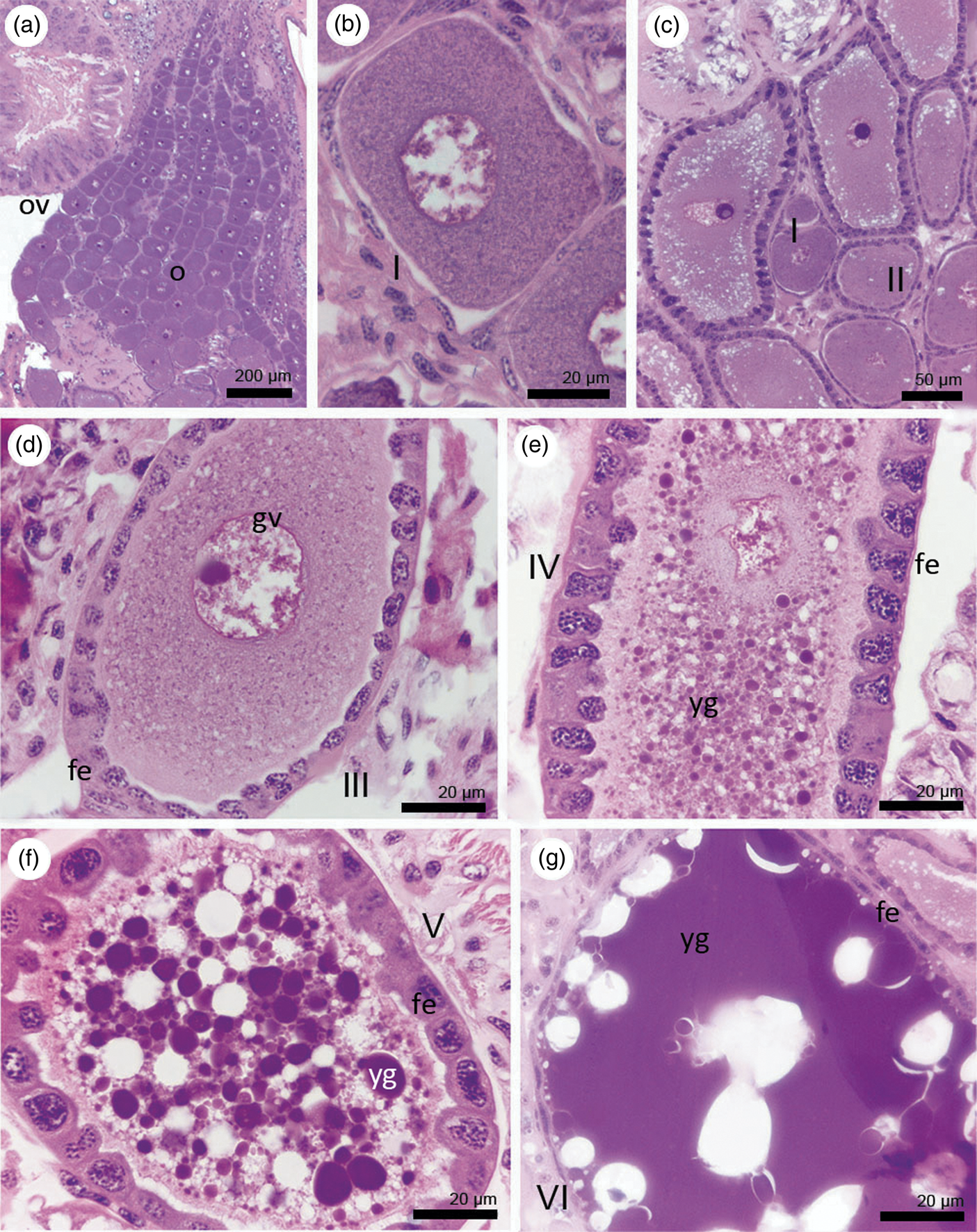
Figure 4. Oogenesis in functional queens of C. gestroi, detailing each developmental stage. (a) General view of the anterior part of ovarioles of a 4-year-old queen. (b) Detail of a previtellogenic oocyte (stage I) of a 10+-year-old queen. Note the flattened follicular epithelium. (c) Previtellogenic (stages I, II, and III) of a 4-year-old queen. (d) Detail of a previtellogenic oocyte of stage III with a conspicuous germinal vesicle and nucleolus. (e) Detail of a vitellogenic oocyte of stage IV with small yolk granules and columnar follicular epithelium. (ep). (f) Detail of a stage V vitellogenic oocyte with many yolk granules and cuboidal follicular epithelium. (g) Detail of a vitellogenic oocyte of stage VI (mature terminal oocyte) with flat follicular epithelium and richness of yolk. Stain: Hematoxylin-eosin. fe = follicular epithelium, gv = germinal vesicle, o = ovarioles, ov = ovary, yg = yolk granule.
Table 1. Number of ovarioles and developmental stages of oocytes in different-aged females of Coptotermes gestroi

± Occurrence or absence varied among individuals of the same caste.
Yolk deposition takes place only in stage IV oocytes, although in a small quantity. In this stage, the oocytes are ovoid and are also enfolded by columnar follicular cells (fig. 4e). Stage V oocytes display a greater amount of deposited yolk and epithelium of cuboidal follicular cells (fig. 4f). Stage VI oocytes, also called mature terminal oocytes, are the largest when compared to any other stage, characterized by a rich deposition of yolk granules and presence of flattened follicular cells enfolding them (fig. 4g). A detailed schematic representation of these developmental stages is shown in fig. 5. The cytoplasm of the previtellogenic oocytes (stages I, II, and III) are basophilic and, due to the yolk deposition, oocytes increase in size and their cytoplasm becomes gradually eosinophilic (stages IV, V, and VI).

Figure 5. Diagrammatic representation of the oogenesis in a functional queen of C. gestroi, with emphasis on the developmental stages of the oocytes. Arrows indicate the follicular epithelium. gv = germinal vesicle, yg = yolk granule.
Fourth-instar nymphs
In these individuals, there were from four to five ovarioles, all of them strongly compacted and composed only of previtellogenic oocytes, which probably belonged to stages I and II (fig. 6a–d).

Figure 6. Ovary development and oogenesis in fourth-instar nymphs of C. gestroi. (a) External view of a fourth-instar nymph. Note the wingbuds of the meso- and metathorax. (b) Total mounting of an ovary of fourth-instar nymph, highlighting the ovarioles. (c, d) Longitudinal sections of an ovary, composed only of previtellogenic oocytes. This structure is shrunk and oocytes are likely to belong to stages I and II. Stain: Hematoxylin-eosin. o = ovariole, oc = oocyte, ov = ovary.
Non-functional neotenics
In non-functional neotenics, ovaries were composed of about 10 ovarioles (fig. 1a–c). The oocyte development in some neotenics, however, resembles that from nymphs, since only previtellogenic oocytes were observed (fig. 1a–c). However, other non-functional neotenics showed oocytes at late developmental stages, but without reaching a terminal stage, similar to some alates (fig. 7a, b).

Figure 7. Comparative oogenesis in non-functional neotenics and alates of C. gestroi. (a, b) Ovary of a non-functional neotenic containing several ovarioles, which contains previtellogenic and vitellogenic oocytes. (c, d) Ovary and ovarioles of an alate female. Note that the terminal oocyte belongs to the stage V. Stain: Toluidine blue. fe = follicular epithelium, gv = germinal vesicle, lo = lateral oviduct, o = ovarioles, ov = ovary, tr = tracheae, V = stage V terminal oocyte, yg = yolk granule.
Alate females
Ovariole number ranged from four to five per ovary (fig. 3a). Oocyte maturation in alates varied among individuals. Some females exhibited only vitellogenic oocytes at stage V, similar to those observed in neotenics (fig. 7c, d), whereas other alates showed stage VI terminal oocytes (fig. 8a, b), resembling those from functional queens (fig. 8c, d).

Figure 8. Vitellogenesis in alates and functional queens (4-year old) of C. gestroi. (a, b) General view and detail of vitellogenic oocytes of an alate female, respectively. Note the occurrence of mature terminal oocytes (stage VI). (c, d) General view and detail of several mature terminal oocytes of a 4-year-old queen, respectively. Stain: Hematoxylin-eosin. fe = follicular epithelium, V = stage V oocyte, VI = stage VI terminal oocyte, yg = yolk granule.
80-d-old queens
During the period of 80 days after colony establishment, the population of the incipient colonies was composed only by larvae, although eggs were also observed. Ovaries of 80-d-old queens presented a conspicuous development, since an increasing number of ovarioles, ranging from five to six, and vitellogenic oocytes (including mature terminal ones) were reported (fig. 3b).
2-year-old queens
The highest number of ovarioles (n = 35) occurred among these queens, which also reflected their mass and colony population (fig. 3c–f). For example, fig. 3d shows the ovarioles containing several terminal oocytes from the heaviest sampled 2-year-old queen (10.3 mg), whose colony contained the highest number of individuals, with 592 workers. Additionally, larger terminal oocytes were observed in these queens, characterized by a remarkable deposition of the chorion (fig. 3f).
Histology of the spermatheca
The spermatheca from different-aged females contained a secretion in the spermathecal lumen of alates and non-functional neotenics, even though spermatozoa were absent (fig. 9a–d). However, spermatheca of 4-year-old queens was more developed than those of alates and neotenics, and contained numerous spherical spermatozoa in the lumen (fig. 9e, f).

Figure 9. Morphological features of the spermatheca among different-aged females of C. gestroi. (a, b). Alate. (c, d) Non-functional neotenic. Note the stored secretion in the lumen of the spermatheca in both females. (e, f) 4-year-old functional queen, showing a developed epithelium and the spermathecal lumen filled with rounded spermatozoa (black arrows). Stain: Hematoxylin-eosin. c = cuticle, ep = epithelium, l = lumen, Mt = Malpighian tubule, mu = musculature, s = secretion.
Oocyte morphometry
Based on measures performed using ImageJ, the cross-sectional area of terminal oocytes was significantly different between alates and functional queens (4-year-old individuals), being 43.52 ± 2.91 mm3 for alates and 73.95 ± 7.27 mm3 for queens (t = 3.8816, df = 6.567, P < 0.01, t test).
Discussion
For insects in general, oogenesis is a crucial process and is influenced by several factors such as nutrition and season (Wheeler, Reference Wheeler1996). Termites have some reproductive peculiarities, which contribute to the success of their colonies, such as queen fecundity associated with longevity and replacement of reproductives (Noirot, Reference Noirot and Engels1990). However, there is a lack of studies monitoring queens during their reproductive life inside the colonies, predominantly due to the cryptic habits of termites.
The current study followed several changes in the gonads, since the ovaries showed variation in the ovariole number and development among different-aged females of C. gestroi (Table 1). Similar to Reticulitermes speratus and Hodotermopsis sjostedti, in which nymphs do not possess vitellogenic oocytes (Maekawa et al., Reference Maekawa, Ishitani, Gotoh, Cornette and Miura2010; Oguchi et al., Reference Oguchi, Shimoji, Hayashi and Miura2016), fourth-instar nymphs of C. gestroi displayed shrunk ovaries composed only of previtellogenic oocytes. Oogenesis in non-functional neotenic females varied, as the ovaries of some sampling females (fig. 1a–c) resembled those of fourth-instar nymphs, while others went through vitellogenesis (fig. 7a, b). Similarly, their non-functionality was especially due to the absence of spermatozoa in the spermatheca and mature terminal oocytes. According to Albino and Costa-Leonardo (Reference Albino and Costa-Leonardo2011), nymphs are present all year round in nests of C. gestroi, and fourth-instar individuals are the most common sampled nymphs. Assuming the high occurrence of fourth-instar nymphs in C. gestroi and their similar ovarian development to those of some non-functional neotenics, we encourage future morphometric assessments to clarify which nymphal instars may differentiate into nymphoid neotenics in this species. Neotenic females are likely to quickly develop their reproductive system as an adaptation to breed inside the nest, since unlike alates, they do not invest in flight apparatus and do not swarm (Oguchi et al., Reference Oguchi, Shimoji, Hayashi and Miura2016). It would explain the higher number of ovarioles in our sampled neotenics when compared to nymphs, even though they were not reproducing. Non-functional neotenics which went through vitellogenesis may have differentiated from later instar nymphs, but it also deserves further investigations.
Alate females of C. gestroi displayed vitellogenic oocytes in later stages (Table 1). However, when compared to functional queens (4-year-old), their terminal oocytes were significantly smaller. It may be explained due to the presence of both stages V and VI oocytes as terminal ones among alate females, whereas only stage VI was identified as terminal one for functional queens. Furthermore, the presence of stage VI oocytes in alate females agrees with reports on R. speratus (Maekawa et al., Reference Maekawa, Ishitani, Gotoh, Cornette and Miura2010). On the other hand, oogenesis in R. flavipes and H. sjostedti went through vitellogenesis, but terminal mature oocytes were absent (Dean and Gold, Reference Dean and Gold2004; Oguchi et al., Reference Oguchi, Shimoji, Hayashi and Miura2016). It suggests that ovary activation and development vary among alates after they leave the natal nest, since this event stimulates female maturation (Greenberg and Stuart, Reference Greenberg and Stuart1979). In some cases, alates may express similar rates of vitellogenin when compared to young queens (Husseneder et al., Reference Husseneder, McGregor, Lang, Collier and Delatte2012), what would explain the occurrence of mature terminal oocytes.
During oogenesis, previtellogenic oocytes display features of increased metabolic activity. Moreover, they grow in size due to the incorporation of different compounds from the hemolymph through endocytic events (Grandi and Chicca, Reference Grandi and Chicca1999). Additionally, the follicular epithelium also undergoes morphological changes, influenced by the increasing number of organelles (Dossi and Cônsoli, Reference Dossi and Cônsoli2014), especially those related to synthesis of precursors of the tunica propria (Grandi and Chicca, Reference Grandi and Chicca1999). Throughout this period, vitellogenin synthesis is influenced by JH titers, which also induce the process of patency of the follicular cells, allowing the assimilation of vitellogenin and other compounds by the oocytes (Dossi and Cônsoli, Reference Dossi and Cônsoli2014; Santos et al., Reference Santos, Humann and Hartfelder2019). Moreover, follicular cells present high synthesis of products which may be incorporated into yolk or facilitate the endocytic uptake of vitellogenin (van Antwerpen and Law, Reference Van Antwerpen and Law1993; Grandi and Chicca, Reference Grandi and Chicca1999). This is a likely reason for the high development of the follicular epithelium, especially during stage IV of vitellogenesis observed in alates and functional queens of C. gestroi.
Termite queens always exhibited ovarioles containing vitellogenic oocytes, including mature terminal ones (Table 1), which showed epithelium composed of flattened follicular cells and reached the maximum size among all oocyte stages. The absence of degenerative ovarioles suggests an active vitellogenesis and therefore egg production. Dean and Gold (Reference Dean and Gold2004) also observed terminal mature oocytes only in 10-d-old queens and tertiary reproductives (neotenics) of Reticulitermes flavipes. It was suggested that the presence of a male and/or food quality promotes ovary maturation in females (Brent and Traniello, Reference Brent and Traniello2001a, Reference Brent and Traniello2002), which could also explain the development of these structures in queens of C. gestroi and R. flavipes after pairing. Moreover, workforce can enhance queen fecundity, since it performs colony labor and therefore increases the energy reserves of the reproductives (Brent and Traniello, Reference Brent and Traniello2001b). It agrees with our observations on colony population and ovary development in 2-yearr-old queens.
Late vitellogenesis is characterized by an intense protein synthesis and transport of compounds associated with the production of vitelline membrane and chorion deposition, a two-layered structure which covers the mature oocyte (Grandi, Reference Grandi1988, Reference Grandi1990; Grandi and Chicca, Reference Grandi and Chicca1999). It is important to highlight the ovary maturation in 80-d-old queens. According to Raina et al. (Reference Raina, Park and Florane2003), after the first oviposition cycle (about 60–70 days after colony foundation), queens of C. formosanus suffer an ovarian atrophy, with absence of pre- and vitellogenic oocytes. In our 80-d-old samples of C. gestroi, ovaries are still developed and composed of vitellogenic oocytes, including terminal ones, after the referred period. Thus, reproductive strategies and fecundity may vary between these congeneric species, and the likely reasons may include the environmental conditions and location where termites were sampled and paired.
Termite colonies and their reproductive dynamics rely on several conditions. Oogenesis in different-aged females of C. gestroi varies considerably, with ovaries developing progressively as females age and turn into swarming and functional reproductives. Among non-functional neotenics, ovarian development was not uniform, as some of them do not go through vitellogenesis. If this variation is a result of different-nymphal instars developing into nymphoid neotenics, it is still unknown. Moreover, the social context (e.g., sociotomy and presence of the royal pair) might influence neotenic maturation. Alate females may show stage V or VI oocytes as terminal ones, which reflects different degrees of ovary activation during swarming events. Functional queens always displayed mature terminal oocytes, including 80-d-old individuals. Future analyses should clarify how ovary maturation among individuals of the same caste and other reproductive features impact colony development.
Acknowledgments
The authors are thankful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico – Grant Number 471922/2013-7) for the financial support.
Conflict of interest
The authors declare none.













